Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Research
Received date: 06 June, 2018
Accepted date: 26 June, 2018
Published date: 03 July, 2018
Citation: Wubneh D, Williams D, Eckert A, Evans C, He P, et al. (2018) Aspirin as Antithrombotic Therapy to Prevent Ischemic Stroke/Embolization in Low Risk Patients with Non-valvular Atrial Fibrillation: A Retrospective Review Community Hospital in Columbus, Ohio. Sch J Appl Sci Res. Vol: 1, Issu: 4 (17 - 21).
Copyright: © 2018 Wubneh D, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Patients with atrial fibrillation have substantial risk of stroke, modified by presence or absence of several risk factors. The risk of atrial fibrillation increases after age 60 with its’ highest rate at age 75 years. The current guideline leaves a wide range of variability to which dose of aspirin is appropriate and effective in low risk patients. Our retrospective study endeavors to answer the question as to which dosage of aspirin, 81mg or 325mg, is effective therapy for low risk AF patients.
Methods: Our group performed a retrospective chart review of clinical records in 122 patients with a mean age of 75. Data from each patient was placed into risk category using CHADS2 score. The study utilized descriptive statistics such as mean, medians, ranges, and standard deviations for continuous variables, and percentages for categorical variables.
Results: It appears from the data there is a 14% risk reduction in stroke events in the patients with 81mg verses the 325mg in patient with a mean age of 75.
Conclusions: The results were not statistically significant, but the raw data did show that a larger group of patients receiving aspirin therapy at the 81mg level did indeed have better outcomes than those at 325mg.
Keywords
Aspirin, Stroke, Arrhythmia, Fibrillation, Atrial.
Abstract
Background: Patients with atrial fibrillation have substantial risk of stroke, modified by presence or absence of several risk factors. The risk of atrial fibrillation increases after age 60 with its’ highest rate at age 75 years. The current guideline leaves a wide range of variability to which dose of aspirin is appropriate and effective in low risk patients. Our retrospective study endeavors to answer the question as to which dosage of aspirin, 81mg or 325mg, is effective therapy for low risk AF patients.
Methods: Our group performed a retrospective chart review of clinical records in 122 patients with a mean age of 75. Data from each patient was placed into risk category using CHADS2 score. The study utilized descriptive statistics such as mean, medians, ranges, and standard deviations for continuous variables, and percentages for categorical variables.
Results: It appears from the data there is a 14% risk reduction in stroke events in the patients with 81mg verses the 325mg in patient with a mean age of 75.
Conclusions: The results were not statistically significant, but the raw data did show that a larger group of patients receiving aspirin therapy at the 81mg level did indeed have better outcomes than those at 325mg.
Keywords
Aspirin, Stroke, Arrhythmia, Fibrillation, Atrial.
Introduction
Atrial fibrillation is the most common type of supraventricular arrhythmia. Patients with atrial fibrillation have substantial risk of stroke, which is modified by presence or absence of several risk factors i.e.., hypertension, diabetes mellitus, and congestive heart failure.
During episodes of atrial fibrillation, there is pooling of blood in the atrial appendage which leads to arterial embolization. Atrial fibrillation affects more than 2.2 million persons in the United States. It is strongly age-dependent, affecting 4% of people over the age of 60 and 8% of people over the age of 80 [1-2]. The cost burden of stroke related atrial fibrillation diagnosis and admissions imposed on hospitals as assessed by Coyne et al. [3] indicated that non-valvular atrial fibrillation is attributed to approximately 350,000 hospitalizations, 5 million office visits, 276,000 emergency room visits, and 234,000 outpatient visits annually within United States. This diagnosis represents a greater burden on inpatient healthcare cost rather than outpatient and continues to increase exponentially without foreseeable boundaries. Thus, stroke-related atrial fibrillation has been a significant contributor to hospital cost [3-4]. According to Center for Disease Control (CDC), it is estimated that as many as 12 million people will be diagnosed with atrial fibrillation by 2050, and about 15% of the patients diagnosed with stroke will be admitted in US hospitals as a result of arterial embolization [5]. Aspirin is considered to be effective in preventing stroke in low risk patients with non-valvular atrial fibrillation. The use of aspirin has been favored by many clinical trials due to its low bleeding risk, and reduced need for patient monitoring. Thus, the updated American College of Cardiology/American Heart Association (ACC/ AHA) guideline recommends the use of aspirin but with a wide dose range.
