Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Research
Received date: 23 May, 2019
Accepted date: 06 June, 2019
Published date: 13 June, 2019
Citation: M’hammed E, Fatiha D, Ayada D, Djeloul B (2019) Evaluation of Reaction of Aza Michael Catalyzed by the Raw Red Clay of Adrar-Algeria Zone, under Solvent- Free Conditions, and at Room Temperature. Sch J Appl Sci Res Vol: 2, Issu: 8 (01-05).
Copyright: © 2019 M’hammed E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This work was conducted to evaluate catalytic efficiency of red clay of Adrar -Algeria zone as an effective green catalyst on the Aza Michael reaction.
The Aza-Michael reaction is the addition of amine to α, β-unsaturated alkenes was carried out using raw red clay from the Adrar-Algeria area as a catalyst to synthesize the new carbon or carbon-heteroatom bond containing products.
The reaction was carried out with favorable conditions, ambient temperature, without solvent, and the protocol likes the environment, so-that the synthesized products provide high yields, and excellent chemo selectivity.
Keywords: Raw Clay, Amines, Alkenes, Environment, Adrar, AzaMichael.
Abstract
This work was conducted to evaluate catalytic efficiency of red clay of Adrar -Algeria zone as an effective green catalyst on the Aza Michael reaction.
The Aza-Michael reaction is the addition of amine to α, β-unsaturated alkenes was carried out using raw red clay from the Adrar-Algeria area as a catalyst to synthesize the new carbon or carbon-heteroatom bond containing products.
The reaction was carried out with favorable conditions, ambient temperature, without solvent, and the protocol likes the environment, so-that the synthesized products provide high yields, and excellent chemo selectivity.
Keywords: Raw Clay, Amines, Alkenes, Environment, Adrar, AzaMichael.
Introduction
Clays are crystalline porous materials, that have been used in many separation processes [1-6], such as in extracting oxygen from the air or separating the Orth, Meta and para isomers of xylene from a mixture [7-15], These processes exploit the properties of adsorption selectivity of in the molecular sieves [16-20], Also clays are among the polides [21], which are used in catalyst industries [19,22-27]. Their fields of application are vast, industrial, sanitary, and environmental, these polides are used in heterogeneous catalysis.
The use of heterogeneous catalysts for organic synthesis is growing rapidly on homogeneous catalytic systems due to their, high stability, ease of handling, recovery and reuse, non-corrosive character, persistent long-term catalytic activity duration, and environmentally friendly [28].
Recently, we reported several organic transformations catalyzed by clay, such as a solid support acid catalyst [29-31]. It has a low production cost, easy to prepare in laboratory and can be stored for a long time without significant loss of catalytic activity However in the research study evaluated the raw clay of the Adrar zone - Algeria as a catalyst in a green synthesis and effective, based on the Aza-Michael reaction [32-34].
Many methods have been described in the literature for synthesis of β-amino, ketones, esters or nitriles. Among the various synthesis methods [35-38], the most frequently used is the conjugated addition of amines, on α, β-unsaturated ketones or esters or nitriles, called AzaMichael reaction [10,39-41]. In general, the Michael addition-1,4 reaction requires basic conditions or very specific conditions [42,43]. The short reaction time, very good chemo selectivity, high efficiency, the ease of purification of the products [44,45], the use of an inexpensive and reusable catalyst, are the main characteristics of this protocol.
| Parameters | The Value | Parameters (obtained at the laboratory level of Bechar clays) | Values |
|---|---|---|---|
| Ph | 3,6 | Moist density | 1,92 t- /-m3 |
| Humidity | 2,14% | Dry density | 1,87 t- /-m³ |
| Swelling index | 1,04% | Saturation level | 17% |
| Liquidity limit (Wl) | 75% |
Table 1: Physical properties of the studied catalysts.
| Chemical compound | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | Cl | F |
|---|---|---|---|---|---|---|---|---|---|
| % mass. | 62,86 | 15,17 | 7,24 | 1,65 | 0,99 | 0,58 | 3,52 | 0,309 | 0,05 |
Table 2: Results of chemical analyzes of Adrar's natural clay.
