Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Review
Received date: 21 July, 2018
Accepted date: 24 August, 2018
Published date: 05 September, 2018
Citation: Elzohry AAM, Foli AME (2018) Basics of Acute Postoperative Pain. Sch J Appl Sci Res. Vol: 1, Issu: 6 (19- 23).
Copyright: © 2018 Elzohry AAM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction and objectivesAcute pain is an important fear for most patients and influences their recovery and overall experience. Poorly treated, it could lead to undesirable effects and patient dissatisfaction. Hence, it is important to understand, assess and treat acute pain effectively. Pain management has been transferred from intraoperative into perioperative period throughout the emergence of modern anesthesiology. Pain management in postoperative period is one of the most essential components of sufficient post-surgical patients care. The objective of this review is to define and demonstrate the risks and different sequela of acute postoperative pain.
Keywords
General anesthesia, Regional anesthesia, Acute postoperative pain, Upper abdominal surgery.
Abstract
Introduction and objectivesAcute pain is an important fear for most patients and influences their recovery and overall experience. Poorly treated, it could lead to undesirable effects and patient dissatisfaction. Hence, it is important to understand, assess and treat acute pain effectively. Pain management has been transferred from intraoperative into perioperative period throughout the emergence of modern anesthesiology. Pain management in postoperative period is one of the most essential components of sufficient post-surgical patients care. The objective of this review is to define and demonstrate the risks and different sequela of acute postoperative pain.
Keywords
General anesthesia, Regional anesthesia, Acute postoperative pain, Upper abdominal surgery.
Introduction
Ronald Melzack said that “By any reasonable code, freedom from pain should be a basic human right, limited only by our knowledge to achieve it”. It is the basic duty of all healthcare professionals to relieve pain, and the most important indication for treating pain after surgery is humanitarian [1].
Acute pain is an important fear for most patients and influences their recovery and overall experience. Poorly treated, it could lead to undesirable effects and patient dissatisfaction. Hence, it is important to understand, assess and treat acute pain effectively [2].
Pain may be classified according to its presumed etiology; nociceptive pain is due to the stimulation of nociceptors by noxious stimuli and neuropathic pain is the result of dysfunction of the nervous system. Pain may also be classified into somatic and visceral pain. An alternative classification is based on duration [3].
Acute pain is defined as ‘pain of recent onset and probable limited duration. It usually has an identifiable temporal and causal relationship to injury or disease’. The point at which acute pain becomes chronic has been suggested at about 12 weeks or when the pain is no longer thought to be due to the initial insult [4].
Pain associated with any surgery can be divided into somatic pain and visceral pain. Therefore, when performing epidural analgesia for abdominal surgeries, both the abdominal wall innervations and the afferent visceral innervations, must be targeted to provide optimal analgesia. The innervations of the abdominal wall has a segmental dermatomal distribution and is supplied by the anterior and lateral cutaneous branches of the ventral rami of the seventh to twelfth intercostal nerves (T7-12) [5].
Pain Perception and Nociceptive Pathways
The ability of the somatosensory system to detect noxious and potentially tissue-damaging stimuli is an important protective mechanism that involves multiple interacting peripheral and central mechanisms. The neural processes underlying the encoding and processing of noxious stimuli are defined as ‘nociception’ [6]. In addition to these sensory effects, the perception and subjective experience of ’pain’ is multi factorial and will be influenced by psychological and environmental factors in every individual.
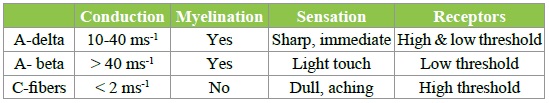
Acute pain perception begins with activation of specific sensory nerves, termed nociceptors. These are unencapsulated free nerve endings that are present in the skin, deep somatic tissue and viscera. Providing the stimulus is suitably intense, high threshold nociceptors will still activate in the absence of actual tissue damage. Nociceptors are probably activated by mechanical distortion of the nerve leading to an increase in H+ and K+ concentration. Nociceptors can be divided into two main classes; A-delta and C fibers. The main properties of these fibres are summarized in the Table 1 below [7].
Table 1: Classification and properties of neurons.