A wide range of clinical interventions have been developed to help prevent arterial embolism as a result of atrial fibrillation, and one of such interventions is the use of antithrombotic therapy. The use of vitamin K antagonist and aspirin have been reported in five (5) randomized controlled trials and proven to be effective in preventing strokes in patients with non-valvular atrial fibrillation and can be quite safe with careful monitoring [6-9].
Patients requiring prevention were placed in one of these categories to ensure maximization of therapy. In addition to the risk stratification index, the ACC/AHA clinical guideline recommends that antithrombotic therapy be initiated to prevent thromboembolism in all patients with AF except in patients with lone AF or existing contraindications [10]. Gage BF et al [11] validate the use of aspirin as an antithrombotic regiment in low risk patients through diagnosis and CHADS2 score stratification selection. In the 2001 Gage study [11], 1733 patients within the age category of 65 to 95 years with a diagnosis of non-rheumatic AF were divided into two treatment groups --- warfarin and aspirin. The Gage et al., [11] study concluded that warfarin therapy was favored when the risk of stroke is high and aspirin was favored when the risk of stroke is low [12-13]. The Gage study also confirmed CHADS2 as an easy-to-use accurate, objective classification scheme that estimates the risk of stroke in elderly patients with AF [11]. Physicians and patients could use CHADS2 to make decisions about antithrombotic therapy based on patient-specific risk of stroke [11]. The Gage study also concluded that CHADS2 score can quantify the risk of stroke for patients who have AF by aiding in selection of antithrombotic therapy [11,14].
Literature Review of Previous Studies
Several pilot studies [2,15-18], on stroke prevention have evaluated the use of aspirin and warfarin as an antithrombotic therapy in patients with atrial fibrillation. The Atrial Fibrillation, Aspirin, Anticoagulation Study (AFASAK) used 75 mg/d of aspirin and found a nonsignificant 18% relative reduction in the risk of ischemic stroke [19]. The European Atrial Fibrillation Trial (EAFT) used 300 mg/d of aspirin and observed a nonsignificant 15% relative reduction in the risk of stroke [20]. The strongest effect of aspirin use was observed in the Stroke Prevention in Atrial Fibrillation I Study (SPAFI) in 1991 [9], where intake of 325 mg/d of aspirin was associated with a 44% relative reduction in the risk of stroke. The SPAFI study was a multicenter, randomized trial which compared 325 mg/day aspirin (double-blind) or warfarin with placebo for prevention of ischemic stroke and systemic embolism (primary events).
The study included 1,330 inpatients and outpatients with constant or intermittent atrial fibrillation in a mean period of 1.3years. From the study, it found that primary event disease occurrence rates in patients assigned to placebo was 6.3% per year, however in assigned to aspirin the disease occurrence rates were reduced by 42%. Within the same SPAFI study, a subgroup of warfarin-eligible patients (most patients were less than 76 years old) were compared to controls and the risk of primary events was also found to be reduced by 67%. Thus, the study concluded overall that both aspirin and warfarin are effective in reducing stroke and systemic embolic events in patients with atrial fibrillation. It should be noted that the warfarin-eligible patients composed of a subset of all aspirin-eligible patients, and therefore the magnitude of reduction in events by warfarin versus aspirin is not a true representation of the effects of aspirin therapy alone. Too few events occurred in warfarin-eligible patients to directly assess the relative benefit of aspirin compared with warfarin and as a result the benefits of aspirin and warfarin were further investigated in a SPAF II study.
