Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Research
Received date: 19 April, 2019
Accepted date: 24 May, 2019
Published date: 31 May, 2019
Citation: Catherine AM, Iortsuun DN, Tafida MA (2019) Distribution Pattern of Pb Among Plant Parts at Selected Growth Stages of Roadside Grown Wheat and Maize at Kadawa, Kano State. Sch J Appl Sci Res Vol: 2, Issu: 7 (12-20).
Copyright: © 2019 Catherine AM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Distribution pattern of Pb in plant tissues (leaf, stem and root) at selected growth stages (germination or seedling stage (15 days), tillering stage (30 days), shooting/booting stage (45 days), heading( maize) and earing (wheat) stage( 60 days), flowering stage( 75 days) and ripening stage (90 days) of two varieties each of wheat- Triticum aestivum L. var. Pavon-76 (SU 1) and Siettecerros (SU 2) and maize – Zea mays L. var. TZEE-Y (yellow maize)(SU 3) and Zea mays everta L. (popcorn) (SU 4) were investigated at Kadawa, Kano State of northern Nigeria. Doruwa Salau (S1) with an average daily traffic density of 19,288 comprises of SU 1 and SU 3 as the experimental sites perpendicular to the Kano- Zaria Highway while SU 2 and SU 4 with a traffic density of 3 are the control sites within the Irrigation Research Station (IRS) (S2). They were selected based on traffic density and distances. Distances from the experimental and control sites to the Kano-Zaria highway is 345m and 2438.28m respectively. Soil and plant samples were collected for one growing harmattan season (December 2008 through to April 2009). Prior to sowing, triplicate soil samples were collected at a depth of 25cm for physico-chemical and Pb level analyses. Triplicates of both the representative plants and corresponding soil samples were collected at a depth of 25cm at the selected growth stages after sowing. Double-beam AAS was used in determining the Pb levels. Results reveals that plant Pb in each of the two varieties of wheat and maize were reportedly higher than the soil Pb indicating atmospheric inputs. Pb level was highest in the leaves at SU 1 and SU 3 and in the roots at SU 2 and SU 4 indicating atmospheric inputs and deposition on the soil respectively. Plant Pb was highest at SU 1 at the 30 days growth stage than at all the sampling units and other growth stages respectively indicating proximity to the highway and dilution of the trace metal levels as growth progresses. Soil Pb before sowing was highest (160mg/kg) at SU 3 and lowest at (40mg/ kg) at SU 1 and SU 4 respectively, which suggests that Pb levels originated from vehicular emissions due to absence of industrial, residential and commercial activities. Individual ANOVA for each sampling units was highly significant for plant parts at SU 2 only and combined ANOVA was highly significant (p=0.01 and 0.05) for sampling units or varieties, growth stages, plant parts and interaction of plant parts, growth stages and sampling units, which remarks that plant parts, growth stages and distances as well as their interactions had a great influence on the distribution of Pb in the crops. Pb (2mg/kg) levels exceeded the WHO permissible limits posing a potential health risks to food crops, livestock and humans.
Keywords
Lead, Growth stages, Wheat, Maize, Popcorn, Plant parts, Traffic density.
Abstract
Distribution pattern of Pb in plant tissues (leaf, stem and root) at selected growth stages (germination or seedling stage (15 days), tillering stage (30 days), shooting/booting stage (45 days), heading( maize) and earing (wheat) stage( 60 days), flowering stage( 75 days) and ripening stage (90 days) of two varieties each of wheat- Triticum aestivum L. var. Pavon-76 (SU 1) and Siettecerros (SU 2) and maize – Zea mays L. var. TZEE-Y (yellow maize)(SU 3) and Zea mays everta L. (popcorn) (SU 4) were investigated at Kadawa, Kano State of northern Nigeria. Doruwa Salau (S1) with an average daily traffic density of 19,288 comprises of SU 1 and SU 3 as the experimental sites perpendicular to the Kano- Zaria Highway while SU 2 and SU 4 with a traffic density of 3 are the control sites within the Irrigation Research Station (IRS) (S2). They were selected based on traffic density and distances. Distances from the experimental and control sites to the Kano-Zaria highway is 345m and 2438.28m respectively. Soil and plant samples were collected for one growing harmattan season (December 2008 through to April 2009). Prior to sowing, triplicate soil samples were collected at a depth of 25cm for physico-chemical and Pb level analyses. Triplicates of both the representative plants and corresponding soil samples were collected at a depth of 25cm at the selected growth stages after sowing. Double-beam AAS was used in determining the Pb levels. Results reveals that plant Pb in each of the two varieties of wheat and maize were reportedly higher than the soil Pb indicating atmospheric inputs. Pb level was highest in the leaves at SU 1 and SU 3 and in the roots at SU 2 and SU 4 indicating atmospheric inputs and deposition on the soil respectively. Plant Pb was highest at SU 1 at the 30 days growth stage than at all the sampling units and other growth stages respectively indicating proximity to the highway and dilution of the trace metal levels as growth progresses. Soil Pb before sowing was highest (160mg/kg) at SU 3 and lowest at (40mg/ kg) at SU 1 and SU 4 respectively, which suggests that Pb levels originated from vehicular emissions due to absence of industrial, residential and commercial activities. Individual ANOVA for each sampling units was highly significant for plant parts at SU 2 only and combined ANOVA was highly significant (p=0.01 and 0.05) for sampling units or varieties, growth stages, plant parts and interaction of plant parts, growth stages and sampling units, which remarks that plant parts, growth stages and distances as well as their interactions had a great influence on the distribution of Pb in the crops. Pb (2mg/kg) levels exceeded the WHO permissible limits posing a potential health risks to food crops, livestock and humans.
Keywords
Lead, Growth stages, Wheat, Maize, Popcorn, Plant parts, Traffic density.
Introduction
There is general agreement that the extent of contamination of the topsoil and vegetation around roadsides are related to the traffic volume and proximity to the highway [1,2]. Excessive accumulation of heavy metals in agricultural land through traffic emissions may results in soil contamination and elevated heavy metal uptake by crops, and thus affects food quality and safety [3,4] and human health [5-7].