Materials and Methods
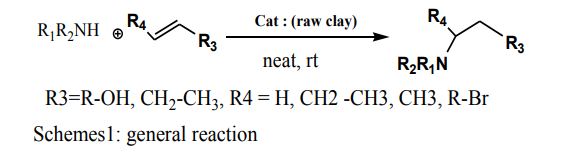
A practical method was developed for the addition of aliphatic or aromatic amines to alkenes, catalyzed by acids or alkalis, which is called Aza-Michael reaction. In an Erlenmeyer Meyer we add (5mmol, 1eq) alkenes on (6mmol, 1.2 equivalents) of amines and during stirring of the mixture we add (0.100 mg) of our catalyst the raw clay, the reaction is without solvent, and at room temperature, we followed the reaction by TLC platelets, until the appearance of the compound after filtered, and washed with dichloromethane, and remove excess amine and excess of the starting reagents by evaporation in a rotary evaporator, and analyzes the products obtained by 1HNMR.
Characterization of our catalyst the natural clay of Adrar -Algeria
The physical properties of the Adrar’s natural clay catalyst is shown in Table 1.
That the result revealed that, the clay type contains a large presence of acid where the pH value was 3.6. The corresponding saturation rate was 17% with a small amount of water depending on the humidity value (2.14%) with a wet and dry density of 1.92 t- /- m3. On the other hand, there is a small IG inflation of 1.06% and liquidity value 75%.
Chemical properties of Adrar’s natural clay catalyst: The chemical properties were obtained according to the Béchar geotechnical laboratory:
Insoluble: 90.86% present the crystalline compounds.
Carbonate: 09.86%
Sulfate: Trace
The results of the chemical analysis of the natural clay sample are given presented in Table 2.
It was found that the predominant constituents of this clay are: silica and alumina. The SiO2/Al2O3 ratio is 4.14. This is explained by the high content of free silica. Some authors present relate to the degree of purity of clay, especially when its value varies between 2 and 5.5 [46]. In particular, the presence of trace oxides such as MgO, CaO, Na2O, and K2O. The presence of a remarkable amount (3.52%) of K2O revealed the presence of an illite phase. Appearance of MgO in the sample may indicate the presence of smectite and a small amount of dolomite in the carbonate-rich clay [46]. Fe2O3 conqtent of the natural clay sample was found to be 7.24%, being the dominant form of iron, iron bound to the silicate lattice (96% of total iron) and the remainder occurred in the form of free iron oxides.
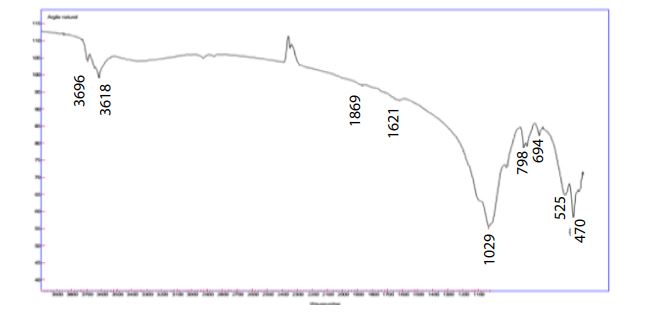
FTIR of characterization: The IR spectra of the sample are in agreement with data from the clay materials literature Jana Madejov, and the spectra show smaller bands around 3696 and 3618 cm-1 which are attributed to elongation of hydroxyl bonds (COH) (Figure 1).
1869cm-1 characteristic with carbonate CO3-2.
1621 cm-1 which is attributed to H-O-H deformation vibrations of water molecules.
The widest and widest band located between 900-1200 cm-1 and centered at 1029 cm-1 corresponds to the valence vibrations of the Si-O bond 798cm-1 pair Al-O and Si-O outside the planes attributed to the impurity of Silica and Quartz.
The 798 cm-1 band is attributed to Si-O-Al stretching vibrations.
The bands centered at 694, 525 and 470 cm-1 are respectively attributed to the deformation vibrations of the Al-OH-Al and Si-O-Al bonds.
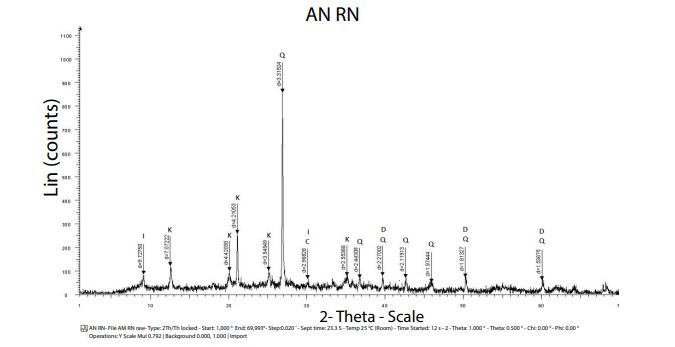
XRD characterization: X-ray diffraction pattern of natural clay is shown in Figure 2.