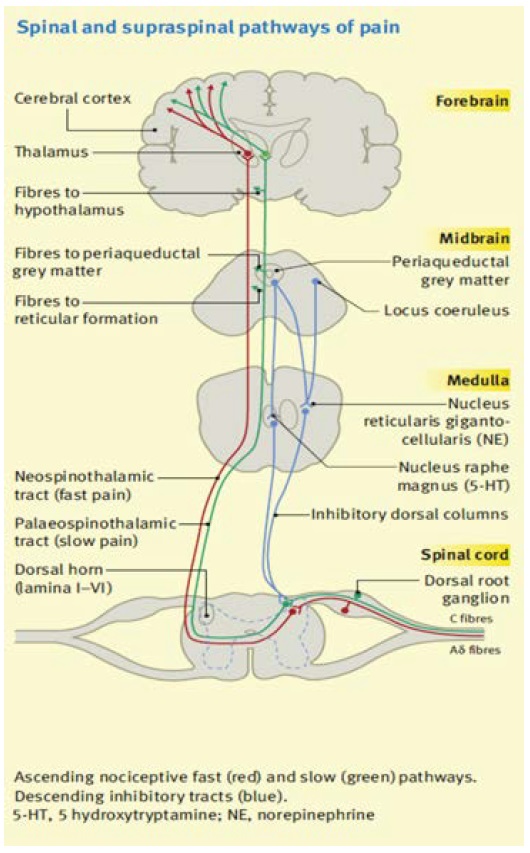
Multiple tracts and centers exist within the central nervous systems which are responsible for the transmission, modulation and perception of noxious stimuli. It is important to realize that these areas should not be considered as fixed or functioning in isolation. Rather, they are subject to change from both descending and ascending pathways and can alter or expand their connections to interact with adjacent nerves [8].
Cell bodies of afferent nerves lie in the DRG with fibres synapsing in the dorsal horn of the spinal cord. The output from the dorsal horn is however dependent on other neuronal input to the synapse. Afferent neurons may divide prior to entering the cord and send branches cephalic or caudal in the longitudinal tract of Lissauer before synapsing with dorsal horn neurons. The result of this being that a single C-fiber afferent may be responsible for innervating dorsal horn neurons at multiple spinal levels [9].
The grey matter of the spinal cord can be divided into ten physiologically and histological distinct layers known as rexed lamina. Laminae 1 to 6 and 10 are the sites that sensory nerves synapse with dorsal horn cells and are important in pain transmission. Laminas 7-9 are involved with motor function Figure 1 [10].
Figure 1: Spinal and Supra spinal pathways of Pain.
Physiology of Pain
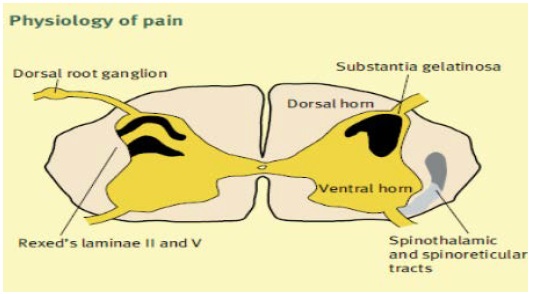
The ability of the somatosensory system to detect noxious and potentially tissue-damaging stimuli is an important protective mechanism that involves multiple interacting peripheral and central mechanisms. The detection of noxious stimuli requires activation of peripheral sensory organs (nociceptors) and transduction into action potentials for conduction to the central nervous system. Nociceptors are stimulated by chemical, thermal or mechanical damage and trigger the nociceptive impulses [11].
Nociceptive primary afferents are widely distributed throughout the body (skin, muscle, joints, viscera, meninges) and comprise both lightly myelinated A-delta fibers (diameter 2-5 mm) and slow-conducting unmyelinated C-fibers (diameter <2 mm). These fibers enter the dorsal horn of the spinal cord and synapse at different sites (Aδ at laminae II and V, C at laminae II). The substantia gelatinosa (lamina II) integrates these inputs and second-order neurons form the ascending spinothalamic and spinoreticular pathways on the contralateral side, Figure 2 [12].
Figure 2: Physiology of pain.
The larger AB fibres conducting “touch” and descending pathways stimulate inhibitory interneurons within the substantia gelatinosa and inhibit C fibre nociceptive inputs. This is the basis of the gate theory of pain. Pain may be modified by altering the neural pathway from its origin at the nociceptor to its interpretation within the central nervous system by various agents. Psychological factors that influence the experience of pain include the processes of attention, other cognitive processes (e.g. memory/ learning, thought processing, beliefs and mood), behavioural responses, and interactions with the person’s environment [13].
Assessment and Measurement of Acute Pain
Pain should be assessed within a bio psychosocial model that recognizes that physiological, psychological and environmental factors influence the overall pain experience. The assessment of acute pain should include a thorough general medical history and physical examination, a specific ‘pain history’ and an evaluation of associated functional impairment along with any side effects of treatment. In acute pain management, assessment must be undertaken at appropriately frequent intervals [14].