Atmospheric emissions from automobiles is a major contributing factor to the abundance of heavy metals in the Nigerian Environment [7,8], and this is much higher than permissible level in some pollution conscious countries [9,10]. Heavy metals from atmospheric deposition are potentially more mobile than those that are ultimately inherited from the geological parent material [11]. The atmospheric deposition of heavy metals on ecosystems occurs mainly in particulate and aerosol forms [12,13]. The area affected by the deposition of heavy metal containing particles is usually much more local than that of gaseous pollutants [7].
Contamination and subsequent pollution of the environment by toxic heavy metals have become an issue of global concern and even more worrisome in the developing countries where research efforts towards monitoring the environment have not been given the desired attention by the stake holders [14]. Studies reveal that the presence of toxic heavy metals like Fe, Pb and Hg reduce soil fertility and agricultural output [15] which have the potential to contaminate crops growing under such irrigation. Lead, being a zootoxic metal has no biological role, but is easily absorbed and accumulated in different parts of a plant [16,17] and is one of the many toxic metals in the environment that needs to be monitored in plants parts used by humans and animals [18] as food and feeds respectively. Several studies indicated that plants have the ability to concentrate Pb [9]. Sridhar [9] also revealed that leaf and root contain more Pb than stem and the contents of Pb in different plant organs were positively correlated to the Pb content in soils. Commonly the Pb does not concentrate in the edible-fruited part of the plant.
Higher plants are usually the first indicators of changes in the chemical and biological composition of natural ecosystem, where other indicators are absent; they have appeal in air and soil pollution monitoring in highly polluted areas [7,19]. Metal accumulation by plants is affected by many factors aside those of anthropogenic sources which directly influence heavy metal concentration on and within plants [20] such as climate, atmospheric deposition, nature of soil upon which the crop is grown, the degree of maturity of the plant at harvesting [21-25], variations in plant species, the growth stage of the plants. It is known that metal sensitivity and toxicity to plants are influenced by not only the concentration and the toxicant types, but also dependent on several developmental stages of the plants [16,17,26,27]. Plants differ in their uptake of heavy metals and their subsequent distribution within plant organs [28]. Plant characteristics that affect the rate of particle deposition and retention include the exposed surface area of the foliage and the presence or absence of leaf hairs [29].
Several researches in Nigeria have been largely focused on the agronomic of agricultural crops, agricultural land use management practices, soil fertility and assessments e.t.c., with less emphasis on the effects of growth stages on Pb levels in plant parts of maize and wheat. Maize and wheat are the most widely used articles of human diet worldwide, and most widely cultivated cereals in Nigeria especially in northern Nigeria. Two popular wheat varieties, Pavon-76 and Siettecerros, are widely cultivated in the northern states of Nigeria especially Kano State. They were chosen for examining the effects of selected growth stages on the concentration of Pb in the plant parts of wheat and maize. Therefore, assessing the concentrations of pollutants in different components of the ecosystem has become an important task in preventing risk to natural life and public health [30]. The aim of this study is to investigate the route of Pb accumulation and distribution of Pb in the soils, leaves, stems and roots of two widely grown varieties each of wheat and maize at selected growth stages in Kadawa, Kano State located in the Sudan savannah region of Nigeria.
Materials and Method
Description of the study area
Historically, Kano State has been a commercial and agricultural state, which was known for the production of groundnuts as well as for its solid mineral deposits. The state has more than 18,684 square kilometres (7,214 sq mi) of cultivable land and is the most extensively irrigated state in Nigeria [31]. Kano has a tradition of irrigated agriculture and is reckoned as the leading hydro-agricultural state in Nigeria. Small-scale irrigation on fadamas has supported crops that require all year round water used for gardening crops for centuries. The distribution network operates under gravity receiving water from the Tiga dam through a 15km long main canal split into east and west branches. Major irrigated crops are wheat, maize, tomatoes and rice [31]. The bulk of the state is classified as the Sudan Savannah and a small portion of Sahel Savannah in the extreme northeastern tip of the State [32]. However, the natural vegetation which consists of the Sudan and the guinea savannah has both been replaced by secondary vegetation. Kano consists of wooded savannah in the south and scrub vegetation in the north and is drained by Kano-Chalawa-Hadejia river system. Kano State has the suitable climatic conditions for dry season maize and wheat cultivation. All the sampling units were selected by principle to represent the level of pollution near major traffic routs and are reference sampling points [33]. The research was conducted at Kadawa across the southern Sudan savannah zone of Kano State.
Kadawa is completely devoid of industrial, commercial activities and scanty residential housings except the presence of the major Highway. The experimental site Doruwa Salau is located perpendicularly to the Kano-Zaria Highway and designated as S1. The Doruwa Salau location is a mix of residential and commercial activities at close proximity to the highway. S1 is located outside the Kadawa irrigation research (IRS) station at a distance of 2438.28metres from the control sites and about 345.79metres from the roadside. Yellow maize farm designated as SU 3 is at a distance of 100metres from the roadside and about 50metres from the Pavon-76 farm designated as SU 1. There had been extensive cultivation of rice, maize, millet, guinea corn, cowpea, garden egg, watermelon and wheat along the roadside. The site had an average daily traffic density of 19,288, being the main exit from Kano State to various major towns of the country, particularly from upper northern States through the middle belt States to southern States and to the country’s capital Abuja. Two sampling units were also selected within the control site, designated as S2 (at a distance of 1934. 61metres from the Kano–Zaria road) is located inside the Irrigation Research Station (IRS), Institute of Agricultural Research (IAR), Kadawa, Kano State, which began operation in 1975. The distance between SU 4, the popcorn farm and SU 2, the Siettecerros farm being the two sampling units is about 250metres. The control site has been extensively used by private institutions, government researchers and both corporate and international research institutes and had an average daily traffic density of 3. The control and experimental sites are located in the village of Kadawa, on the outskirt of Kano City, and are a mix of research, scanty rural settlements and commercial maize, wheat and vegetable farming.