K: Kaolinite, I: Illite, Q: Quartz, C: Calcite, D: Dolomite.
The preliminary examination of the diffractogram of natural clay reveals the presence of the following minerals: Kaolinite (K), Illite (I), Quartz (Q), Dolimite (D) and Calcite (C).
The major crystalline phases contained in this natural clay are composed of the following minerals: Kaolinite (K) and Illite (I). The dominant clay mineral is Kaolinite, characterized by an intense peak at (d=4.21A°, 2θ=21.093) and a series of peaks of varying intensities presented in the Table 3.
Crystalline impurities (non-clay minerals) consist mainly of quartz, calcite and dolomite (Table 4).
From these results it can be shown that this natural clay is comparable to that of type 7 A°, which is a Kaolinite.
Figure 1: IR spectrum of our catalyst Adrar pure clay.
Figure 2: X-ray diffract gram of our catalyst Adrar's natural clay.
Figure 3: Schemes 1: General reaction.
| Kaolinite | Illite | ||||||
|---|---|---|---|---|---|---|---|
| 2θ (°) | 12,537 | 20,075 | 21,093 | 25,153 | 35,143 | 9,096 | 30,114 |
| d (A°) | 7,072 | 4,420 | 4,210 | 3,545 | 2,553 | 9,727 | 2,968 |
Table 3: Diffraction angle and inter-reticular distances of clay phases
| Quartz | |||||||
|---|---|---|---|---|---|---|---|
| 2θ (°) | 26,865 | 36,772 | 40,029 | 42,675 | 46,013 | 50,321 | 60,133 |
| d (A°) | 3,315 | 2,44 | 2,270 | 2,119 | 1,974 | 1,813 | 1,538 |
| Dolomite | Calcite | |||
|---|---|---|---|---|
| 2θ (°) | 40,029 | 50,321 | 60,133 | 30,114 |
| d (A°) | 2,27 | 1,813 | 1,538 | 2,968 |
Table 4: Diffraction angle and inter-reticular distances of impurities.
Results and Discussion
From these raw Adrar Clay analysis results we have carried out our work of testing the catalytic efficiency of our Catalyst raw clay from Adrar - Algeria, with a cost effective and efficient method able to satisfy the objective of AzaMichael addition, nucleophilic addition of a carbanion on an α, β-unsaturated carbonyl compound, for the synthesis of a variety of nitrogen-containing products, bioactive natural products, antibiotics, and chiral auxiliaries, and the formation of CC or C-heteroatom bonds (Figure 3 and Table 5).
Conclusion
According to these excellent results, we can conclude that the use of an inexpensive and reusable catalyst such as Red Clay of the Adrar-Algeria zone, to synthesize the new Carbon-Carbon or Carbon-Heteroatom bond containing products according to the recommendations of the reaction Aza Michael, of addition of amines to α, β-unsaturated alkenes, is satisfying the objective.
The reaction was solvent free, with short time, at room temperature, with excellent chemical selectivity, high product yields and easy purification, are the main features of this elegant protocol.