The SPAFII study was age-dependent and compared the differential effects of aspirin 325mg/day and warfarin [6] in 715 patients under the age of 75 years old and 385 patients older than 75 years old. The SPAF II study concluded that warfarin may be more effective than aspirin for prevention of ischemic stroke in patients with atrial fibrillation, but the absolute reduction in stroke rate by warfarin is small. Younger patients without risk factors had a low rate of stroke when treated with aspirin [6]. In older patients the rate of stroke (ischemic and hemorrhagic) was substantial, irrespective of which agent was given [6] (Table 1).
Table 1: Compares 81mg group to the 325mg group. CVA - Cerebrovascular accident; TIA - Transient ischemic attack.
| Aspirin Group | Stroke (CVA, TIA) | Total Patients | |
|---|---|---|---|
| NO | YES | ||
| 81mg | 40 | 54(57%) | 94 |
| 325mg | 14 | 14(50%) | 28 |
| Total | 54 | 68 | 122 |
Therefore, patient age and the inherent risk of thromboembolism should be considered in the choice of antithrombotic prophylaxis for patients with non-valvular atrial fibrillation [6].
In addition to SPAF II age-dependent study [6], an analysis of major bleeding was considered in five pooled randomized controlled trials published in The Archives of Internal Medicine (AIM), 1994 edition [7]. This pooled analysis evaluated the embolic rates per age group with or without risk factors in warfarin treated patients and placebo. It showed that in the placebo arm, patients between 65-75 years of age carried an annual embolic rate of 5.7% and patients over 75 years of age had 8.1% in comparison to the warfarin group with 1.7% annual embolic rate regardless of age. The SPAFII study and the AIM 1994 analysis further established that the treatment protocol for patients over the age 75 warfarin is the prophylactic treatment of choice to prevent embolic events. With an established age criterion for initiating warfarin therapy, clear parameters were now present in ascertaining which aspirin dose based on a specific patient population would be effective in preventing embolic events that lead to strokes. An examination of the pooled analysis of the three trials [6], the European Atrial Fibrillation trial [21], the second phase of the SPAFII [1] and The Danish AFASAK (Copenhagen Atrial Fibrillation, Aspirin, Anticoagulation) trial [22] (which is part of pool data from five randomized trials) [7], attempted to answer this important issue regarding stroke prevention in patient with AF [22,23]. Abers [23] reported in special article to the EAFT study in which he documented a mild but statistically not significant benefit of aspirin for prevention of stroke (risk reduction, 16%), which was very similar to the degree of aspirin benefit documented with 75 mg of aspirin in the AFASAK study [21-23]. Abers et al [23] reported that when all three trials were examined there was a 20%-25% aggregate stroke risk reduction attributable to aspirin verses the placebo but still no clear relationship to which aspirin dose was more effective.
In 1999, SPAF III trial evaluated of 1044 patients with AF and at least one thromboembolic risk factor (congestive heart failure or left ventricular fractional shortening ≤ 25%, previous thromboembolism, systolic blood pressure of more than 160 mm Hg at study enrolment, or being a woman aged over 75 years) to determine the effectiveness of aspirin to warfarin. In the SPAFIII trial study patients were randomly assigned either a combination of low-intensity, fixed-dose warfarin (international normalized ratio [INR] 1·2–1·5 for initial dose adjustment) and aspirin (325 mg/day) or adjusted-dose warfarin (INR 2·0–3·0). It was determined from the study that combination therapy was inadequate to prevent stroke in high risk patients. However, observations from the SPAF III trial provided a report for a longitudinal study in which 892 low risk atrial fibrillation (AF) patients were treated with aspirin alone [3].
