Journal Name: Anaesthesiology and Pain Medicine
Article Type: Clinical Trial
Received date: 02 January, 2020
Accepted date: 21 January, 2020
Published date: 27 January, 2020
Citation: Mounaouaz, Attia H, Zbidi B, Masmoudi K, Bannour I, et al (2020) Effect Of Ventilatory Management On Postoperative Pain After Laparoscopic Surgery: A Prospective Randomized Trial. Anaesth Pain Med 2020. Vol: 2, Issu: 1 (04-08).
Copyright: © 2020 Rajendram R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Laparoscopic pneumoperitoneum affect respiratory gas exchanges (PaCO2, EtCO2, pH and bicarbonate) leading to respiratory acidosis and hypercapnia that can amplify postoperative pain which mechanisms are multiple. Ventilator management may interfere with the inflammatory mechanism of pain.
Patients and methods: we conducted a prospective randomized trial including patients with physical status ASA I or II and proposed for laparoscopic cholecystectomy.
Thirty not premeditated patients aged more than 18 years, were randomized to be allocated to:
• Group VC: classic ventilation 8cc/Kg of ideal body weight (IBW), PEP=5, I/E=1/2
• Group VP: protective ventilation 6cc/Kg of IBW, PEP=8, I/E=1/2, and hyperventilation at closure time.
Anesthesiologists in charged with post-operative evaluation are blinded to the patient’s allocation.
The primary outcome was the quality of postoperative analgesia. VAS was measured at H0, H2, H6, H12 and H24. The secondary outcome was enhanced post-operative recovery.
Results: We found that protective ventilation associated with hyperventilation at the end of laparoscopic surgery can significantly reduce postoperative pain, and morphine consumption leading to an enhanced post-operative recovery.
Conclusion: Protective ventilation associated with end surgery hyperventilation may reduce post-operative pain and allow an enhanced recovery. However, a larger sample size is needed to have more representative results. Dosage of inflammation’s mediators should be considered.
Keywords
Cholecystectomies, Laparoscopic, Carbone Dioxide, Postoperative Pain, Hyperventilation
Abstract
Introduction: Laparoscopic pneumoperitoneum affect respiratory gas exchanges (PaCO2, EtCO2, pH and bicarbonate) leading to respiratory acidosis and hypercapnia that can amplify postoperative pain which mechanisms are multiple. Ventilator management may interfere with the inflammatory mechanism of pain.
Patients and methods: we conducted a prospective randomized trial including patients with physical status ASA I or II and proposed for laparoscopic cholecystectomy.
Thirty not premeditated patients aged more than 18 years, were randomized to be allocated to:
• Group VC: classic ventilation 8cc/Kg of ideal body weight (IBW), PEP=5, I/E=1/2
• Group VP: protective ventilation 6cc/Kg of IBW, PEP=8, I/E=1/2, and hyperventilation at closure time.
Anesthesiologists in charged with post-operative evaluation are blinded to the patient’s allocation.
The primary outcome was the quality of postoperative analgesia. VAS was measured at H0, H2, H6, H12 and H24. The secondary outcome was enhanced post-operative recovery.
Results: We found that protective ventilation associated with hyperventilation at the end of laparoscopic surgery can significantly reduce postoperative pain, and morphine consumption leading to an enhanced post-operative recovery.
Conclusion: Protective ventilation associated with end surgery hyperventilation may reduce post-operative pain and allow an enhanced recovery. However, a larger sample size is needed to have more representative results. Dosage of inflammation’s mediators should be considered.
Keywords
Cholecystectomies, Laparoscopic, Carbone Dioxide, Postoperative Pain, Hyperventilation
Introduction
Despite of allowing a best surgical view, laparoscopic pneumoperitoneum with CO2 can affect respiratory gas exchanges (PaCO2, EtCO2, pH and bicarbonate) leading to respiratory acidosis and hypercapnia with physiological consequences [1]. To overcome these unwanted effects, we have to adjust minute ventilation during intraoperative period. Ten to 15% increment in minute volume keeps the PaCO2 levels in acceptable range during and after CO2 pneumoperitoneum [2].
Nevertheless, there is no study that evaluates the importance of hyperventilation on postoperative pain and rehabilitation. In this study we aimed to study the effects of combination of protective ventilation associated with hyperventilation at the end of surgery on the post operative pain scale and enhanced recovery.