Both sites are significant for dry season irrigation farming and have irrigation channels connected to the sub-channels that networked the Tiga dam from the Hadejia – Jama’are River Basin Dam and provides water for dry season irrigation of farmlands within and outside (close to the Highway) the irrigation research station, Kadawa. The average daily vehicular traffic densities from S1 and S2 were estimated by direct counting of the number of vehicles between 7am to 8am in the morning, at 12 noon to 1pm and at 4pm to 5pm in the afternoon and in the evening respectively. The average of the three estimates was obtained representing the average daily traffic density during preliminary survey. The same measurement was repeated before the collection of soil and plant samples and at the end of the sampling. The total average daily traffic density was then estimated.
Prior to sampling of soil and crops, the global positioning system (GPS) was used in recording the four coordinates for each sampling units (SU 1, SU 2, SU 3 and SU 4) of the study area as shown in Table 1.
Determination of Physico-chemical properties of soil
Prior to sowing of the two varieties each of wheat and maize, a pre-survey of the characteristics of the study area was conducted and four replicates per sampling site of soil samples were collected at a depth of 25cm using soil auger. A total of sixteen (16) soil samples were collected between the months of January 2007 – October 2008 before sowing between the months of November to December 2009. Physico-chemical parameters of soil samples were determined; soil pH was determined using a standardised pH meter [34], soil particle-size distribution was determined according to [35], organic matter and organic carbon content was determined according to Walkley and Black [36] and Nelson and Sommers [37]. Cation exchange capacity was analysed according to Black [38].
Soil and plant sampling and analyses
A total of 216 plant samples and 144 corresponding soil samples were collected in duplicates and triplicates respectively from four sampling units. Two varieties each of wheat and maize namely, Triticum aestivum L., var. Pavon-76 and Siettecerros, Zea mays L. var. TZEE-Y (yellow maize) and Zea mays everta L. were collected in a randomised block design setup. Both the soil and plant samples were collected fortnightly at the 15 days, 30 days, 45 days, 60 days, 75 days and 90 days which are the germination or seedling, tillering, jointing/booting, heading/earing, flowering and ripening stages respectively. Corresponding soil samples were collected in duplicates at a vertical depth of 25cm, carefully packed into polyethene bags and transported to the laboratory. In laboratory, the roots of each cereal crop were carefully separated from the soil particles samples by washing under running tapwater and were separated into root, stem and leaf and then air-dried at room temperature. The dried plant samples were ground, using a grinding mill model Foss CyclotecTM 1093 based on TecatorTMtechnology and then kept in clean polyethylene bags for analysis. The soil samples were air dried at room temperature, ground in an agate mortar, sieved through 22 mm mesh sieve, and then kept in clean polyethylene bags for analysis. The ground plant samples were well packaged and Pb was determined using the double beam spectrophotometer at National Animal Production Research Institute (NAPRI), Ahmadu Bello University, Zaria.
Double beam atomic absorption spectrophotometer and sample preparation procedures
Approximately1g of ground dried plant sample was weighed into a digestion vessel. 10 mL of concentrated HNO3 was added into it and allowed to stand overnight. The mixture was carefully placed on a hot plate and heated for 4 hours at 1250C until the production of red NO2 fumes has ceased and the entire solids has dis-appeared, and a transparent solution is obtained. Next, HCl and distilled water in a ratio of 1:1 was added to the digested sample and the mixture transferred to the digester again for 30 minutes. The beaker was then removed from the hot plate and allowed to cool. A small amount (2-4 ml) of 70% HClO4 was added, heated
| Sampling Units | GPS readings |
|---|---|
| Control Site SU 2 | A=N11O38.68’; E008O38.167’; Elevation=1615ft |
| B=N11O 38.626’; E008O25.815’; Elevation=1620ft | |
| C=N11O38.698’; E008O25.841; Elevation=1608ft | |
| D=N11O38.646’; E008O25.866’; Elevation=1614ft | |
| SU 4 | A=N11O38.601’; E008O25.916’; Elevation=1615ft |
| B=N11O38.547’; E008O25.937’; Elevation=1608ft | |
| C=N11O38.608’; E008O25.932’; Elevation=1606ft | |
| D=N11O38.554’; E008O25.954’; Elevation=1609ft | |
| EXPERIMENTAL SITE SU 1 and SU 3 | A=N11O39.776’; E008O25.191; Elevation=1627ft |
| B=N11O39.742’; E008O25.207’; Elevation=1635ft | |
| C=N11O39.759’; E008O25.240’; Elevation=1616ft | |
| D=N11O39.790’; E008O25.230’; Elevation=1626ft |
Table 1:GPS readings of the four Sampling Units.
| Site | % Sand | % Silt | % Clay | Textural Class | pH H20 1:1 | HCL 1:1 | % OC | % OM | CEC |
|---|---|---|---|---|---|---|---|---|---|
| SU 1 | 62. 9 | 15. 2 | 21. 9 | Sandy Clay loam | 6. 41 | 5. 77 | 0. 88 | 1. 50 | 7. 25 |
| SU 2 | 75. 5 | 12. 4 | 12. 1 | Sandy loam | 5. 48 | 4. 66 | 0. 65 | 1. 12 | 5. 9 |
| SU 3 | 71.6 | 13.5 | 14. 9 | Sandy loam | 5.75 | 4. 86 | 1. 82 | 1. 05 | 6. 3 |
| SU 4 | 60. 5 | 17. 2 | 22. 3 | Sandy clay bloam | 6. 21 | 5. 40 | 0. 78 | 1. 36 | 8. 01 |
Table 2:Physicochemical Parameters of Soils from Doruwa Salau at close proximity tothe Kano- Zaria road and from the Irrigation Research Station before sowing.
SU 1=Wheat (Pavon-76) on DoruwaSalau at close proximity to the Kano-Zaria road
SU 2=Wheat (Siettecerros) at the Control Site (Irrigation Research Station-IRS), Kadawa
SU 3=Yellow Maize on DoruwaSalau at close proximity to the Kano –Zaria road
SU 4=Popcorn at the Control Site (Irrigation Research Station-IRS), Kadawa
| Sampling units | Soil Pb levels | |
|---|---|---|
| Before sowing (mg/kg) | After sowing (mg/kg) | |
| SU 1 | 40 | 360 |
| SU 2 | 80 | 80 |
| SU 3 | 160 | 360 |
| SU 4 | 40 | ND |
Table 3:Soil Pb at the four Sampling Units at Kadawa.