This study concluded that warfarin reduces the risk of strokes in patient that were high risk and whose ischemic strokes were cardioembolic in nature; while aspirin significantly reduces low-risk AF patients for noncardioembolic strokes [8]. But again, in all the studies presented there is no specific evidence with regard to the evaluation of optimal dosage of aspirin necessary to reduce non-valvular AF non-cardioembolic strokes. This dosage description is pivotal to providing guidance since the range of treatment to prevent non-ischemic non-cardiac strokes in low risk patients at risk is from 81mg to 650mg per day. There is a need to do further studies to definitively clarify the dose of aspirin in preventing atrial fibrillation ischemic strokes [6-9].
Study Design and Procedures
Research Question
Which specific dose of aspirin (81mg versus 325mg) should be consistently recommended as an antithrombotic therapy by clinicians to effectively prevent of Strokes/ Transient ischemic attacks in patients with low risk nonvalvular atrial fibrillation?
Method
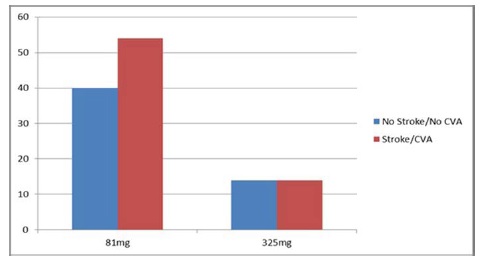
Our group performed a retrospective chart review of clinical records in 122 patients (60=Male; 62=Female) with a mean age of 75.82 admitted to Doctors Hospital in Columbus, OH from 2007 to 2011. The patients were for ischemic events (Stroke or TIA) while on aspirin therapy of 81mg or 325mg. The investigators were provided with password protected computer access and patient information was stored in secured facility with limited access. Patients records were reviewed with regard to their aspirin use (Figure 1). A total of 97% of the patients in the study stated they used aspirin frequently. With regard to dosage, all data collection and storage process strictly followed health insurance portability and accountability act (HIPAA) guidelines in compliance with 21 CFR 46.115(b). All patient health information accessed was erased or destroyed per hospital protocol at conclusion of the study. The medical records were accessed and reviewed for ICD-9 codes (atrial fibrillation, altered mental status, weakness, paralysis, ischemic stroke, and transient ischemic attack) along with pertinent demographic, diagnosis, radiographic (CT or MRI) examinations. All clinical data obtained were recorded on a structured data collection form indicating dose of aspirin. Data from each patient was placed into risk category using CHADS2 score and aspirin dose use was stratified by age, sex, medical record number, and any negative outcomes such as bleeding complications and death.
Figure 1: Relative Risk of the Stroke/TIA, CVA between the 81mg group and the 325 mg group. There is no difference in the relative risk of 14% Stroke/TIA, CVA in the 325 mg group. However, there is a relative risk reduction of the 81mg group. CVA – Cerebrovascular accident.
Population
The population consisted of 122 patients stratified as either CHADS 2 score of zero (low risk patients) or CHADS 2 score of one (moderate risk patients) diagnosed with atrial fibrillation on either aspirin 81mg or 325mg.
Inclusion Criteria
Patients with diagnosis of atrial fibrillation with altered mental status or weakness, as evident on CT or MRI. Patients diagnosed with transient ischemic attack, but a neurological symptom was resolved within 24 hours. Patients stratified as CHADS 2 score of 0 or 1 [10,13-14]. Patients with atrial fibrillation on any of the described aspirin therapy (dosages) (81mg or 325mg).
Exclusion Criteria
Patients with atrial fibrillation on other forms of anticoagulation (enoxaparin, unfractionated heparin, and fondaparinux). Patients with contraindication to aspirin therapy due to history of peptic ulcer disease or bleeding disorders or allergy.
Baseline characteristics
This retrospective study utilized demographic data from the study variables: age, sex, diagnosis, date of diagnosis, aspirin dose group (81mg and 325mg), CHADS 2 score, admission units (emergency department, intensive care unit, and general medical floor), and negative outcomes such as stroke, transient ischemic stroke (TIA) or death.