Patients and Methods
We conducted a prospective randomized observerblinded study realized during March 2019 conducted in visceral surgery operating room involving 35 patients After obtaining their approval and written informed consent, patients are Aged more than 18 years, of physical status American Society of Anesthesiologist (ASA) I and II and scheduled for laparoscopic cholecystectomy. We excluded Patients who presented surgical incident requiring conversion to laparotomy or anesthetic complication which need postoperative ventilation. Not premeditated patients are randomized to: Groupe VC: classic ventilation 8cc/Kg of ideal body weight (IBW), PEP=5, I/E=1/2 or Groupe VP: protective ventilation 6cc/Kg of IBW, PEP=8, I/E=1/2, and hyperventilation at closure time (until EtCO2=28-30mmHg).
The anesthesiologist who’s charged by the evaluation of the postoperative period was not informed of peroperative ventilation strategy. All patients were hospitalized one day before intervention. The pre-anesthetic visit allowed checking any modification of clinical status of the patient and the application of the rules of fast. No premedication was given. In the operating room, standard monitoring including electrocardiography, pulse oximeter, and noninvasive blood pressure was used. Anesthetic induction was achieved with propofol 2mg/kg and remifentanil 1μg/kg /min. Intubation was facilitated with cisatracrium 0,15mg/Kg and additional dose 0,05mg/kg if needed to keep a single twitch on the train-of-four stimulation of the ulnar nerve. Maintenance of anesthesia was provided with Isoflurane and a continuous infusion of remifentanil which the dose was adapted to the anesthesia nociception index (ANI). The ANI value was maintained between 50 and 70. Bispectral index (BIS) was attached to monitor anesthetic depth throughout the operation. The BIS value was maintained between 40 and 60. In both groups, the ventilator mode was volume-controlled ventilation, inspiration to expiration ratio of 1:2 and fraction of inspired oxygen (FiO2) of 0, 5 in the medical air. These ventilator parameters were maintained throughout the study and the end-tidal CO2 (EtCO2) was kept between 35 and 40mmHg by adjusting respiratory rate, whenever necessary.
In group VC, ventilation was performed with VT of 8ml/ Kg (IBW) and Peep of 5mm Hg till the end of surgery.
In group VP, patients received VT of 6ml/Kg (IBW) with PEEP of 8, recruitment maneuvers every 30 minutes, plateau pressure < 20 cm of water and at the closure time we proceed to a manual hyperventilation until EtCO2 reaches 28-30 mmHg. Pneumoperitoneum was achieved by introduction of a veress needle at the umbilicus and CO2 insufflations. The intra-abdominal pressure was maintained between 10 and 12mmHg during surgery. Patient’s position was changed with 30° reverse Trendelenburg and 20° left lateral tift to improve surgical access. Intra-abdominal pressure was decreased every time a significant increase in airway pressure was noted and ventilator parameters were unchanged. Auscultation of both lung fields was performed to rule out one-lung-ventilation during pneumoperitoneum. Postoperative analgesia was anticipated by 1g of paracetamol and 20mg of nefopam in both groups. During the 24 first hours, the administration of 1g paracetamol was repeated every 6 hours and 20 mg of Nefopam every 8 hours.
In the postoperative period, the postoperative pain was evaluated by Visual analogical scale (VAS) at H0, H2, H6, H12 and H24. The primary outcome of this study was the quality of postoperative analgesia. VAS was measured at H0, H2, H6, H12 and H24. The secondary outcome was the quality of postoperative rehabilitation
Statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables (age, sex, BMI, pneumoperitoeum and anesthesia duration) were analyzed with t test or Mann Whitney test. Repeated –measures analysis of variance (ANOVA) was used to analyze changes in respiratory, anesthetic and hemodynamic parameters over time, using time as the between-subject factor. Data were expressed as the median± variance or number (%). A P values< 0, 05 were considered to indicate statistical significance.
