Journal Name: Scholar Journal of Applied Sciences and Research
Article Type: Research
Received date: 16 July 2018
Accepted date: 28 July, 2018
Published date: 12 August, 2018
Citation: Lawal G, Bidaisee S, Ducey J (2018) One Health Effectiveness in Managing Zoonoses. Sch J Appl Sci Res. Vol: 1, Issu: 5(25-29).
Copyright: © 2018 Lawal G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Zoonoses or zoonotic diseases refer to more than diseases that can be transmitted from animals to humans. They represent an important part of infectious diseases in all aspects of medicine and have been implicated in many emerging and re-emerging diseases. Measures to curtail zoonotic disease are mostly centered around microbiological or laboratory techniques. However, the prevalence of zoonotic diseases continues to remain high. A One Health approach provides a breakthrough method towards preventing and reducing the occurrence of zoonotic diseases by integrating human, animal, and environmental health. One Health highlights the importance of investigating health across all species of animals. It also explores the impact of economic, political, social and cultural sectors on public health both of humans and of animals.
Abstract
Zoonoses or zoonotic diseases refer to more than diseases that can be transmitted from animals to humans. They represent an important part of infectious diseases in all aspects of medicine and have been implicated in many emerging and re-emerging diseases. Measures to curtail zoonotic disease are mostly centered around microbiological or laboratory techniques. However, the prevalence of zoonotic diseases continues to remain high. A One Health approach provides a breakthrough method towards preventing and reducing the occurrence of zoonotic diseases by integrating human, animal, and environmental health. One Health highlights the importance of investigating health across all species of animals. It also explores the impact of economic, political, social and cultural sectors on public health both of humans and of animals.
Introduction
The Zoonoses Perspective and One Health
Zoonotic diseases are a burden on healthcare systems globally, especially underdeveloped nations. According to the United States Centers for Disease Control and Prevention, zoonoses represent 60% of infectious diseases and 75% of new or emerging infectious disease [1]. Zoonotic diseases continue to be on the rise despite current interventions. Continued support for interdisciplinary approaches to zoonotic management with implementation of emerging novel interventions as well as curtailing irresponsible and detrimental management strategies.
The concept of One Health originated in the 1980s and has consistently been promoted by researchers and physicians [2]. Robert Koch’s investigation of anthrax demonstrated the link between microorganisms and the disease. He established Bacillus anthracispresent in animal blood of animals could be transferred into healthy animals by injection with infected blood. In four years, anthrax caused the deaths of as much as 528 persons and 56,000 livestock [3]. Though some animals only serve as intermediate hosts in disease transmission and do not always directly infect humans [4]. Koch’s experiment informs of possible transmission of infectious agents being passed directly from animals to humans via inoculation [3]. Other methods of transmission include ingestion, inhalation and so on [5]. Many factors contributing to zoonoses and their effects on human health need to be studied in to expand our understanding of how the human-animal relationship can detriment human health. Governments, health departments and communities were not able to manage the spread of anthrax until Koch’s discovery, which highlights the importance of research into zoonotic diseases, especially in a One Health approach to public health.
One health is a comprehensive, global approach to emphasize the importance of interdisciplinary communication in all facets of public healthcare for humans, animal welfare and management, and environmental management and conservation [6]. One Health has been described as integrative thinking of human and animal health [7]. Its goal is to increase cooperation between the government, communities and health workers, especially physicians and veterinarians, to better manage the issue of zoonotic diseases. Mazet et al highlights the interplay of human, animal and environmental health [8]. One Health approaches promote interventions and policies that aim to collaborate all parts of human health [8].
With emerging and remerging diseases, One Health inclusion and emphasis of zoonoses has become increasing important. Researchers such as Calvin Schwabe revived the concept of One Health through his book “Veterinary Medicine and Human Health” [9]. He advocated for integrative work between veterinary and human medicine [9]. Strategies to overcome emerging and re-emerging zoonotic disease must include collaboration between animal, human, and environmental health [6]. Otherwise, challenges will continue to arise inhibiting the control of zoonotic diseases.