SU 1=Wheat (Pavon-76) on Doruwa Salau at close proximity to the Kano-Zaria road
SU 2=Wheat (Siettecerros) at the Control Site (Irrigation Research Station-IRS), Kadawa
SU 3=Yellow Maize on Doruw aSalau at close proximity to the Kano –Zaria road
SU 4=Popcorn at the Control Site ( Irrigation Research Station-IRS), Kadawa
slowly at a low temperature and allowed to evaporate to a small volume. The cooled sample was then transferred into a 50-ml flask and diluted to the appropriate volume with distilled or deionised water then filtered through filter paper No 1. Determination of trace metals was done using the double beam atomic absorption spectrophotometer (model Shimadzu AA 650) after calibrating the equipment with stock standard solution of 1000 mg/L for Cd, 1.000 g of cadmium metal was dissolved in a minimum volume of (1+1) HCl and diluted to 1 liter with 1% (v/v) HCl while, the stock standard solution of 1000 mg/L for Pb was prepared by dissolving 1.598 g of lead nitrate -Pb(NO3)2- in 1% (v/v) HNO3 and diluted to 1 liter with 1% (v/v) HNO3. The acid digestion produces a clear solution without loss of any of the elements to be determined. A combination of nitric acid and perchloric acid is especially useful for the complete destruction of fats and proteins in biological samples [39].
Results and Discussion
Physico-chemical parameters: Physico-chemical analyses of the soil samples prior to sowing are shown in Table 1. The pH values in the study areas ranged from 5.07 to 5.80 suggesting that all the soils were basically acidic in nature (Table 1). The control site depicted mean pH value of 5.44 while the experimental site has mean pH value of 5.70. Soil pH, nature of soil and climatic changes affect the rate of uptake of metals by plants [40]. Metal mobility has been shown to increase with decreasing soil pH. Hence, moderate pH values observed in the present study could lead to increased mobility of metals in the soil, thus explaining higher metal levels in the plant parts of the cereal crops than in the soil. Cations exchange capacity (CEC) ranged from 5.9 at SU 2 to 8.01 at SU 4 both at the control site. CEC is a measure of how many cations can be retained on soil surfaces. It affects many aspects of soil chemistry and is used as a measure of soil fertility. It indicates the capacity of the soil to retain several nutrients (K+, NH4+, Ca2+) in plantavailable form as well as the capacity to retain pollutant cations (Pb2+). The high CEC of 7.25 to 8.01 and high organic carbon of 0.88 to 0.78 and organic matter of 1.50 to 1.36 at SU 1 and SU 2 respectively is due to the sandy clay loam texture of the soils. Organic matter makes a very significant contribution to cation exchange, due to its large number of charged functional groups. CEC is typically higher near the soil surface, where organic matter content is highest and declines with depth. The CEC of organic matter is highly pHdependent (Table 2).
Pb Levels in soils before sowing and during growth and development
The levels of soil Pb during the development of the four cereal crops, from germination or seedling to ripening stages were higher than soil Pb before sowing (Table 3). SU 1 and SU 3 at the experimental site had the highest Pb levels than at the SU 2 of the control site. Soil Pb was not detected at SU 4, the farthest sampling unit from the Kano-Zaria Highway.
The uptake of metals from the soil depends on different factors such as the soluble content in it, soil pH, plant growth stages, types of species, fertilizers and soil [41,42]. High soil Pb during growth and development than before sowing could be related to the organic matter contents determined before sowing (Table 2) and further enrichment of the soil organic matter contents during growth and development. When plants die and decay, the heavy metals taken into the plants are redistributed and the soil is enriched with the pollutants [43]. Lead (Pb) occurs naturally in all soils, in concentrations ranging from 1 to 200 mg/kg, with a mean of 15 mg/kg [44]. Normal mean content of Pb crustal rocks is usually about 13ppm. Although the total amount of Pb used is highest in batteries, but the gasoline additives are the major Pb discharges.
Pb Levels in soils before sowing and during growth and development
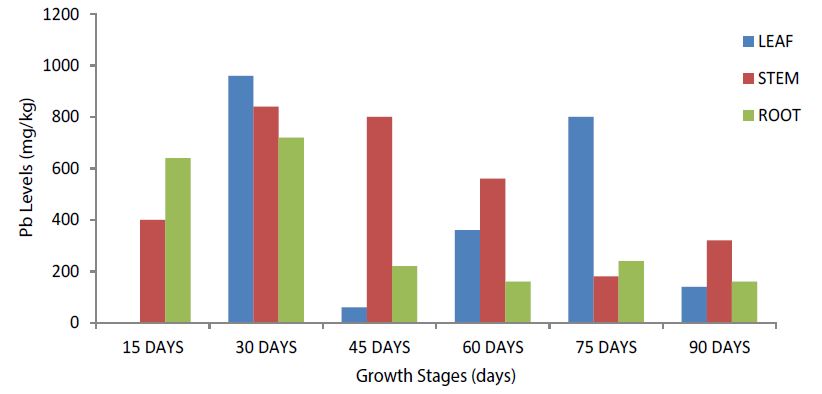
Leaf, stem and root Pb levels at the selected growth stages of Pavon-76, Siettecerros, yellow maize and popcorn are shown in Figures 1-4. Total Pb level in Pavon-76 was highest in the leaves and stems at the selected growth stages than in the roots (Figure 1) and absent at the 15-days growth stage. The 30-days growth stage recorded the highest Pb levels in the leaf, stem and root than at the other growth stages. Leaf Pb was highest at the 30 and 75 days growth stages (Figure 1).