Sometimes associated factors such as hyperalgesia, the stress response (e.g. plasma cortisol concentrations), behavioural responses (e.g. facial expression), functional impairment (e.g. coughing, ambulation) or physiological responses (e.g. changes in heart rate) may provide additional information [15].
The assessment of acute pain should include a thorough general medical history and physical examination, a specific ‘pain history’ and an evaluation of associated functional impairment. In acute pain management, assessment must be undertaken at appropriately frequent intervals. At these times, evaluation of pain intensity, functional impact, and side effects of treatment must be undertaken and recorded using tools and scales that are consistent, valid and reliable [16].
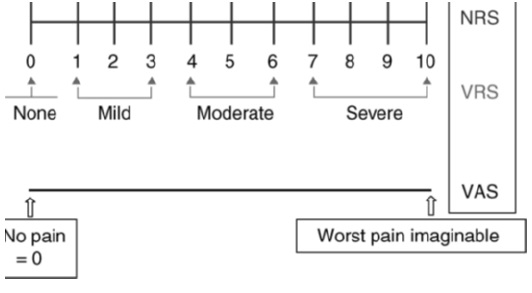
The well-known visual analogue scale (VAS) and numeric rating scale (NRS) for assessment of pain intensity agree well and are equally sensitive in assessing acute pain after surgery, and they are both superior to a four-point verbal categorical rating scale (VRS). They may be used for worst, least, or average pain over the last 24 h, or during the last week [17].
Assessment of pain immediately after surgery can be more difficult and lead to greater inter patient variability in pain scores because of transient anesthetic-related cognitive impairment and decreases in visual acuity. (Machata et al., 2009) Figure 3.
Figure 3: Commonly used one-dimensional pain intensity scales: the 11-point NRS, the VAS from no pain [0] to worst pain imaginable [10] and the four-point categorical verbal rating scale (VRS).
Adverse Physiological and Psychological Aspects of Pain
Clinically significant injury such as surgeries responses can lead to a range of physiological effects which may lead to adverse clinical effects. Patients at greatest risk of adverse outcomes from unrelieved acute pain include very young or elderly patients, those with concurrent medical illnesses and those undergoing major surgery [18].
Sustained acute nociceptive input, as occurs after surgery, can also have a major influence on psychological function, which may in turn alter pain perception. Failure to relieve acute pain may result in increasing anxiety, inability to sleep, demoralization, a feeling of helplessness, loss of control, inability to think and interact with others -in the most extreme situations, where patients can no longer communicate, effectively they have lost their autonomy [19].
Aggressive perioperative pain prevention can yield both short-term and long-term benefits as unrelieved pain affects patient recovery, prolongs hospital stays, increases hospital morbidity, and adds to the burden of growing healthcare costs. In total, there are many important reasons for aggressive acute pain management [20].
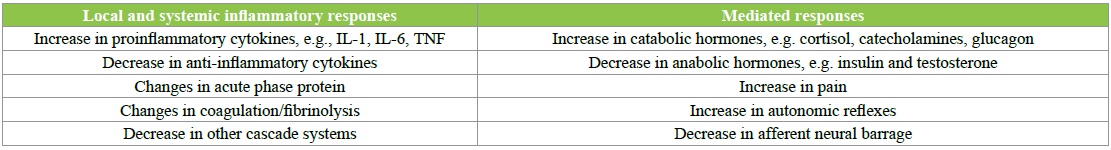
Postoperative pain is considered a form of acute pain secondary to surgical trauma and is associated with an inflammatory reaction and subsequent pathway of afferent neuronal signals. Acute postoperative pain is associated with autonomic, endocrine-metabolic, physiological, and behavioural responses summarized in following Table 2 [21].
Table 2: Effects of surgery, including local and systemic inflammatory responses and mediated responses.
Acute surgical pain causes a global sympathetic response capable of increasing heart rate, peripheral vascular resistance, blood pressure, and subsequently cardiac output. This sympathetic cascade can increase the oxygen demand of the myocardium and potentiate myocardial ischemia, especially in patients with preexisting coronary artery disease Table 3 [22].
Table 3: Adverse effects of undertreated acute pain.