Materials and Methods
This paper represents a reflective perspective of the role of zoonoses on the concept of One Health. The paper seeks to comment on the importance of zoonoses as a priority of One Health. A review of literature was conducted to evaluate the impact of various human-animal relationships with respect to zoonoses. Internet search engine, Google scholar, along research databases: PubMed, CINAHL, Cochrane Database and the National Institutes of Health, were used to appraise One Health approaches to zoonotic disease management. Search terms and key words included: zoonoses, infectious diseases, one health, global health, public health, environmental health, genetically modified organisms, antibiotics administration to animals, infectious disease prevalence and treatment, human and animal global population, agriculture, food distribution and safety. Review of peer reviewed articles, case reports, government as well as professional documentation reveals themes and topics to determine the scope of One Health and the involvement of zoonoses as well as other themes.
Search for research articles were initially broad using the “One Health” keyword. Articles selected for review were screened using criteria for articles published between year 2000 to year 2018 which included the terminology of One Health and zoonoses. In addition, articles that referenced animal-human and animal-animal zoonotic disease transmission were selected. 23 peer reviewed articles, case reports, government depositions and international databases were eventually selected from a little over 70 resources that were initially reviewed.
Results and Discussion
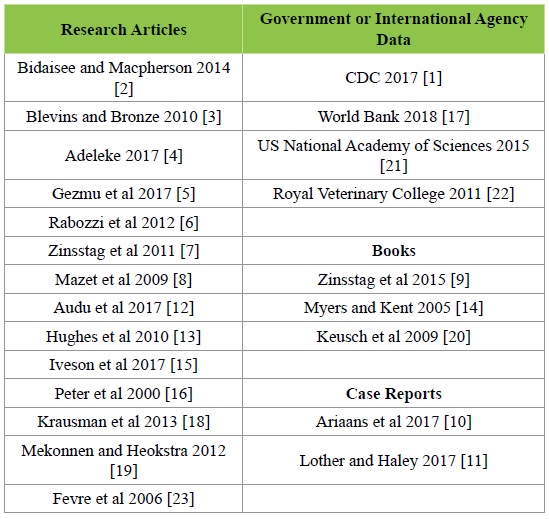
Table 1 below demonstrates the breakdown of the various resources utilized from this review. Ultimately included were 14 research articles, 2 case reports, 3 books regarding public health policy, and data from 4 governmental or quasigovernmental agencies.
Table 1: Resources for Review.
The Blind Spot: Human and Animal Interactions
Research trends show that scientists, researchers and health organizations around the world have spent decades focusing on the prevention of zoonotic diseases through the use of antimicrobial drugs and diagnostic techniques. Emphasis is placed on measures easily quantified within a laboratory setting. This focus overlooks other vital considerations necessary in order to aptly address zoonotic diseases.
Linking particular zoonoses with particular species has been an important aspect of public health for some time. Examples include: histoplasmosis linked with contact with bats habitats while spelunking or volcano boarding [10], rickettsial diseases common with stings from the dermacentor species while mountain climbing [11], or rabies when bitten by an infected dog [12]. The HIV pandemic resulted from simian primate hunters being inoculated by the simian immunodeficiency virus in the early 20th Century [13]. These examples demonstrate successes in determining the link between human diseases and wild animal hosts.
There’s so much attention focused towards wild animals that create a vulnerability to overlook other animals which commonly interact with people. While it is important to be mindful of exposure to zoonoses from unfamiliar animals, it remains pertinent to address the animals which habitually contact humans daily. Animals can be found in many households assisting in many ways and serving many purposes. These roles include companionship as well as service animals. While the presence of animals amongst humans is not entirely detrimental, the association between humans and animals remains contributory to the expanding distribution of zoonotic diseases. Provision of healthcare needs to account for not only include the human patients, but animals surrounding them as well.
Population Dynamics
An additional consideration is the population dynamics between humans and animals. Depicted in Figure 1 below, human population has experienced an exponential increase over the years. Myers et al quantifies the ratio of the animal population compared to that of the human population. It is stated that there are 23 billion animals to 6.4 billion humans [14]. In other words, there are three times as many animals as humans. While human population has doubled, the domestic animal population has tripled over the years since 1960 [14]. This increases the incidence as well as the frequency of interactions between humans and various animals.