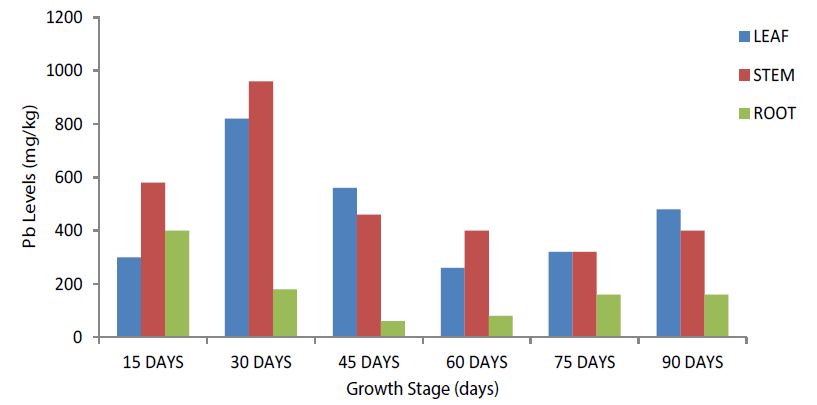
The total Pb level was highest in the leaves and stems at the selected growth stages than in the roots. The 30-days growth stage recorded the highest Pb levels in the leaves and stems while the 15-days growth stage recorded the highest Pb levels in the root (Figure 2).
Figure 1:Total Pb Levels among the selected growth stages in the Leaf, Stem and Root of Pavon-76 nearest to the Kano-Zaria road (Experimental Site).
Figure 2:Total Pb Levels among the selected growth stages in the Leaf, Stem and Root of Siettecerros at the IRS (Control Site).
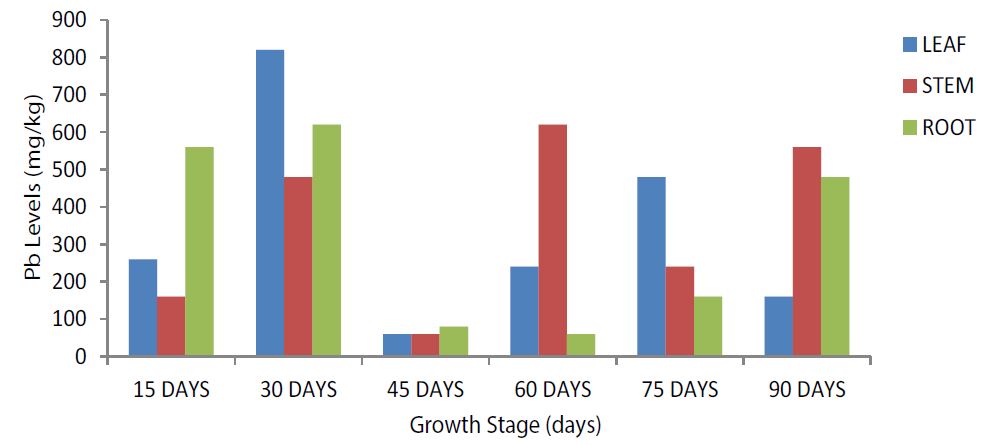
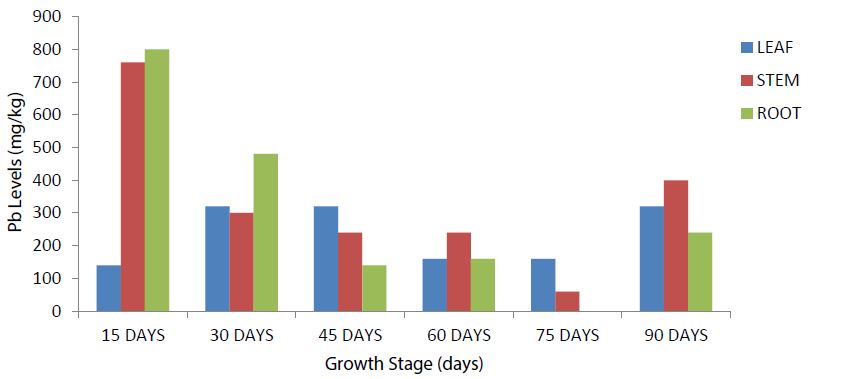
The highest leaf, root and stem Pb levels was evident at the 30-days and 60-days growth stages respectively. The 45-days growth stage recorded the lowest Pb levels in the leaf, stem and root (Figure 3). Stem and root Pb levels was highest at the 15-days while the leaves, stems and roots Pb levels were invariably higher at the 30- and 90-days growth stages (Figure 4). Root Pb level was not observed in the plant sample during analysis at the 75-days growth stage which also recorded the lowest stem Pb levels (Figure 4).
Pb levels in the soil and plant samples varied to a great extent from sample to sample, site to site, plant part to plant part and from growth stage to growth stage.
Uptake and accumulation of elements by plants may follow two different paths i.e., through the roots and foliar surface [44] including deposition of particulate matter on the plant leaves [45]. Uptake and accumulation of Pb in the different plant parts of the cereal crops varied significantly and was above the tolerable limit (WHO safe limits (2 mg kg-1)) at all the sampling units. This suggests that the agricultural produce from these sites maybe potentially
hazardous for human and livestock consumption since the only anthropogenic activity at the study area is the presence of a major highway. This study is in contrast to other studies that in terrestrial ecosystems, soil act as the main source of heavy metal transference to agricultural products [46]. The pH of soil and the levels of organic matter can also promote Pb uptake by the leaves. The Pb content of most plant species is normally in the range of 0.5-3ppm. For certain species however, the Pb toxicity level is very high. This may create a rather dangerous situation because such plants may show no toxic symptoms and apparently looks healthy at Pb levels which are hazardous for human consumption.
Although Pb has no biological role in animals, plants, and microorganisms [47], it forms a bond with the sulfihydryl group of proteins, and hence can disrupt the metabolism and biological activities of many proteins and has caused cancer in kidneys of rodents. According to the Codex Alimentarius Commission of the Joint FAO/WHO Food Standards [48], the maximum level for Pb in most vegetables is 0.1 mg kg-1 on fresh weight basis and in comparison with this present study revealed higher levels in the four cereals above the WHO safe limits (2 mg kg-1).
Figure 3:Total Pb Levels among the selected growth stages in the Leaf, Stem and Root of Zea mays L. var. TZEE-yellow maize at the Kano- Zaria road (Experimental Site).