Result
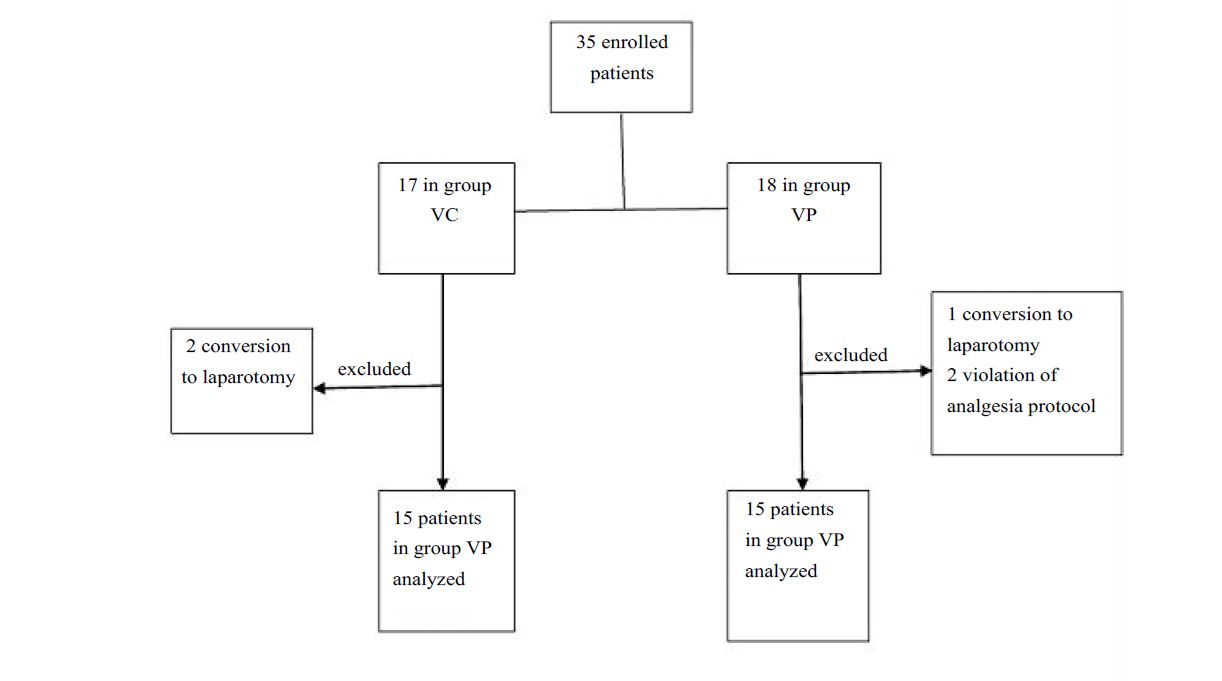
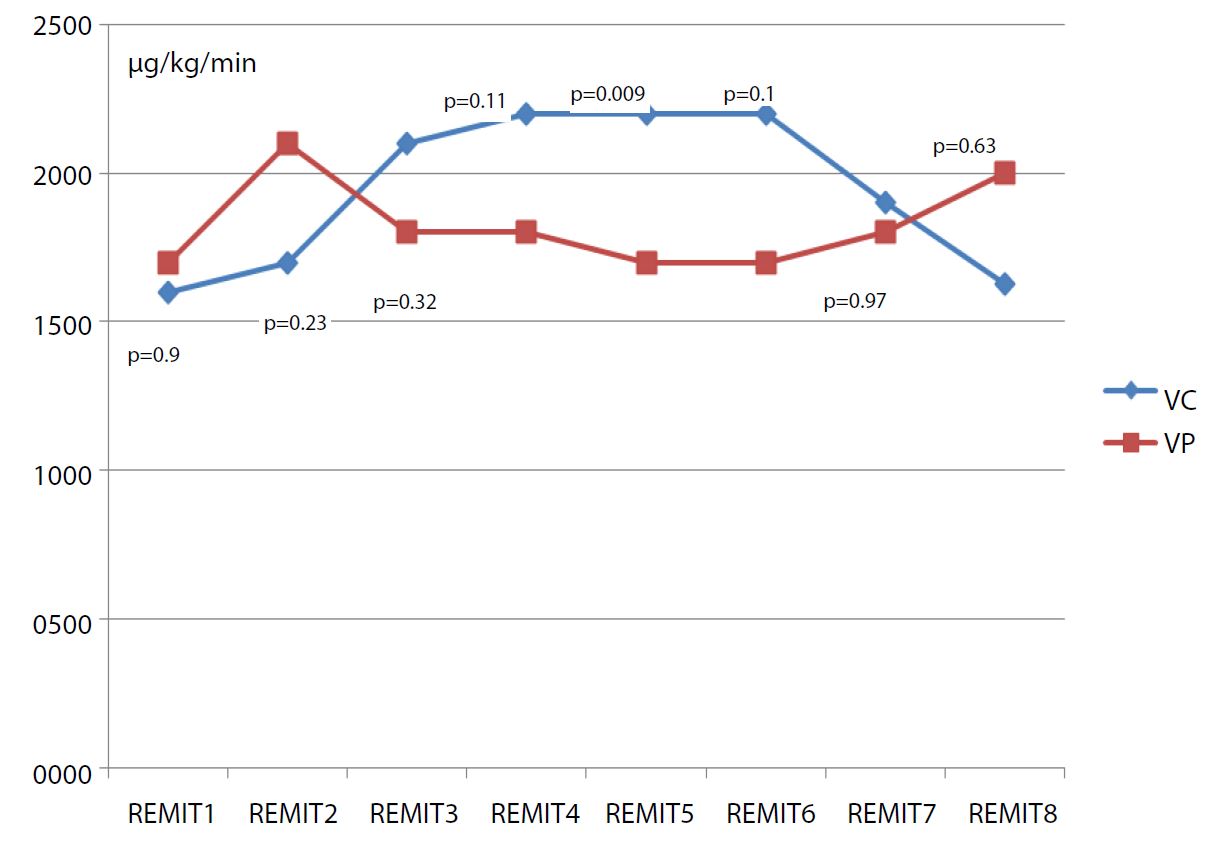
We allocated 35 patients in this study only 15 per group are analyzed (Figure 1), demographic characteristics and duration of surgery and anesthesia are comparable between two groups (Table I). During preoperative period, hemodynamic profile was comparable. SBP and HR were similar at all anesthetic times. The mean Remifentanil consumption during the surgical procedure was comparable (Figure 2). There is no difference of capnia and oxygenation in the preoperative period.
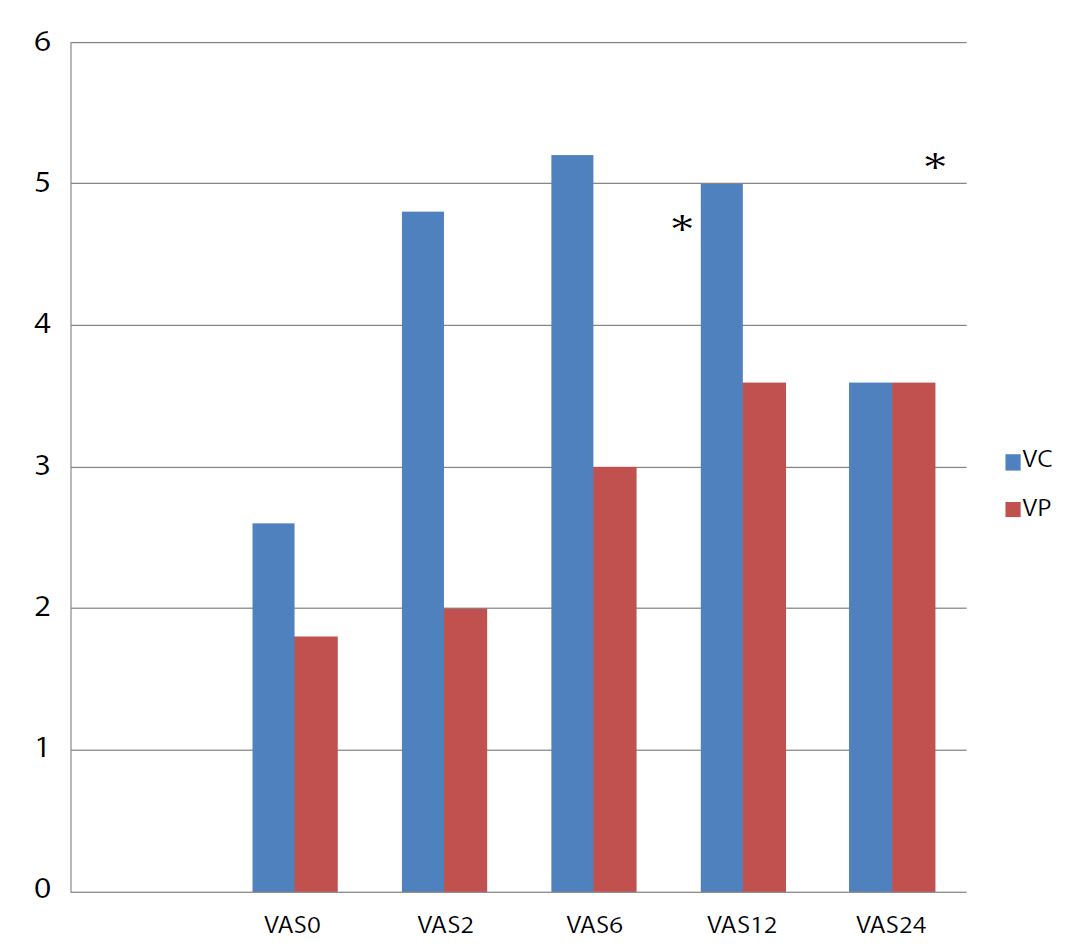
Postoperative analgesia was better in VP group. Statistical difference was significant only at the second and the sixth postoperative hour (p<0,001) (Figure 3). Four patients (13,3%) in group VC needed morphine in postoperative period versus no patient in group VP (p<0,001). The mean time to first rise up was 7,6±1,3 h in group VP versus 16±2,1h in group VC. This difference was statistically significant (p=0,04). The mean time to restore transit was shorter in group VP 8,8±2,7 h versus 20,8±3,1h in group VC (p=0,01). No pulmonary complication was noted in both groups.