Statistical analysis
SPSS 22.0 to do the statistics. The study power was set at 80% to detect a significant difference in dose efficacy and evaluated at 5% (p value of p<0.05 as the significance level). The study utilized descriptive statistics such as mean, medians, ranges, and standard deviations for continuous variables, and percentages for categorical variables. A baseline comparison between two groups was done using Pearson’s chi- square test for categorical data and a t-test for continuous data. The outcomes of negative events were evaluated by hazard ratio (HR). Statistical significance was determined using a two-sided p-value. If the p value was greater than the alpha value, then the test was not significant. All data obtained and analyzed was based on intention- totreat.
Results
It appears from the data below there is a 14% risk reduction in stroke events in the patients with 81mg verses the 325mg in patient with a mean age of 75. This was consistent with the Abers EAFT study [21] in which a mild but statistically not significant benefit of aspirin for prevention of stroke (risk reduction, 16%) and very similar to the degree of aspirin benefit with 75 mg reported in the AFASAK study [21,22].
Discussion
Based on previous studies [6-9], the prevalence of strokes/TIA in patients diagnosed with atrial fibrillation is associated with advancing age. In addition, age is evaluated as a risk factor and found that patients less than 65 years old are at low risk of developing stroke when aspirin is given for prophylaxis, and patients greater than 75 years old are at substantially higher risk of developing stroke with or without risk factors. When analyzing bleeding risks in low risk patients on aspirin therapy alone at a dose of 325mg/day of in five pooled trials5 it was found that the risk of bleeding was substantial in patients in the high-risk group rather than in the low risk group. The bleeding risk event occurred at a rate of 2.2% per year versus 0.5% (95% CI, 1.6%-3.0. Thus, the Gage et al., study favors the use of aspirin in low risk patients due to less bleeding risks and a reduced need for patient monitoring. Similarly, three atrial fibrillation studies directly compared aspirin to placebo: it was concluded that 20%-25% reduction of stroke/TIA events was attributed to aspirin. All three trials [7] were in support aspirin treatment group providing a lower the risk of developing stroke, decrease mortality, and improve quality of life in patients younger than 65 years of age. However, when compiling the evidence of aspirin treatment there was a limited relationship to which dose maximized benefit. Our study explored the efficacy of aspirin’s effectiveness in order to establish a dose specific efficacy in low risk patients.
The results from our study did not present a statistically significant level but the raw data did show that a larger group of patients receiving aspirin therapy at the 81mg level did indeed have better outcomes than those at 325mg. Based on the previous studies [6-9], it is evident that age, and risk factors should be considered when choosing antithrombotic prophylaxis in atrial fibrillation and as a result the ACC/ AHA recommends the use of aspirin prophylactic therapy in low risk patients. Physicians and other health care professionals should consider patient’s risk factors prior to recommendation and low dose aspirin at 81mg should be recommended if the benefits outweigh the risk.
Study Limitations
There are some limitations of our study which may or may not have played a role in data collection and its interpretation. The patient demographic was not stratified by race or social economic status. The socioeconomic impact regarding access and compliancy to take aspirin (in spite of the fact that it is an over the counter medication) may have been impacted given the fact patient still need the necessary income to acquire the medication. As a result, income will unequivocally affect compliance rates. We also did not quantify how compliant patients were with regard to daily usage a shortcoming we assumed as part of a retrospective study.
Conclusion
The overall goal of our study has been meeting to provide information regarding the specific dose age of aspirin for low risk AF patients under 65years old. In addition, we hope that our result added to the dialogue of support for the treatment of low risk patient with aspirin. In addition, our study support the recommendation that ubiquitous dosing of the aspirin in the low risk population is be evaluated, and this evaluation results in patient health benefit due to specific dosing. So where do we go from here? The initiation of future studies on a larger scale, to further quantify the benefit of aspirin use in thromboembolic prevention is needed. Our study, though small and done at the community hospital level, shows promise that with more studies, perhaps we will be able to further support aspirin use primarily at higher dosages. A larger study will provide more robust data and additional confirmation with the opportunity to examine its statistical significance.