Figure 4:Total Pb Levels among the selected growth stages in the Leaf, Stem and Root of Zea mays everta L. (popcorn) at the IRS (Control Site).
Leaf and root contain more Pb than stem and the contents of Pb in different plant organs were positively correlated to the Pb content in soils [9]. In contrast to this study, the amounts of Pb in leaves and stems were higher than in the roots. One possible explanation of this situation is that the Pb uptake arose from the aerial deposition of Pb particulate from vehicular emission exhausts. About 80% of Pb in petrol escapes through the exhaust pipe as particulate [49]. This could also be attributed to the larger exposure area of the leaves than the other parts to wind action, the surface adsorption of particulate matter and high rate of assimilation of heavy metals from the environment agreed with the findings of Keane et al. [50] and Al-Kateeb and Leilah [51]. This also agrees with other reports that metal concentrations in leaves are usually much higher than those in grain and other plant parts [45,52]. Plant characteristics that affect the rate of particle deposition and retention include the exposed surface area of the foliage and the presence or absence of leaf hairs [29]. Earlier works by Little and Wiffen [53] observed that rough or hairy leaf surfaces were more efficient at collecting aerosols than smooth surfaces, explained by the increased surface area and projection of roughness elements through the leaf-air boundary layer [29].
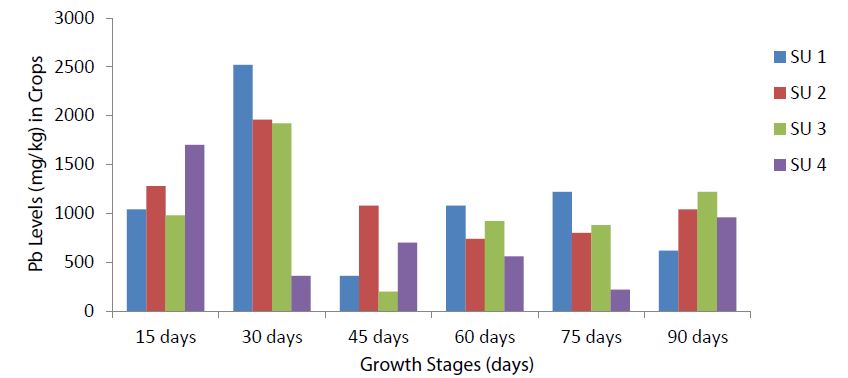
Comparison of plant Pb among the four sampling units
The 30-days growth stage recorded the highest Pb levels in Triticum aestivum L. var. Pavon-76, Siettecerros and Zea mays L. (yellow maize) at SU 1, SU 2 and SU 3 respectively (Figure 5). Crop Pb was progressively fluctuating from the 45-days to the 75-days growth stages with a slight increase at the 90-days (Figure 5). The 15-days and 30-days growth stages had the highest Pb levels at all the sampling units. SU 1 had the highest Pb levels at the 30-days growth stage followed by the 60-days and 75-days growth stages. The lowest Pb levels was observed at the 45-days growth stage (Figure 5).
The 15-days and 30-days growth stages which are the germination or seedling and tillering stages respectively are the periods of vegetative phase of plant development where the plants are busy carrying out photosynthesis and accumulating resources that will be needed for flowering and reproduction [31]. Germination is the awakening of a seed (embryo) from a resting stage which involves the harnessing of energy stored in the seed and activation of the components in the environment [54]. During tillering, the plant begins to tiller producing axilliary or side roots. Tillering is an important and distinct stage in the development of wheat. Over the growing season the biomass of plant increases, diluting the chemical concentration within plant tissues relative to the flux of chemical uptake, and increasing the aboveground canopy for interception of aerial deposition [29].
Figure 5:Pb Levels in crops grown at the four sampling units among the selected growth stages.
| Source of Variation | DF | SS | MS | F-ratio | Significant | |
|---|---|---|---|---|---|---|
| Plant Parts (P-1) | 2 | 445688.89 | 222844.44 | 10.126 | ** | * |
| Sampling Units/Variety (Su/V – 1) | 3 | 8245336.96 | 2748445.65 | 124.894 | ** | * |
| Growth Stage (G-1) | 5 | 2209022.21 | 441804.44 | 20.076 | ** | * |
| P x Su Interaction (P-1) (Su-1) | 6 | 8144765.28 | 1357460.88 | 61.685 | ** | * |
| G x Su Interaction (G- 1) (Su-1) | 15 | 13332568.06 | 888837.87 | 40.390 | ** | * |
| P × G × Su Interaction (P-1) (G-1) (Su-1) | 30 | 11916834.71 | 397227.82 | 18.050 | ** | * |
Table 4:Combined analysis of variance.
The individual ANOVA investigation of the effects of growth stages and plant parts (leaf, stem and root) on Pb levels at the four sampling units was non-significance. However, combined ANOVA for Pb levels was highly significant (P=0.05 and 0.01) at the sampling units, plant parts, growth stages and interactions of the three parameters. Table 4 shows the level of significant for all the combined factors.
The highly significant (P=0.05 and 0.01) ANOVA values for Pb at plant parts (leaf, stem and root), sampling units or varieties, growth stages and interaction of the three factors shows that the levels of Pb in the two varieties of wheat and maize were influenced by a combination of those factors as well as the distances from the highway [55].
Conclusion
Wheat and maize are referred to as the “big three” among cereal crops because of their worldwide consumption as the basic staple foods. Hence, a continuous control and evaluation is required on their safety to human health.The concentration of Pb in the plant parts of Triticum aestivum L. var. Pavon-76, var. Siettecerros, Zea mays L. var. TZEE-Y yellow maize and Zea mays everta was higher than the concentration of Pb in the soil revealing atmospheric inputs. Pb was highest in Pavon-76 at the 30-days growth stage than at all the sampling units indicating proximity to the Kano-Zaria Highway and the increased meristematic activities at tillering (30-days growth) stage resulted to high Pb absorption. The highly significant values of the effects of plant parts on the concentration of Pb in Siettecerros indicate the variety ability to accumulate Pb, despite the distance (1934.61metres) between SU 2 to the highway. The highly significant value of the combined ANOVA remarks that the distribution of Pb levels in the plant parts of the four crops was a complex interplay of growth stages, plant parts, sampling units. Therefore, assessing the concentrations of pollutants in different components of the ecosystem has become an important task in preventing risk to natural life and public health.