Pectral Data
Product 01
Product 02
Product 03
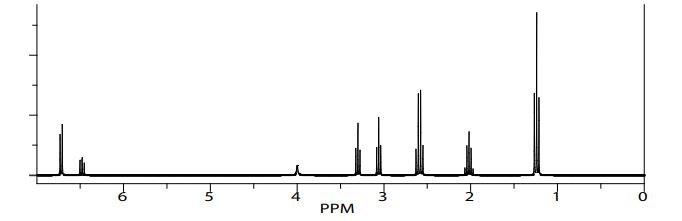
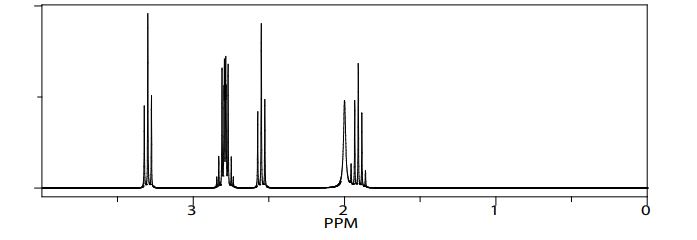
Product 01: RF=0.69 (Hexane-EtoAc): 2/1 1HNMR (CDCl3, 300 MHz) δ: 1-1.52 (t, 2H) ;1.79 (m,2H); 3.30(t, 2H); 4.00(s, 1H); 6.34 (d, 2H); 6.58(t, 1H); 7.04(m, 2H)
Product 02: RF=0.66 (Hexane-EtoAc): 2/1 1HNMR (CDCl3, 300 MHz) δ: 1.24 (s, 6H); 2.02 (m, 2H); 2.59(q, 4H); 3.06(q,2H); 3.30 (t, 2H); 4.0(s, 1H); 6.48(t, 2H); 6.72(d, 4H)
Product 03: RF=0.74 (Hexane-EtoAc): 2/1 1HNMR (CDCl3, 300 MHz) δ: 1.91 (m, 2H); 2.00 (s, 3H); 2.55(t, 2H); 2.77(t,2H); 2.81 (t, 2H); 3.30(t, 2H)
Carretero MI (2002) Clay minerals and their beneficial effects upon human health: a review, applied Clay Science 21: 155-163.[ Ref ]
Carretero MI, Pozo M (2009) Clay and non-clay minerals in the pharmaceutical industry Part I. Excipients and medical applications. Applied Clay Science 46: 73-80.[ Ref ]
Chavanne P (2011) 200 remèdes à l’argile, éditions First, dépôt légal: aout.[ Ref ]
Choy J, Choi S, Oh J, Park T (2007) Clay minerals and layered double hydroxides for novel biological applications. Applied Clay Science 36: 122-132.[ Ref ]
Belt ST, Guy Allard W, Rintatalo J, Johns LA, Van Dun ACT, et al. (2000) Clay and acid catalyzed isomerization and cyclization of highly branched isoprenoid (HBI) alkenes: implications for sedimentary reactions and distributions. Geochim Cosmochim Acta 64: 3337-3345.[ Ref ]
Choudhary VR, Jha R, Narkhede VS (2005) In-Mg-hydrotalcite anionic clay as catalyst or catalyst precursor for Friedel-Crafts type benzylation reactions. J Mol Catal A Chem 239: 76-81.[ Ref ]
Dabbagh HA, Teimouri A, NajafiChermahini A (2007) Environmentally friendly efficient synthesis and mechanism of triazenes derived from cyclic amines on clays HZSM-5 and sulfated zirconia. Appl Catal 76: 24- 33.[ Ref ]
Nagendrappa G (2011) Applied Clay Science 53: 106-138.[ Ref ]
Ehsan AM, Ehsan S, Khan S, Khan MS (2006) Friedel-Crafts benzylation using clay mineral catalysts and new synthesis of metal complex dyes. J Chem Soc Pak 28: 489-493.[ Ref ]
Gültekin Z (2004) Iron (III)-doped montmorillonite catalysis of alkenes bearing sulfoxide groups in Diels-Alder reactions. Clay Miner 39: 345- 348.[ Ref ]
Ait Barka E, Eullaffroy P, Clement C, Vernet G (2004) Chitosan improves development, and protects Vitisvinifera L against Botrytis cinerea. Plant Cell Rep 22: 608-614.[ Ref ]
Ait Barka E, Gognies S, Nowak J, Audran JC, Belarbi A (2002) Inhibitory effect of endophyte bacteria on Botrytis cinereal and its influence to promote the grapevine growth. Biol Control 24: 135-142.[ Ref ]
Alabouvette C, Lemanceau P, Steinberg C (1996) Biological control of Fusarium wilts: opportunities for developing a commercial product. In: Hall R (Ed), Principles and Practice of Managing Soilborne Plant Pathogens. American Phytopathological Press St Paul Minn; pp: 192- 212.[ Ref ]
Badalyan SM, Innocenti G, Garibyan NG (2002) Antagonistic activity of xylotrophic mushrooms against pathogens of cereals in dual culture. Phytopathol. Mediterr 41: 200-225.[ Ref ]
