Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Meta-analysis
Received date: 18 December, 2018
Accepted date: 10 January, 2019
Published date: 14 January, 2019
Citation: Likar LM (2019) Background Ionized Radiation Battery Energy Nuclear. Sch J Appl Sci Res Vol: 2, Issu: 1 (15-24).
Copyright: © 2019 Likar LM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Natural nuclear decay of radioactive elements forms the Earth’s background radiation, which envelopes all living things. In some places, concentrated deposits of radioactive decay elements forms what is called low level radiation. This is the case when extracting and processing rare earth elements.1
Of power sources of a high energy value for the creation of electrical power for the industrial economic benefit of ever larger populations of people. Only the nuclear fission branch of nuclear energy has been achieved for levels of electrical power generation sufficient for those levels of human economic growth. Yet all this power generated for massive, ever growing human wealth, has its origins in the natural properties of these tiny little, out of sight out of mind sub, atomic particles; (Qualitative)₂. Electron mass being 9.1 x10⁻³¹kg ₃(Quantitative)₂.
The simplest low energy, nuclear battery, harnesses the products of the natural decay process of radioactive elements. Where these decay products are of opposite electrical charge, the manipulation of these opposite electrical charged sub atomic particles, allows a controlled electron flow sufficient to produce a working electrical current.
With the electrical attractive force of the negative charged beta (electron) particle {x2}, to the opposite dual positive charged alpha particle, causing the two sub atomic particles to combine into the neutrally charged, stable, full outer, valance shell, noble gas Helium.4 & 23
In nature all the lighter atoms and molecules prefer a full stable, outer electron shell of eight electrons (octet). Even salts in solution with opposing attractive electrical charges (positive cations & negative anions) have full, stable, outer shells of eight electrons. ₅Tiny little atoms like the stable, inert, chemically unreactive helium atom; has a full, stable outer electron shell of two (duplet). This fundamental chemistry principle found in nature strongly suggests that the alpha particle which forms the helium nucleus (two protons + two neutrons) will accept two beta particles as two electrons to become a helium gas atom.
By the Formula: 2β⁻+ ⁴/₂α²⁺→⁴/₂He(𝔤)+ Q/t {Q/t=𝐼} ₆
Nuclear fission & nuclear fusion research has provided humans with the tools of elemental transmutation. 7 Converting one elemental substance into another elemental substance. Just as cosmic rays convert some of the atmosphere of Carbon12 into radioactive carbon14. 8
In a two cell electrochemistry battery, excess electrons produced by the ‘oxidation reaction cell’ are attracted through an external wire to the positive ions produced by the positive ions produced at the ‘reduction reaction cell’.9
By creating short lived, high energy radioactive isotopes (in a nuclear reactor environment) which produce decay material of opposing electrical charge.10
A positive electrode and a negative electrode, for a two cell nuclear battery may be created. Where β⁻ created in negative nuclear decay cell moves along negative electrode to external wire, and is attracted to positive sub atomic particles at positive electrode, located in positive nuclear decay material. 11With the Helium gas electro-chemically manufactured, created product. 11 Similar to natural occurring Helium from natural radioactive processes in the Earth23
But why stop there?
By regulating and restricting the proportion of electrons to alpha particles in a closed environment it can be postulated that diatomic helium can be formed. Either type of covalent bonded Helium can be theoretically predicted. (е- ⍺²⁺ е- е- ⍺²⁺ е-) = He₂ diatomic Helium Molecule. Or (е- ⍺²⁺⍺²⁺ е-)²⁺ = (He₂)²⁺ Helium diatomic complex ion molecule. ₁₃
But why stop there?
Theoretically, as well as the electrical manipulation of the alpha particle to produce the helium atom; combined with nuclear transmutation techniques; various Helium isotopes are feasible. Helium isotopes are the forerunner to the science of economically viable nuclear fusion. Producing enough helium isotopes to provide commercially viable amounts is the next goal. From low energy nuclear batteries, nuclear transmutation of elements, use of radioactive waste products as source material for fusion reactors; is a pure scientific research goal. 14 With significant future commercial implications.