Acknowledgements
We would like to acknowledge Doctors Hospital in Columbus, Ohio for providing the resources necessary to conduct this this study.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosures
The Author(s) declare(s) that there is no conflict of interest.
Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, et al. (2004) Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 110: 1042-1046 [ Ref ]
Hart RG (1999) Warfarin in atrial fibrillation: underused in the elderly, often inappropriately used in the young (editorial). Heart 82: 539-540. [ Ref ]
Coyne KS, Paramore C, Grandy S (2006) Assessing the direct costs of treating Non-valvular Atrial Fibrillation in the United States. Value in Health 9: 348-356. [ Ref ]
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. (2010) Heart Disease and Stroke Statistics 2010 update: a report from the American Heart Association. Circulation 121: e91. [ Ref ]
Go AS, Hylek EM, Phillips KA, Borowsky LH, Henault LE, et al. (2000) Implications of stroke risk criteria on the anticoagulation decision in nonvalvular atrial fibrillation: the anticoagulation and risk factors in the Atrial Fibrillation (ATRIA) study. Circulation 102: 11-13. [ Ref ]
Stroke Prevention in Atrial Fibrillation investigators (1994) Warfarin versus Aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 343: 687-691. [ Ref ]
Atrial Fibrillation Investigators (1994) Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 154: 1449-1457. [ Ref ]
The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators (1998) Patients with Nonvalvular Atrial Fibrillation at Low Risk of Stroke during Treatment with Aspirin (SPAF III study). JAMA 279: 1273-1277. [ Ref ]
Stroke Prevention in Atrial Fibrillation Investigators (1991) Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 84: 527. [ Ref ]
Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, et al. (2011) ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123: 104-123. [ Ref ]
Gage BF, Waterman AD, Shannon W, Boechler M, Rich, MW, et al. (2001) Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285: 2864-2870. [ Ref ]
Gage BF, Cardinalli AB, Albers GW, Owens DK, et al. (1995) Costeffectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 274: 1839-1845. [ Ref ]
Laupacis A, Albers G, Dalen J, Dunn MI, Jacobson AK (1998) Antithrombotic therapy in atrial fibrillation. Chest 114: 579S-589S. [ Ref ]
Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, et al. (2004) “Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin”. Circulation 110: 2287-2292. [ Ref ]
Atrial Fibrillation Investigators (1994) Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Arch Intern Med 154: 1449-1157. [ Ref ]
Hart RG, Benavente O, McBride R, Pearce LA (1999) Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a metaanalysis. Ann Intern Med 131: 492-501. [ Ref ]
The European Atrial Fibrillation Trial Study Group (1993) Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 342: 1255-1262. [ Ref ]
Laupacis A, Boysen G, Connolly S, Ezekowitz M, Hart B, et al. (1997) The efficacy of aspirin in patients with atrial fibrillation: analysis of pooled data from 3 randomized trials. Arch Intern Med 157: 1237-1240. [ Ref ]
Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B (1989) Placebo controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. Lancet 1: 175-178. [ Ref ]
EAFT Study Group 1993) Secondary prevention of vascular events in patients with nonrheumatic atrial fibrillation and a recent transient ischaemic attack or minor ischaemic stroke. Lancet 342: 1255-1262. [ Ref ]
European Atrial Fibrillation Trial Study Group (1993) Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 342: 1255-1262. [ Ref ]
Petersen P, Godtfredsen J, Boysen G, Andersen ED, Andersen B (1989) Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: The Copenhagen AFASAK study. Lancet 1: 175-179. [ Ref ]
Albers GW (1994) Atrial Fibrillation and Stroke Three New Studies, Three Remaining Questions. Arch Intern Med 154: 1443-1448. [ Ref ]