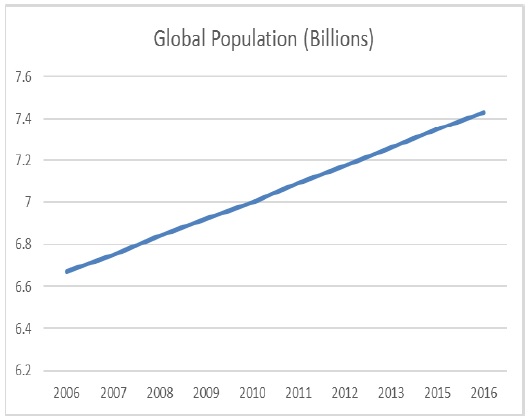
Figure 1: Total human population in the world, 2006 – 2016 [17].
In addition to the increasing population, human migration across the globe in also on the rise [15]. Migration of humans to increase accommodations and living space or during tourism is occurring at a rapid rate such that it has led to an invasion of humans into animal territory [15]. To further buttress these effects, Peter et al shows the interaction observed between wildlife, domestic animal, and human populations using the host-parasite ecological continuum [8]. The article discusses the correlation between disease emergence and a change in the ecology of host and pathogen, emphasizing the emergence of viruses such as Marburg, Ebola and HIV due to the intrusion of humans in to animal habitat[16]. In summary, increasing human population increases animal population, leading to increased risk of the emergence or reemergence of zoonotic disease [16].
Figure 1 shows the increased in human population from 2006-2016[17]. In 2006, total human population increased by 1.2% to 6,676,244,478 people. This rate was maintained over the next decade resulting in a global population of 7,429,764,000 in 2016[17].
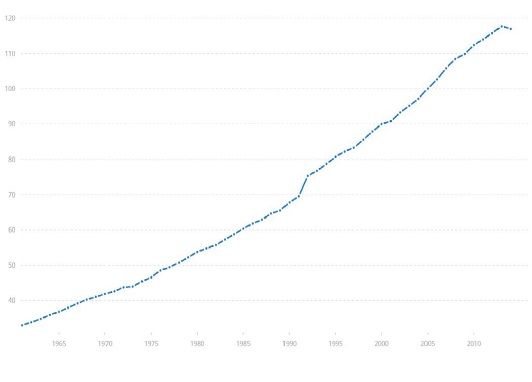
Figure 2 shows displays the global livestock production index relative to 2004-2006 and the dramatic increase since the beginning of collecting such data by the World Bank. In approximately the same decade of population increase we just reviewed the global livestock index has steadily increased to levels 16.984% higher than in 2004[17].
Figure 2: Livestock Production Index (2004-2006=100), 1961 – 2014 [17].
The Food System – How Human Food Production Contributes to Zoonoses
With the surge in human population, there has been a corresponding increase in the demand for food products including meat. This dependence on animal products globally means world’s demand for meat is projected to double [18]. Myers et al describes human reliance on animal products such as meat, milk, hides, wool and so on, by emphasizing the increase in the global production as well as consumption of animal products over recent decades [14]. He projects that this demand will continue to increase. According to a 2005 article by the Food and Agricultural Organization of the United Nations (FAO, UN), global meat production doubled between year 1980 and 2004 [8], and this trend will persist considering the anticipated expansion of meat production through year 2050 [19]. Capacity to meet the upsurge in the demand for animal products has been achieved with improved methods of husbandry both in the number of animals generated and how much product can be generated from them [20]. In addition, genetic augmentation in biotechnology is being considered as a method to augment contemporary breeding techniques to achieve greater overall production and sustainability from each animal [20]. Antimicrobials are also being administered to animals to enhance productivity [21].
Domesticated animals do not forage but rather live in “bed and breakfast” conditions, being fed in pens with feeds laced with antibiotics [21]. Antimicrobial use in rearing domestic animals is overzealous, accounting for approximately 80% of the yearly antimicrobial consumption in the United States [21]. This reckless use of antibiotics contributed to the increased prevalence of drug-resistant pathogens [21]. These animals are being genetically adapted for faster growth and production of more meat, without consideration of the environmental and public health implications which can result from these types of management.