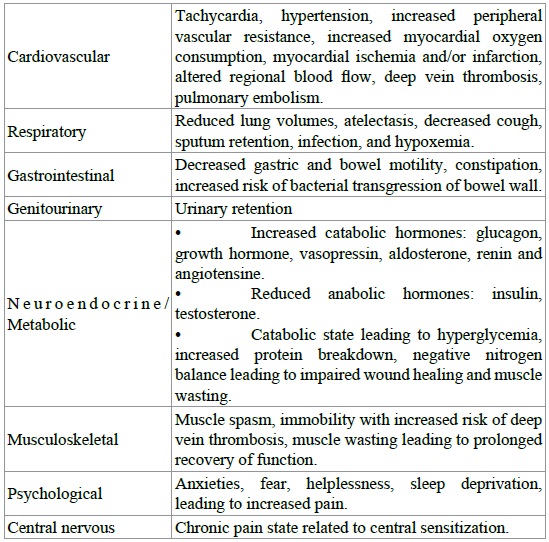
Acute pain has also been found to decrease limb blood flow by directing blood away from skin and viscera and toward vital organs. This decrease in extremity blood flow may impair wound healing and increase muscle spasm. Postoperative pain may also reduce patient mobility, promoting venous stasis. Increases in fibrinogen and platelet activation related to surgical trauma will increase blood coagulability. These factors together increase the risk of venous thromboembolism [23].
Data suggest that “the use of epidural anesthesia in the perioperative period results in less platelet activation and significantly better fibrinolytic function” thereby resulting in significant protection against Thromboembolic complications. This protection also may be related to the systemic effect of local anesthetics, which have been shown to have an antithrombotic effect [24].
Perioperative cardiovascular adverse events may result in considerably prolonged postoperative stay. Cardiovascular events warranting unanticipated hospital admissions are infrequent. Cardiovascular events occur with higher frequency among patients with pre-existing cardiovascular diseases (e.g. hypertension, congestive heart failure). Increasing age is also associated with higher incidence of cardiovascular conditions among elderly patients [25].
Pain management
Non-pharmacological methods of pain relief
- Preoperative explanation and education
- Relaxation therapy
- Hypnosis
- Cold or heat
- Splinting of wounds
- Transcutaneous electrical nerve stimulation (TENS).
Pharmacological and interventional methods of pain relief
There are many techniques available for management of postoperative pain including:
- Intravenous administration of opioids and non-steroidal anti-inflammatory drugs, alpha-adrenergic drugs;
- Infiltration of local anesthetics;
- Nerve blocks;
- Epidural techniques;
- Intratechal technique [26].
All of these techniques have benefits and risks (advantage and disadvantage) Patient control analgesia (PCA) is one of the most common techniques for postoperative pain management. This device is under patient control intermittently or continuously, and infuses IV opioids or non- opioids. The use of intravenous opiates is still limited because of side effects such as respiratory depression [27].
Pre-emptive Analgesia
The administration of analgesia before surgery (preemptively) may be effective in reducing the postoperative pain from surgery by preventing the peripheral and central sensitization caused initially by surgical incision and later by inflammatory injury. There are studies that support the effectiveness of pre-emptive analgesia. However, other studies concluded that there was a lack of evidence for preemptive treatment with NSAlD’s, intravenous opioids, and ketamine, peripheral local anesthetics, and caudal analgesia [28].
The effectiveness of preemptive analgesia is likely to remain a controversial issue for some time. Despite this, the preoperative administration of non-opioid analgesia, such as NSAlDs, ketamine, and local anesthetics, prior to surgical incision is an important component in reducing postoperative pain scores and analgesic requirements in the first 24 h after discharge [29].
Regional anesthesia and analgesia can be used to significantly reduce postoperative pain scores and spare the use of systemic opioids. Regional anesthesia can be performed at the neuraxis (epidural and intrathecal), the nerve root (paravertebral), and the peripheral nerve (transversus abdominis plane) level. Local anesthetic deposition at these sites will selectively block nerve conduction and result in different analgesic and side effect profiles [30].
Conclusion
Acute postoperative pain is multifactorial with complicated Pathophysiology, but careful history and good assessment lead to ideal selection of treatment plane either medications or interventions according to operation performed. Interventional methods are favourable due to many reasons; avoiding chronic pain and decreased side effects of systematic medications as opioids.