Abstract
Natural nuclear decay of radioactive elements forms the Earth’s background radiation, which envelopes all living things. In some places, concentrated deposits of radioactive decay elements forms what is called low level radiation. This is the case when extracting and processing rare earth elements.1
Of power sources of a high energy value for the creation of electrical power for the industrial economic benefit of ever larger populations of people. Only the nuclear fission branch of nuclear energy has been achieved for levels of electrical power generation sufficient for those levels of human economic growth. Yet all this power generated for massive, ever growing human wealth, has its origins in the natural properties of these tiny little, out of sight out of mind sub, atomic particles; (Qualitative)₂. Electron mass being 9.1 x10⁻³¹kg ₃(Quantitative)₂.
The simplest low energy, nuclear battery, harnesses the products of the natural decay process of radioactive elements. Where these decay products are of opposite electrical charge, the manipulation of these opposite electrical charged sub atomic particles, allows a controlled electron flow sufficient to produce a working electrical current.
With the electrical attractive force of the negative charged beta (electron) particle {x2}, to the opposite dual positive charged alpha particle, causing the two sub atomic particles to combine into the neutrally charged, stable, full outer, valance shell, noble gas Helium.4 & 23
In nature all the lighter atoms and molecules prefer a full stable, outer electron shell of eight electrons (octet). Even salts in solution with opposing attractive electrical charges (positive cations & negative anions) have full, stable, outer shells of eight electrons. ₅Tiny little atoms like the stable, inert, chemically unreactive helium atom; has a full, stable outer electron shell of two (duplet). This fundamental chemistry principle found in nature strongly suggests that the alpha particle which forms the helium nucleus (two protons + two neutrons) will accept two beta particles as two electrons to become a helium gas atom.
By the Formula: 2β⁻+ ⁴/₂α²⁺→⁴/₂He(𝔤)+ Q/t {Q/t=𝐼} ₆
Nuclear fission & nuclear fusion research has provided humans with the tools of elemental transmutation. 7 Converting one elemental substance into another elemental substance. Just as cosmic rays convert some of the atmosphere of Carbon12 into radioactive carbon14. 8
In a two cell electrochemistry battery, excess electrons produced by the ‘oxidation reaction cell’ are attracted through an external wire to the positive ions produced by the positive ions produced at the ‘reduction reaction cell’.9
By creating short lived, high energy radioactive isotopes (in a nuclear reactor environment) which produce decay material of opposing electrical charge.10
A positive electrode and a negative electrode, for a two cell nuclear battery may be created. Where β⁻ created in negative nuclear decay cell moves along negative electrode to external wire, and is attracted to positive sub atomic particles at positive electrode, located in positive nuclear decay material. 11With the Helium gas electro-chemically manufactured, created product. 11 Similar to natural occurring Helium from natural radioactive processes in the Earth23
But why stop there?
By regulating and restricting the proportion of electrons to alpha particles in a closed environment it can be postulated that diatomic helium can be formed. Either type of covalent bonded Helium can be theoretically predicted. (е- ⍺²⁺ е- е- ⍺²⁺ е-) = He₂ diatomic Helium Molecule. Or (е- ⍺²⁺⍺²⁺ е-)²⁺ = (He₂)²⁺ Helium diatomic complex ion molecule. ₁₃
But why stop there?
Theoretically, as well as the electrical manipulation of the alpha particle to produce the helium atom; combined with nuclear transmutation techniques; various Helium isotopes are feasible. Helium isotopes are the forerunner to the science of economically viable nuclear fusion. Producing enough helium isotopes to provide commercially viable amounts is the next goal. From low energy nuclear batteries, nuclear transmutation of elements, use of radioactive waste products as source material for fusion reactors; is a pure scientific research goal. 14 With significant future commercial implications.