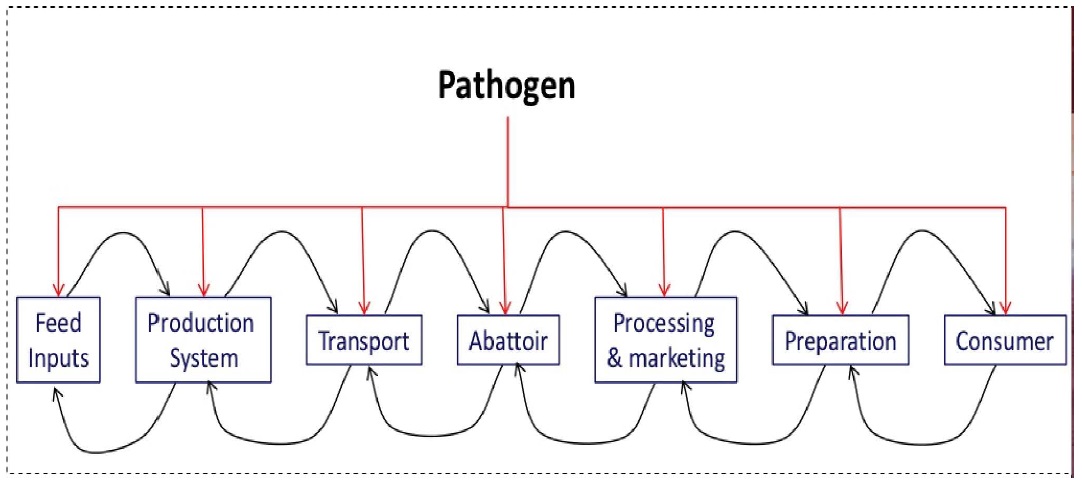
Many pathogens develop animals and can be passed to humans. Furthermore, these pathogens in some cases can also be transferred between animals. When considering passage of pathogens between animals, we need to also analyze the chain and interconnectivity existing between animals during production, distribution and marketing of animal products. The algorithm in Figure 3 helps to visualize this relationship and how pathogens can take advantage of each stage involve in livestock food system[22].
Figure 3: Relationship between livestock food systems and pathogen [22].
It is also important to consider the geographical location of origin where animal products are exported from. Somalia, for example is known to export livestock in large quantities. According to Fevre et al, 75 000 head of cattle move from Somalia to Kenya on a yearly basis, and up to 850 000 goats are sent from Somalia to the Middle East [23]. Since Somalia is known to be endemic for many infectious diseases; including both zoonotic and non-zoonotic infectious diseases such as anthrax, babesiosis, brucellosis, bovine and caprine pleuropneumonia, hand, foot and mouth disease (HFMD), heartwater, rabies, Rift Valley Fever, as well as trypanosomiasis [14]. As a result, transportation of animal products from these areas during distribution and marketing may also serve as a vehicle for transmission of infectious diseases.
There is an increasing interconnectedness between the countries of the world. A simple breakfast meal may have come from different parts of the world, which further reiterates the need to strive towards creating a One World One Health One Medicine strategy in eliminating zoonotic diseases. Actions taking into consideration the economic, political, social, and cultural sectors, including their effects on food system need to be implemented to decrease the risk of transmission during distribution of animal products. Contact should also be limited between wild animals and domestic animals to limit transmission to humans.
Conclusions
One Health, Vision 2020: The concept of One Health One Medicine is increasingly embraced. It is being practiced globally and plays an important role in the management of zoonotic diseases. The One Health approach and zoonoses reporting has led to a significant improvement in issues of population dynamics plaguing human health. However, developing nations still represent a deficit in the global spread of the One Health model of intervention and prevention where zoonotic diseases are more prevalent and cause even greater detriments.
Also, food system management poses another challenge to mitigating zoonotic diseases. A more collective effort and an approach that is systems-based should be implemented, instead of just focusing on zoonotic epidemic control. There are social, economic, and environmental risks involved in the manufacturing, distribution and marketing of animal products. Every step of each of these processes represents a potential target for interventions to minimize these risks. Health certification of animals should be required and be based on laboratory test results. Animals that fail laboratory tests should be quarantined. Inspection of animal products at the ports and markets before distribution should be carried out thoroughly. Exportation and importation regulations need to be implemented and enforced. The efforts of policy makers and the government organizations will go a long way in curbing the issue of administering antibiotics and ensuring appropriate management of gene modification to animals.