Ho YB, Tai KM (1988) Elevated Levels of Pb and other Metals in Roadside Soil and Grass and their use to monitor aerial metal deposition in Hong Kong. Environmental Pollution 49: 37-51.[ Ref ]
Anongo MC, Bako SP, Ezealor AU (2005) Trace Metal Contents in relation to Population of Microorganisms in Soils along some Highways in Nigeria’s Guinea Savanna Asian Journal of Biological Sciences 5: 1- 4.[ Ref ]
Garcia R, Millan E (1998) Assessment of Cd, Pb and Zn contamination in roadside Soil and grasses from Gipuzkoi (Spain). Chemosphere 37: 1615-1625.[ Ref ]
Emamverdian A, Ding Y (2017) Effects of heavy metals’ toxicity on plants and enhancement of plant defense mechanisms of Si-mediation “Review” International Journal of Environmental & Agriculture Research (IJOEAR) 3: 41-51.[ Ref ]
Bako SP, Bhwankot ES, Ezealor AU, Chia AM, Funtua II (2009) Human Health Implications of heavy metal contents in parts of Maize (Zea mays L) plants cultivated along highways in Nigeria’s Guinea Savanna. In Soil Remediation Series: Pollution Science, Technology and Abatement. Lukas Aachen and Paul Eichmann (eds); pp: 345-356.[ Ref ]
Bako SP, Bhwankot ES, Ezealor AU, Chia AM, Funtua II (2009) Human Health Implications of heavy metal contents in parts of Cowpea (Vigna unguiculata L Walp) plants cultivated along highways in Nigeria’s Guinea Savanna. In Soil Remediation Series: Pollution Science, Technology and Abatement. Lukas Aachen and Paul Eichmann (eds); pp: 357-368.[ Ref ]
Anongo MC, Bako SP, Iortsuun DN, Japhet WS (2015) Trace Metal Contents at Selected Growth Stages of Wheat and Maize Grown on Roadside Soil at Kadawa Kano State, Nigeria. PhD Thesis. Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria.[ Ref ]
Onianwa PC, Ajayi SO (1987) Heavy Metal Contents of Epiphytic Acrocarpus Mosses within Inhabited Sites in Southwest Nigeria. Environment International 13: 191-196.[ Ref ]
Sridhar MKC (2001) Environmental Lead Levels in African Cities. Heavy Metal Research Group, Division of Environmental Health, College of Medicine University of Ibadan, Ibadan, Nigeria; pp: 1-9.[ Ref ]
Rim-Rukeh A, Okokoyo PA (2003) Blood Pb levels Amongst Commercial Motorcycles in Warri and Environs. Journal of Science and Technology Research 2: 8-11.[ Ref ]
Chlopecka A, Bacon JR, Wilson MJ, Kay J (1996) Forms of Cadmium, Lead and Zinc in Contaminated Soils from South West Poland Journal of Environmental Quality 25: 69-79.[ Ref ]
Ross SM (1994a) Retention, transformation and mobility of toxic metals in soils. In: Ross SM (ed) Toxic metals in soil - plant systems. John Wiley, Chichester; pp: 63-152.[ Ref ]
Luttermann A, Freedman B (2000) Risks to forests in heavily polluted regions. In: Innes JL, Oleksyn J (eds) Forest dynamics in heavily polluted regions. CABI Publishing, Wallingford; pp: 9-26.[ Ref ]
Oluyemi EA, Feuyit G, Oyekunle JAO, Ogunfowokan AO (2008) Seasonal variations in heavy metal concentrations in soil and some selected crops at a landfill in Nigeria. African Journal of Environmental Science and Technology 2: 089-096.[ Ref ]
Lokhande RS, Kelkar N (1999) Studies on Heavy Metals in water of Vasai Creek, Maharashtra. Indian Journal of Environment Protection 19: 664- 668.[ Ref ]
Anongo MC, Bako SP, Iortsuun DN, Japhet WS (2017) Pb Uptake in Roadside Grown Wheat (Triticum aestivum L) in the Sudan Savannah, Kano State. Journal of Environmental and Analytical Toxicology 7: 470.[ Ref ]
Nas FS, Ali M (2018) The effect of lead on plants in terms of growing and biochemical parameters: a review. MOJ Ecology & Environmental Sciences 3: 265-268.[ Ref ]
Alloway BJ (1995) Heavy Metals in Soils. [2nd edtn] Blackie Academic and Professional, London.[ Ref ]
Mulgrew A, Williams P (2005) Biomonitoring of Air Quality using plants. Air Hygiene Report No-10.[ Ref ]
Muchuweti M, Birkett JW, Chinyanga E, Zvauya R, Scrimshaw MD, et al. (2006) Heavy Metals Content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agriculture, Ecosystem and Environment 112: 41-48.[ Ref ]
Lake DL, Kirk PW, Lester JN (1984) The Fractionation, Characterization and Speciation of heavy metals in sewage sludge and sewage sludge amended soils: a review. Journal of Environmental Quality 13: 175-183.[ Ref ]
Scott D, Keoghan JM, Alian RE (1996) Native and low-input grasses at New Zealand high country perspective. New Zealand Journal of Agricultural Research 39: 499-512.[ Ref ]
Voutsa D, Grimanis A, Samara C (1996) Heavy elements in vegetables grown in an industrial area in relation to soil and air particulate matter. Environmental Pollution 94: 325-335.[ Ref ]
Margaret TO (1998) Impact of Waste Discharge in a Coastal Zone. A Case Study of Lagos State; pp: 327-343.[ Ref ]
Omoloye AA (2009) Field accumulation risks of heavy metals and uptake effects on the biology of Sitophilus zeamais (Coleoptera: Curculionidae). African Scientist 10: 75-88.[ Ref ]
Liu JG, Liang JS, Li KQ, Zhang ZJ, Yu BY, et al. (2005) Correlations between cadmium and Mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 52: 1467-1473.[ Ref ]