Theory
• Will a positive charge be attracted to a negative charge? Yes
Will a positive ion from a radioactive element, from that radioactive element’s decay process be attracted to a negative ion from its radioactive element, from that radioactive element’s decay process. Yes
So an alpha particle with 2 positive charges (2 protons) in its nucleus would be attracted to 2 negatively charged ions or 2 free electrons. Yes. But an alpha particle with 2 protons in its nucleus and 2 neutrons in its nucleus is the Helium nucleus! Therefore adding 2 negatively charged electrons to an alpha particle with 2 positively charged protons should almost certainly create a Helium atom.
• Helium is created in the sun.
• Helium three deposits on the moon are a more suitable fuel for nuclear fusion
• Helium deposits occur in some deposits on Earth and are assumed to have been left on Earth 5 billion years ago at Earth’s beginning.
• The Helium deposits on the Earth that were assumed to have been created in a previous sun and were picked up in the gravitational forces during Earth’s creation 5 billion years ago.
What if a radioactive deposit rich in trapped alpha particle decay element material. Was exposed to salt solutions rich in positive and negative ions in solution. Would the alpha (positively charged with 2 protons) be more electronegative than other elements, and therefore accept the outer shell valance electrons more readily the some of the positive ions in solution?
• Therefore there is sufficient theoretical evidence to speculate on a nuclear battery based on at least two radioactive elements of opposing decay charges, such as one radioactive decay element yielding a positively charged alpha particle while the second radioactive element yielding the negatively decay product, the beta particle, (single electron at the speed of light). With the obvious byproduct being the neutrally charged, inert gas, unreactive, Noble gas, Helium gas!
• Taking the above theory a step further. As Helium 3 is found on the moon. Theoretically Helium 3 is an easier reactant to use in nuclear fusion reactions. Can a suitable form of Helium be manufactured on earth, (a) artificially from naturally radioactive decay products like the alpha particle, or (b) from alpha yielding radioactive byproducts from the commercial nuclear process, or (C) a new process additional and within a current commercial nuclear reactor?
Commercial Applications
• There is NOT enough charged particles available per second to create enough current from either uranium 238 or uranium 235. Longer the half life less radioactive particles released in a short time period. Fast radioactive decay elements release more ionized radiation in a short period so are more dangerous to tissue, but release a greater number of ionized charged particles in a short time period. Such as 90Strontium.
There is barely enough charged particles available per second to create enough current from radium226, to create the bulky alpha half, of an opposing charge, ionization nuclear battery.
One kilo of radium produces only enough alpha particles from radioactive decay to provide conservatively about 8 micro amps of positive charge.
Therefore a country would need to have plentiful supplies of positive ion, alpha yielding radium, along with plentiful supplies of a radioactive decay element yielding negative charged, beta particles. Or a nuclear industry with available suitable radioactive waste elements with the ability to separate elements that release opposing ionized charged particles.
• As rooms of fridge size batteries would be required to supply the alpha component of the very slow forming, and very low volume of helium gas manufacture. The amount of inert helium gas product from radium is too low, over too long a period of time, to be economic. Unless a more rapid decay alpha particle were extracted from a synthetically created element from a nuclear reactor.
• Uranium 235. Uranium 238. Thorium 232. Radium 226. Are all considered low level radioactive materials. However all of these radioactive elements will cause cancer over time and cause death and a reduced life expectancy from cancer caused by radiation exposure. These are NOT for residential or commercial use.
A nuclear power company or a military installation could use medium level radioactive decay elements in a nuclear battery process, to produce a greater volume of charged particle from a lesser volume of radioactive element material.
How much Helium Gas?