Cousins MJ, Brennan F, Carr DB (2004) Pain relief. A universal human right. Pain 112: 1-4. [ Ref ]
Kumar S, Gupta R, Kaleem AM, Pandey A (2014) Mitigation of pain and anaesthetic drugs. OA Anaesthetics 2:2. [ Ref ]
Sriwatanakul K, Kelvie W, Lasagna L (1999) The quantification of pain: an analysis of words used to describe pain and analgesia in clinical trials. Clin Pharmacol Ther 32: 143-148. [ Ref ]
American Society of Anesthesiologists Task Force on Acute Pain Management (2012) Practice guidelines for acute pain management the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 116: 248-273. [ Ref ]
Flisberg P, Rudin Α, Linnér R, Lundberg CJ (2003) Pain relief and safety after major surgery. A prospective study of epidural and intravenous analgesia in 2696 patients. Acta Anaesthesiol Scand 47: 457-465. [ Ref ]
Cousins M and Power I (1999) Acute postoperative pain. In Wall PD, Melzack R, editors. Textbook of pain. (4th edn) Edinburgh, Churchill Livingstone, pp. 447-491. [ Ref ]
Van den Beuken-van Everdingen MH, De Rijke JM, Kessels AG, Schouten HC, Van Kleef M, et al. (2007) Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 18: 1437-1449. [ Ref ]
Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367: 1618-1625. [ Ref ]
Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 248: 189-198. [ Ref ]
Macintyre PE and Schug SA (2007) Adverse effects of undertreated severe acute pain. In Acute pain management: a practical guide. (3rd edn), Elsevier, Edinburgh, pp. 1-7. [ Ref ]
Habler H-J, Janig W, Koltzenberg M (1990) Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545-562. [ Ref ]
Lehmann KA (2005) Recent developments in patient-controlled analgesia J Pain Symptom Manage 29: S72-89. [ Ref ]
Raj P (2001) Celiac Plexus/Splanchnic Nerve Blocks. Tech Reg Anesth Pain Manag 5: 102-115. [ Ref ]
Southall JL, Beddall C, Raphael JH (2006) Cost utility analysis of intrathecal pump implant for chronic nonmalignant low back pain. Neuromodulation 9: 156-157. [ Ref ]
Machata AM, Kabon B, Willschke H, Fässler K, Gustorff B, et al. (2009) A new instrument for pain assessment in the immediate postoperative period. Anaesthesia 64: 392-398. [ Ref ]
Grond S, Zech D, Diefenbach C, Radbruch L, Lehmann KA (1996) Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain 64: 107-114. [ Ref ]
Macintyre PE and Schug SA (2007) Adverse effects of undertreated severe acute pain. In Acute pain management: a practical guide. (3rd edn) Elsevier, Edinburgh, pp. 1-7. [ Ref ]
Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM (2010) APM: SE Working Group of the Australian and New Zealand College of Anesthetists and Faculty of Pain Medicine. Acute Pain Management: Scientific Evidence. (3rd edn), ANZCA & FPM, Melbourne. [ Ref ]
Bailey PL, Egan TD, Stanley TH (2011) Intravenous opioid anesthetic. In: Miller RD, (eds) Anesthesia. (5th edn), Pa: Churchill Livingstone, Philadelphia, pp. 273-386. [ Ref ]
Lansky AJ, Stone GW (2010) Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ Cardiovasc Inters 3: 602- 610. [ Ref ]
Liu SS, Wu CL (2007) Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 104: 689-702. [ Ref ]
Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA Jr, et al. (2003) Efficacy of postoperative epidural analgesia versus systemic opioids: ametaanalysis. JAMA 290: 2455-2463. [ Ref ]
Woodtorde JM, Fielding JR (1975) Pain and cancer. In: Weisenberg M, (eds) Pain, Clinical and Experimental Perspectives. St. Louis, CV Mosby, pp. I-II. [ Ref ]
Bouman EA, Theunissen M, Bons SA, van Mook WN, Gramke HF, et al. (2014) Reduced incidence of chronic postsurgical pain after epidural analgesia for abdominal surgery. Pain Pract 14: E76-84. [ Ref ]
Cook TM, Counsel D, Wildsmith JAW (2009) Major complications of central neuraxial block: report on the 3rd National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 102: 179-190. [ Ref ]
Bujedo BM, Santos SG, Azpiazu AU (2012) A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag 8: 177-192. [ Ref ]
Koneti KK, Jones M (2013) Management of acute pain .Surgery 31: 77-83. [ Ref ]
Retsky M, Rogers R, Demicheli R, Hrushesky WJ, Gukas I, et al. (2012) NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat 134: 881-888. [ Ref ]
McQuay HJ, Barden J, Moore RA (2003) Clinically important changeswhat’s important and whose change is it anyway? J Pain Symptom Manage 25: 395-396. [ Ref ]
Niraj G and Rowbotham J (2011) Persistent postoperative pain: where are we now? Br J Anaesth 107: 25-29. [ Ref ]