More than ever, it is of paramount significance to highlight the importance of a One Health attitude towards reducing zoonotic diseases. The government, community and practitioners in all fields of medicine, including allopathic, osteopathic and veterinary medicine need to merge efforts and approaches in the management of zoonotic diseases.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Centers for Disease Control and Prevention (CDC). Zoonotic Diseases. July 2017. [ Ref ]
Bidaisee S and Macpherson CNL (2014) Zoonoses and One Health: A Review of the Literature. Journal of Parasitology Research. [ Ref ]
Blevins SM and Bronz, M. (2010) Robert Koch and the ‘golden age’ of bacteriology. International Journal of Infectious Diseases 14: 744-751. [ Ref ]
Adeleke MA (2017) Delineation of potential urban urogenital schistosomiasis transmission areas in Osogbo, south-west Nigeria. Nigerian Journal of Parasitology 38. [ Ref ]
Gezmu M, Bsrat A, Mekuria A (2017) Assessment of Community Knowledge, Attitude and Practice on Zoonotic Disease in and Around Dodola Town, West Arsi Zone, Ethiopia. Ethiopian Journal of Veterinary Science and Animal Production 1: 57-65. [ Ref ]
Rabozzi G, Bonizzi L, Crespi E, Somaruga C, Sokooti M, et al. (2012) Emerging Zoonoses: the “One Health Approach.” Safety and Health at Work 3: 77-83. [ Ref ]
Zinsstag J, Schelling E, Waltner-Toews D, Tanner M (2011) From “one medicine” to “one health” and systemic approaches to health and well-being. Preventive Veterinary Medicine 101: 148-156. [ Ref ]
Mazet J, Clifford DL, Coppolillo PB, Deolalikar AB, Erickson JD, et al. (2009) A “One Health” Approach to Address Emerging Zoonoses: The HALI Project in Tanzania. PLoS Med 6: e1000190. [ Ref ]
Zinsstag J, Schelling E, Waltner-Toews D, Whittaker M, Tanner M (2015) One Health: The Theory and Practice of Integrated Health Approaches. CABI Publishing. [ Ref ]
Ariaans M, Valladares MJ, Keuter M, Verweij P, Van der Ven AJ, et al. (2017) Fever and arthralgia after ‘volcano boarding’ in Nicaragua. Travel Medicine and Infectious Disease 16: 68-69. [ Ref ]
Lother SA and Haley L (2017) Tick paralysis. CMAJ 189. [ Ref ]
Audu SW, Adawa D, Mshebwala PP, Simon Y, Yakubu B (2017) Field and Laboratory Detection of Rabies Antigens in Saliva and Brains of Dogs in Nigeria: An Approach Using Rapid Immunochromatographic Test. Journal of Microbes & Microbiology Techniques 1: 104. [ Ref ]
Hughes JM, Wilson ME, Pike BL, Saylors KE, Fair JN, et al. (2010) The Origin and Prevention of Pandemics. Clinical Infectious Diseases 50: 1636- 1640. [ Ref ]
Myers N and Kent J (2005) The New Atlas of Planet Management. Berkeley: University of California press. [ Ref ]
Iveson JB, Bradshaw SD, Smith DW (2017) The movement of humans and the spread of Salmonella into existing and pristine ecosystems. Microbiology Australia. [ Ref ]
Peter D, Andrew AC, Alex DH (2000) Emerging infectious diseases of wildlife; threats to biodiversity and human health. Science Compass 443: 9. [ Ref ]
Statistics (nd) Retrieved February 1, 2018, from http://datatopics.worldbank. org/ health/population https://data.worldbank.org/indicator/AG.PRD.LVSK. XD?end=2014&start=1961&type=points&view=chart [ Ref ]
Krausman F, Erb K, Simone G, Haberi H, Bondeau A, et al. (2013) Global human appropriation of net primary production doubled in the 20th century. PNAS 110: 10324-10329. [ Ref ]
Mekonnen MM and Hoekstra AY (2012) A Global Assessment of the Water Footprint of Farm Animal Products. Springer International Publishing 15: 401-415. [ Ref ]
Keusch GT, Pappaioanou M, Gonzalez MC (2009) Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. Chapter 3. National Academies Press (US). [ Ref ]
VanBoeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, et al. (2015) Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences of the United States of America 112: 5649- 5654. [ Ref ]
Rushton J, Yrjo-Koskinene A, Knight-Jones T, Marshall E, Onono J, et al. (2011) People, livestock, trade and animal disease: How can we improve the management of risk? Royal Veterinary College. [ Ref ]
Fevre EM, Bronsvoort BMC, Hamilton KA, Cleveland S (2006) Animal movements and the spread of infectious diseases. Trends in Microbiology 14: 125-131. [ Ref ]