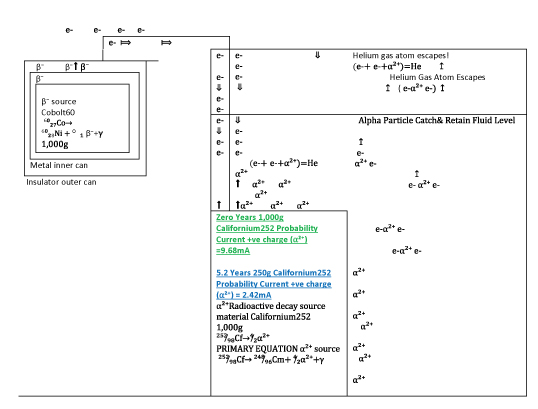
Californium
²⁵²̸₉₈Cf→ ²⁴⁸̸₉₆Cm+ ⁴̸₂α²⁺ +γ 2.6 years half life
²⁵²̸₉₈Cf→⁴̸₂α 1 : 1
1,000kg ²⁵²̸₉₈Cf breaks down to 500 kg in 2.6 years.
mol ²⁵²̸₉₈Cf = m/M= 5.0 x 10⁵ grams/252= 1.98 x 10³mol
mol ratio 1 : 1
grams ⁴̸₂α = m x M = 1.98 x 10³mol x 4 = 7.93 x 10³ grams.
Or 7.9 kilograms ⁴̸₂He gas from 500 kg ²⁵²̸₉₈Cf over 2.6 years!
Radium
226Ra → 222Rn + 4/2α With a half-life of 1,600 years.
1 : 1 : 1
1,000kg 226Ra breaks down to 500 kg in 1,600 years.
mol ratio 1 : 1
mol ²²⁶̸₈₈Ra=m/M = 5.0 x 10⁵ grams/226 = 2.21 x 10³ mol.
Grams ⁴̸₂α = m x M = 2.21 x 10³mol x 4 = 8.85 x 10³grams
Or 8.85 kilograms⁴̸₂He gas from 500 kg ²²⁶̸₈₈Ra But it takes 1,600 years.
It takes 1,600 years for 8.85 kilograms ⁴̸₂He gas to accumulate from 500 kg ²²⁶̸₈₈Ra
(Figure 1 and equations; Figure 2 and equations)
Background Ionized Radiation Battery Energy Nuclear
Theory
Taking the above theory a step further. As Helium 3 is found on the moon. Theoretically Helium 3 is an easier reactant to use in nuclear fusion reactions. Can a suitable form of Helium be manufactured on earth, (a) artificially from naturally radioactive decay products like the alpha particle, or (b) from alpha yielding radioactive byproducts from the commercial nuclear process, or (C) a new process additional and within a current commercial nuclear reactor?(b)
Method 1
• Restrict the quantity of electrons applied to fluid containing the ⍺²⁺by controlling the number of amps per second from the power supply. To a quantity of electrons less than required to make a complete helium molecule from the alpha particles.
• Restrict the quantity of alpha particles applied to the fluid in the fluid containing that captures the ⍺²⁺particles, by closing off the ⍺⁺Radioactive decay source material in a separate internal enclosure.
• Reduce the ratio of electrons available for the number of alpha particles available each trial. And measure if any of the alpha particles have formed a covalent bonded Helium molecule.
• What is the percentage of the alpha particles that have formed a covalent bonded Helium molecule; to normal Helium atoms. If any? Two types of covalent bonded Helium predicted. (е- ⍺²⁺ е- е- ⍺²⁺ е-) = He₂ diatomic Helium Molecule; Or (е- ⍺²⁺⍺²⁺ е-)²⁺ = (He₂)²⁺ Helium diatomic complex ion molecule.
(e)If either of the two new Helium molecules form they may offer new chemical compounds and new types of nuclear fusion fuel.
The first Experiment was to determine if the alpha particle with two neutrons and two protons as its nucleus would accept two foreign sourced electrons and go from a dual positive charged ion to a neutral charged atom with a stable, first outer shell of two electrons.
In this second Experiment the volume or number of electrons is restricted so:
only a percentage of alpha particles will form neutral charged atoms with stable outer shell of two electrons.
In a closed restricted electron environment will two alpha particles (both with dual charges) share two electrons to form a stable first shell of two; and exist as a positively charged diatomic ion? What is the probable percentage of remaining alpha particles, created helium atoms, and diatomic helium molecules?