Hatamzadeh A, Sharaf ARN, Vafaei MH, Salehi M, Ahmadi G (2012) Effect of some heavy metals (Fe, Cu and Pb) On seed germination and incipient seedling growth of Festuca rubra sp commutate (Chewing fescue). International Journal of Agriculture and Crop Sciences 4: 1068-1073.[ Ref ]
Pal S, Singh HB, Rakshit A (2013) Potential of different crop species for Ni and Cd phytoremediation in peri-urban areas of Varanasi district, India with more than twenty years of wastewater irrigation history. Italian Journal of Agronomy 8: 58-63.[ Ref ]
Collins CD, Martin I, Fryer M (2006) Evaluation of models for predicting plant uptake of chemicals from soil Environment Agency Science Report- SC050021/SR.[ Ref ]
Rahimi G, Kolahchi R, Charkhabi A (2017) Uptake and Translocation of Some Heavy Metals by Rice Crop (Oryza Sativa) In Paddy Soils Agriculture (Poľnohospodárstvo) 63: 163-175.[ Ref ]
Kano State: Natural Resources and Potential for Development1998-2013.[ Ref ]
Abba A (1991) Wheat Production in Kano State. In Wheat in Nigeria: Production, Processing and Utilization. Rayar AL, Kaigama BK, Olukosi JO, Anaso AB (eds) LCPI, IAR and UNIMA1D.[ Ref ]
Misurovic A (1998) Monitoring Programs in Montenegro: PI center for Ecotoxicological Research of Montenegro (CETI).[ Ref ]
Lieneweg F (1954) Electronic pH Determination. John Wiley and Sons Inc New York.[ Ref ]
Bouyoucos GH (1962) Hydrometer Method improved for making Particle-size Analysis of Soils. Agronomy Journal 53: 464.[ Ref ]
Walkley A, Black IA (1965) An examination of the Degtrajeff Method for determination of soil organic matter and a proposed Chromic acid Titration Method. Soil Science 37: 29-38.[ Ref ]
Nelson DW, Sommers LE (1975) A rapid and accurate method for estimating organic carbon in the soil. Proceedings of the Indiana Academy of Science 84: 456-462.[ Ref ]
Black CA (1965) Methods of Soil Analysis. Parts 1 and 2, American Society of Agronomy, Madison, Wisconsin; pp: 552-562.[ Ref ]
Amoo A, Ogbonnaya CI, Ojediran J (2004) Movement of some heavy metals in poorly drained Fadama soils in the Southern Guinea savannah zone of Nigeria. Food, Agriculture and Environment 2: 378-380.[ Ref ]
Alloway BJ, Ayres CD (1997) Chemical Principles of Environmental Pollution. [2nd Edtn] Blackie Academia and Professional, London.[ Ref ]
Ismail BS, Farihah K, Khairiah J (2005) Bioaccumulation of heavy metals in vegetables from selected agricultural areas. Bulletin of Environmental Contamination and Toxicology 74: 320-327.[ Ref ]
Sharma RK, Agrawal M, Marshall F (2006) Heavy metals contamination in Vegetables grown in wastewater irrigated areas of Varanasi, India Bulletin of Environmental Contamination Toxicology 77: 312-318.[ Ref ]
Sawidis T, Chettri MK, Papaionnou A, Zachariadis G, Stratis J (2001) A study of Metal distribution from lignite fuels using trees as biological monitors. Ecotoxicology and Environmental Safety 48: 27-35.[ Ref ]
Chirenje T, Ma L, Reeves M, Szulczewski M (2004) Lead Distribution in Near-Surface Soils of Two Florida Cities: Gainesville and Miami. Geoderma 119: 113-120.[ Ref ]
Reuben KD, Akan JC, Akan FI, Sodipo OA (2008) Elemental Content in Plant Sample of Croton Zambesicus from Mubi, Adamawa State, Nigeria. Continental Journal of Applied Sciences 3: 46-50.[ Ref ]
Ashraf U, Kanu AS, Deng Q, Mo Z, Pan S, et al. (2017) Lead (Pb) Toxicity; Physio-Biochemical Mechanisms, Grain Yield, Quality, and Pb Distribution Proportions in Scented Rice. Frontiers Plant Science.[ Ref ]
Sobolev and Begonia (2008) Effects of heavy metal contamination upon soil microbes: lead-induced changes in general and denitrifying microbial communities as evidenced by molecular markers. Int J Environ Res Public Health 5: 450-456.[ Ref ]
Joint FAO/WHO Food Standards (2006) Codex Alimentarius Commission: The Hague, Netherlands.[ Ref ]
Ogbuagu JO, Nwokoye AOC, Ogburubi I (2001) Application of Additives in Diesel Fuels as an Emission Control Strategy. African Journal of Environmental Studies 2: 146-151.[ Ref ]
Keane B, Collier MH, Shann JR, Rogstad SH (2001) Metal contents of dandelion (Taraxacumofficinale) leaves in relation to soil contamination and airborne particulate matter. Science of the Total Environment 281: 63-78.[ Ref ]
Al-Khateeb SA, Leilah AA (2005) Heavy metals accumulation in the natural vegetation of Eastern province of Saudi Arabia. Journal of Biological Sciences 5: 707-712.[ Ref ]
Jung MC, Thornton I (1996) Heavy metal contamination of soils and plants in the vicinity of a lead-zinc mine, Korea. Applied Geochemistry 11: 3-9.[ Ref ]
Little P, Wiffen RD (1977) Emission and Deposition of Petrol Engine Exhaust Pb-I. Deposition of Exhaust Pb to Plant and Soil Surfaces. Atmospheric Environment 11: 437-447.[ Ref ]
Wisconsin Fast Plants (1998) Germination: Launching the Seed. University of Wisconsin-Madison College. College of Agricultural and Life Sciences, Department of Plant.[ Ref ]
Sharma S, Sharma P, Siddiqui S, Bhattacharyya AK (2010) Effect of Metal Pickling and Electroplating Industrial Sludge-Borne Heavy Metals on Wheat (Triticum aestivum) Seedling Growth. Nature and Science 8: 1- 8.[ Ref ]