Hatimi A (1989) Etude de la receptivit´ e des sols de deux palmeraies marocaines au Bayoud. These de troisi` eme cycle, Universit` e Cadi Ayyad, Marrakech; pp: 58.[ Ref ]
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15: 353-378.[ Ref ]
Laux P, Baysal O, Zeller W (2002) Biological control of fire blight by using Rahnellaaquatilis Ra39 and Pseudomonas spec. R1 Acta Hort 590: 225-230.[ Ref ]
Hadrami I, Ramos T, El Bellaj M, El Idrissi-Tourane A, Macheix JJ (1997) A sinapic derivative as induced defense compound of date palm against Fusarium oxysporumf sp albedinis, the agent causing Bayoud disease. J Phytopathol 145: 329-333.[ Ref ]
Mukherjee C, Misra AK (2007) Letters in Organic Chemistry 4: 54-59.[ Ref ]
Azizi N, Saidi MR (2004) Tetrahedron 60: 383-387.[ Ref ]
Reddy BM, Patil MK, Reddy BT (2008) Catal Lett 126: 413-418.[ Ref ]
Wang Y, Yan-Qin Y, Rong S (2009) Molecules 14: 4779-4789.[ Ref ]
Jung ME (1991) In Comprehensive Organic Synthesis; Trost BM, Fleming I Eds Pergamon: Oxford.[ Ref ]
Perlmutter P (1992) Conjugate Addition Reactions in Organic Synthesis; Tetrahedron Organic Chemistry Series; Pergamon: Oxford 9: 114.[ Ref ]
Bartoli G, Cimarelli C, Marcantoni E, Palmieri G, Petrini MJ (1994) Org Chem 59: 5328.[ Ref ]
Liu M, Sibi MP (2002) Tetrahedron 58: 7991.[ Ref ]
Dintzner MR, Morse KM, McClelland KM, Coligado DM (2004) Investigation of the montmorillonite clay-catalyzed [1, 3] shift reaction of 3-methyl-2-butenyl phenyl ether. Tetrahedron Lett 45: 79-81.[ Ref ]
Leadbeater NE, Pillsbury SJ, Shanahan E, Williams VA (2005) Tetrahedron 61: 3565.[ Ref ]
Arend M, Westermann B, Angew RN (1998) Chem Int Ed Engl 37: 1044.[ Ref ]
Gomtsyan A, Koening RJ, Lee CH (2001) J Org Chem 66: 3613.[ Ref ]
Gomtsyan A (2002) Org Lett 11.[ Ref ]
Davies SG, McCarthy TD, Synlett (1995) 700.[ Ref ]
Bull SD, Davies SG, Delgado-Ballester S, Fenton G, Kelly PM, et al. (2000) 1257.[ Ref ]
Jenner G (1995) Tetrahedron Lett 36: 233.[ Ref ]
Surendra K, Krishnaveni NS, Sridhar R, Rao KR (2006) Tetrahedron Lett 47: 2125.[ Ref ]
Amore KM, Leadbeater NE, Miller TA, Schmink JR (2006) Tetrahedron Lett 47: 8583.[ Ref ]
Polshettiwar V, Varma RS (2007) Tetrahedron Lett 48: 8735.[ Ref ]
Bhanushali MJ, Nandurkar NS, Jagtap SR, Bhanage BM (2008) Catal Commun 9: 189.[ Ref ]
Hazarika MK, Parajuli R, Phukan P (2007) Synthesis of parabens using montmorillonite K10 clay as catalyst: a green protocol. Indian J Chem Technol 14: 104-106.[ Ref ]
Wallis PJ, Gates WP, Patti AF, Scott JL, Teoh E (2007) Assessing and improving the catalytic activity of K10 montmorillonite. Green Chem 9: 980-986.[ Ref ]
Vogels RJMJ, Kloprogge JT, Geus JW (2005) Catalytic activity of synthetic saponite clays: effects of tetrahedral and octahedral composition. J Catal 231: 443-452.[ Ref ]
Varma RS (2002) Clay and clay supported reagents in organic synthesis. Tetrahedron 58: 1235-1255.[ Ref ]
Reddy P, Bandichhor R (2013) Tetrahedron Lett 54: 3911-3915.[ Ref ]
Itoh J, Fuchibe K, Akiyama T (2008) Angew Chem 120: 4080-4084.[ Ref ]
Ganesh M, Seidel D (2008) J Am Chem Soc 130: 16464-16465.[ Ref ]
Wu J, Li X, Wan B (2011) Org Lett 13: 4834-4837.[ Ref ]