Therefore, which is the fundamental force. For an atom or molecule to create a stable, FULL outer electron shell, or the electromagnetic forces of attraction to be balanced. Remembering, that in chemistry, positive and negative charged ions (cations & anions respectively), exist in solution as common salts with stable FULL outer shells of eight (octet). So, some alpha particles should become diatomic helium molecules with a FULL electron outer shell of two.
(Figure 3)
Method 2
(a)Restrict the quantity of electrons applied to fluid containing the ⍺²⁺by controlling the number of phosphorous atoms with excess electrons in silicon semiconductor material. To a quantity of electrons less than required to make a complete helium molecule from the alpha particles.
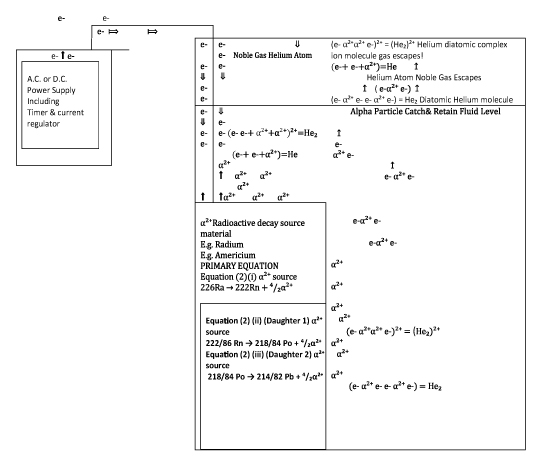
Figure 1:Diagrammatic Representation of A Background Ionization Radiation (Opposite Charged Sub Atomic Particles) /Nuclear Energy Battery Experiment to determine if viable, a long-lasting battery, with enough current from opposing charged particles, from radioactive decay elements (with helium atoms as byproduct)
Figure 2:Construction Diagram of Experiment Of ‘The Creation Of The Helium Atom’. By Combining The Radioactive, Positively Charged Ion, Alpha Particle (Derived from Radioactive Decay Element); With Negatively Charged Electrons from An External Source. The Helium Atom Is Theoretically Created.
(b) Restrict the quantity of alpha particles applied to the fluid in the fluid containing that captures the ⍺²⁺particles, by closing off the ⍺⁺Radioactive decay source material in a separate internal enclosure.
(C) Reduce the ratio of electrons available for the number of alpha particles available each trial. By choosing smaller and smaller pieces of silicon semiconductor material doped with phosphorous atoms. And measure if any of the alpha particles have formed a covalent bonded Helium molecule.
(d) What is the percentage of the alpha particles that have formed a covalent bonded Helium molecule; to normal Helium atoms. If any? Two types of covalent bonded Helium predicted. (е- ⍺²⁺ е- е- ⍺²⁺ е-) = He₂ diatomic Helium Molecule; Or (е- ⍺²⁺⍺²⁺ е-)²⁺ = (He₂)²⁺ Helium diatomic complex ion molecule.
Figure 3:Theoretical Experiment for The Creation of a Helium Diatomic Molecule. By Combining The Radioactive, Positively Charged Ion, Alpha Particle (Derived from Radioactive Decay Element); With A Smaller Number of Negatively Charged Electrons from An External Source. The Ratio of Electrons Is Restricted to Alpha Particles Is Restricted. The Ratio of Electrons Is Restricted by The Equipment’s Adjustable Current Flow. The Positive Charged Ion Alpha Particles Compete for The Restricted Number of Electrons from The Reduced Current Levels into The Closed System
(e)If either of the two new Helium molecules form they may offer new chemical compounds and new types of nuclear fusion fuel.
(Figure 3,4 and 5; Tables 1,2)
There are many theoretical & practical varieties of engineering designs for this particular concept of a nuclear battery. However, the concept itself on which the engineering designs (theoretical & practical) is NOT engineer reversible. As a concept is the foundation principle on which any and all engineering designs are based. The concept on which this nuclear battery is based, is opposite charged electrical sub atomic particles are attracted to each other. Therefore, opposite charged electrical sub atomic particles from radioactive decay material are attracted to each other; sufficient to produce a usable flow of electrons (current, I; ranging in amps to milli-amps to nano-amps etc.) By manipulating physical conditions (engineering), negatively charged beta particles emitted (from radioactive decay material) at near light speed, can be utilized as a flow of electrons (negative current flow, I), when attracted to oppositely positively charged sub atomic proton particles (twin protons combined with twin neutrons from alpha particles, from radioactive decay material, positive current flow, I).
By the Formula: 2β⁻+ ⁴/₂α²⁺ →⁴/₂He(𝔤)+ Q/t {Q/t=𝐼}
I don’t consider these concepts as patentable, however this document is original and I have declared this original document an open copyright & open patent document anyway.
Conclusion
Opposite charged ions from radioactive decaying elements, theoretically should provide enough current (charged particles per second), and an electrical potential difference, to perform electrical work. From micro-amps to milliamps. But common naturally occurring radioactive alpha isotopes, have too long a half-life to provide practical low amps of power.
Figure 4:Experiment: The Creation of a Helium Diatomic Molecule from the Radioactive Ion Alpha Particle Base.
Unless a basketball court of fridge size nuclear batteries is considered more practical than say a small creek hydroelectric unit.
Above or below ground.
Synthetically created elements from a nuclear process or nuclear reactor, such as Californium²⁵²̸₉₈Cf with a half-life of 2.6 years. Still looks like the only possible viable economic ancillary nuclear reactor process.
As the alpha particle consists of 2 positive charged protons + two neutrons, the alpha particle will attract negative ions in the air and water, as well as negative charged beta particles. The radioactive decay product alpha particle is also the exact equivalent of the Helium nucleus, 2 protons + 2 neutrons. Not only should the positively charged alpha particle attract a negatively charged beta particle or electron, capturing that beta particle or electron, should see the alpha particle becoming a neutral charged Helium atom. By the Formula: 2β⁻+ ⁴/₂α²⁺→⁴/₂He(𝔤)+ Q/t{Q/t=𝐼}
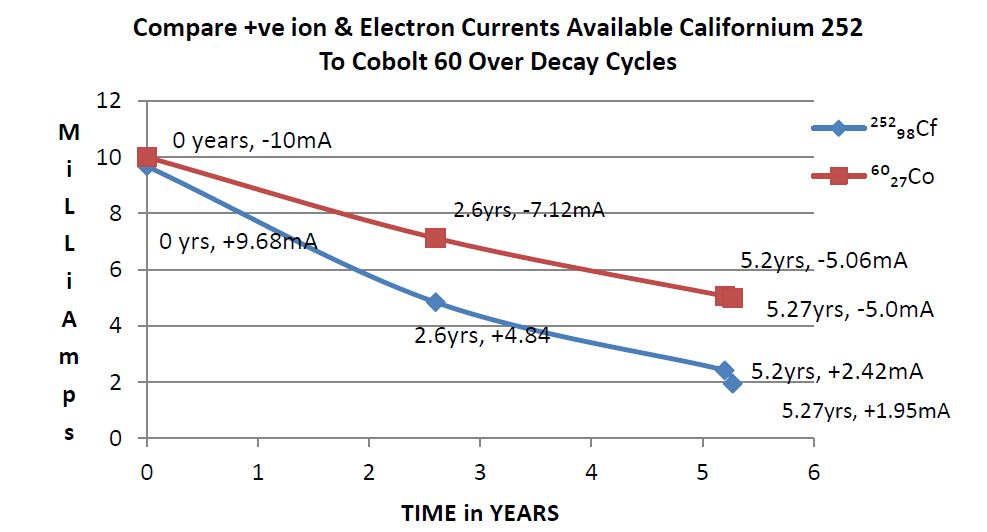
Figure 5:Compare +ve ion & Electron Currents Available Californium 252 To Cobolt 60 Over Decay Cycles.
| Time in Years | Mass Start Cycle | Mass Per Second Available Radioactive Decay Material | NEGATIVE Charged Particles Beta- Electrons Available Per Second | 60 ̸27 Co Negative Current Flow Amps |
|---|---|---|---|---|
| Initial | 1,000g 60 ̸27Co | 6.0170x10-6grams/sec of mass 60/27 Co material | 6.017038 x10¹6 beta particles per second | Negative charge 1.0028x10-² amps =10.0mA |
| 2.6 years | 710.42g 60 ̸27Co | 4.274624 x10-6grams/sec of mass 60 ̸27Co material | 4.274624 x1beta particles per second | Negative charge 7.1243739 x10-³ amps =7.12mA |
| 5.2 years | 504.69910 grams | 3.0367938 x10-6grams/sec or mass 60 ̸27Co material | 3.0367938 x10¹6 Beta particles Per second | Negative charge 5.061323 x10-³ amps =5.06 mA |
| 5.27 years (half-life 60 ̸27Co ) | 500g 60 ̸27Co | 3.008796 x10-6grams/sec of mass 60 ̸27Co material | 3.008796 x10¹6 beta particles per second | Negative charge 5.01466x10-³ amps =5.0mA |
Table 1: Available Negative Charge (Beta/Electrons) in Amps and mA from the Radioactive Decay Cycle of a Given Mass of 60 ̸27Co See appendix for calculation & calculation method Nuclear Decay Ionization Battery 60 ̸27Co NEGATIVE Potential Current Calculation
| Time in Years | Mass Start Cycle | Mass Per Second Available Radioactive Decay Material | POSITIVE Charged Particles Alpha Twin Protons Available Per Second | ²⁵²̸₉₈Cf Positive Current Flow Amps |
|---|---|---|---|---|
| Initial | 1,000g²⁵²̸₉₈Cf | 1.219607 x10⁻⁵grams/sec. Mass ²⁵²̸₉₈Cf material | 2.903827 x1016of alpha particles per second | Positive charge of 9.679423 x10⁻³ amps = 9.68mA |
| 2.6 years (halflife) | 500g²⁵²̸₉₈Cf | 6.0980369 x10⁻⁶grams/sec. Mass ²⁵²̸₉₈Cf material | 1.451913 x1016 alpha particles per second | Positive charge of 4.8397118 x10⁻³ amps = 4.84mA |
| 5.2 years | 250g ²⁵²̸₉₈Cf | 3.049018 x10⁻⁶grams/sec. Mass ²⁵²̸₉₈Cf material | 7.259567 x1015 alpha particles per second | Positive charge of 4.8397118 x10⁻³ amps = 2.42mA. |
| 5.27 years | 201.708 g ²⁵²̸₉₈Cf | 2.4600456 x10⁻⁶ grams/sec. Mass ²⁵²̸₉₈Cf material | 5.8572515 x1015 alpha particles per second | Positive charge of 1.9524 x10⁻³ amps = 1.95mA. |
Table 2: Available Positive Charge (Alpha/Proton Pairs) In Amps and mA from the Radioactive Decay Cycle of a Given Mass of ²⁵²̸₉₈Cf See appendix for calculation & calculation method Nuclear Decay Ionization Battery ²⁵²̸₉₈Cf POSITIVE Potential Current Calculation
Figure 6:Experiment to determine if viable, long lasting battery, with enough current from radioactive decay elements, producing opposing charged ions, with a helium atom byproduct/ Cobolt60-Californium252-Nuclear Ionized Radiation Battery 1,000g Cobolt60 Zero Years Probability Current –ve charge (е-) =10mA 504.7 grams Cobolt60 At 5.2 Years Probability Current –ve charge (е-) =5.06mA
particle is also the exact equivalent of the Helium nucleus, 2 protons + 2 neutrons. Not only should the positively charged alpha particle attract a negatively charged beta particle or electron, capturing that beta particle or electron, should see the alpha particle becoming a neutral charged Helium atom. By the Formula: 2β⁻+ ⁴/₂α²⁺→⁴/₂He(𝔤)+ Q/t{Q/t=𝐼}
Only experimentation will reveal if this theory is correct
Secondly if it does prove possible to create the Helium atom in the lab, or commercially from nuclear waste. Are there other Helium isotopes, or even molecules that can be constructed from the radioactive decay alpha positively charged particle.
Could either or both types of covalent bonded Helium be theoretically predicted? (е- ⍺²⁺ е- е- ⍺²⁺ е-) = He₂ diatomic Helium Molecule. Or (е- ⍺²⁺⍺²⁺ е-)²⁺ = (He₂)²⁺ Helium diatomic complex ion molecule.
Possible fusion reaction products
⁴̸₂He₂+⁴̸₂He₂→¹⁶̸₈O Terra forming possibilities
2²̸₁H+⁴̸₂He₂ →⁸̸₄Be +⁴̸₂He
²̸₁H+⁴̸₂He₂→⁶̸₃Li+⁴̸₂He
2²̸₁H+⁴̸₂He₂→⁶̸₃Li₂
Only experimentation will reveal if this theory is correct
Acknowledgement
I acknowledge this is all my original work and by publishing I acknowledge my work now becomes an open patent, and anyone who wishes is free to do further theoretical and or practical work based on my presentation of work.
Victorian (2011) Teacher notes Frankston TAFE. VCE Chemistry 1&2.[ Ref ]
Victorian (2011) Chemical Connections Frankston TAFE. VCE Chemistry 1&2.[ Ref ]
Victorian (2011) Teacher notes Box TAFE. VCE Physics 1&2[ Ref ]
Victorian (2011) Nelson Physics Storen/Martine Box Hill TAFE. VCE Physics 1&2.[ Ref ]
Victorian (2012) VCE Chemistry 3&4. Teacher notes Box Hill TAFE[ Ref ]
Victorian (2012) Chemistry 2 Heinemann Pearson Box Hill TAFE. VCE Chemistry[ Ref ]
Smith (1987) Conquering Chemistry. Mc Graw Hill[ Ref ]
Victorian Diploma Of Laboratory Technology, Chemistry Teacher notes, Box Hill TAFE[ Ref ]
Bachelor Of Science Chemistry Major 1st Year Swinburne University:[ Ref ]
CHE10001_Lecturer notes (Professor Feng, Dr Eldridge, Dr Malherbe) Reference Text Book Chemistry 12th Edition Chang/Goldsby, McGraw Hill,[ Ref ]
Fundamentals Of Analytical Chemistry 9E, Skoog/West_ Brooks Cloe Cengage Learning.[ Ref ]
CHE10002_Lecturer notes:(Professor Feng, Dr Eldridge, Dr Malherbe) Reference Text Book Chemistry 12th Edition Chang/Goldsby, McGraw Hill,[ Ref ]
Fundamentals Of Analytical Chemistry 9E, Skoog/West_ Brooks Cloe Cengage Learning.[ Ref ]
Extra: Schuam’s Outlines Beginning Chemistry 2nd Edition Goldberg Mc Graw Hill[ Ref ]
Advanced Physics For You 2nd Edition, Johnson/Hewett/Holt/Miller, Oxford University Press.[ Ref ]
Proposal Paper, 4 pages, 4th July 2016,(Hand Written Notes, NO Internet). Addressed to One Professor, Three Doctors, at both Swinburne University’s Chemistry & Physics Departments.[ Ref ]
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/ Isotopes_of_radium July 2018[ Ref ]
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/ Strontium-90 July 2018[ Ref ]
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/ Radium-223 July 2018[ Ref ]
file:///H:/Decay%20Nuclear%20Elements/978-3-642-02586-0_ BookBackMatter.pdf[ Ref ]