Table 1:Two group demographic characteristics and duration of surgery.
| parameters | Group VC (n=15) | Group VP (n=15) | p |
|---|---|---|---|
| Age (year)* | 55,6±3,9 | 52,8±15,5 | 0,7 |
| Sex (M/F) (n) | 4/15 | 2/15 | 0,2 |
| BMI (Kg/m2)* | 27,6±3,5 | 24±2,12 | 0,08 |
| ASA I (n, %) II (n, %) | 7 (40,6%) 8(59,4%) | 8(59,4%) 7(40,6%) | 0,1 |
| Duration of surgery (min)* | 90±24,4 | 106±20,6 | 0,2 |
| Duration of anesthesia* (min) | 72±24 | 82±13 | 0,4 |
Figure 1:Flow Chart.
Figure 2:Mean Remifentanil consumption at different anesthesia’s time
Figure 3:Evolution of median VAS in the postoperative period *significant difference
Discussion
In this study we focused on the effect of protective peroperative ventilation combined with hyperventilation at the end of surgery. We found that postoperative pain and morphine consumption were reduced significantly. In fact, postoperative pain following laparoscopic cholecystectomy is multifactorial with complex mechanisms and has been the subject of numerous studies [3, 6]. It results from 2 mechanisms: nociception and inflammation.
Nociception in cholecystectomy causes moderate pain. However, the part of inflammation is more important. Conventionally, carbon dioxide (CO2) insufflation used during a laparoscopy procedure, may affect stress responses [7]. It leads to acidification of the peritoneum by formation of carbonic acid, and space is created between the liver and the diaphragm, which might be the cause of visceral and shoulder tip pain.
In this study we investigated the effect of ventilatory management on reducing the part of pain caused by inflammation and the resulting enhanced recovery. Lung-protective ventilator strategies are preferred to lessen regional end-inspiratory stretch so that pulmonary damage and levels of inflammatory mediators could be decreased [8]. Han et al. [7] reported that levels of inflammatory mediators including TNF-alpha, IL-6, IL-10, and heat shock protein 70 (Hsp70) were significantly higher in conventional gynecological laparoscopy assisted by CO2 insufflation compared to abdominal wall-lifting methods. Wrigge et al. [9] evaluated the effect of traditional VT with zero or low PEEP versus low VT with a PEEP of 10 cm H2O in patients with normal lungs during thoracic and abdominal surgery. They reported higher levels of inflammatory mediators in the serum and in tracheal aspiration material during and after mechanical ventilation. However, no significant differences were reported in the levels of inflammatory markers between both groups. Similarly, Memthsoudis et al. [10] also showed that no significant differences were present in the plasma levels of inflammatory mediators between two groups (lower VT and higher PEEP vs. higher VT and zero PEEP) of a total of 26 patients. Contrary to these former studies, Wolthuis et al. [7] reported that ventilation with lower VT alleviates an increase in the level of inflammatory mediators in the BAL fluid as compared with higher VT. With these findings, they suggested that ventilation with higher VT was associated with bigger proinflammatory stimuli in healthy lungs [7]. Different results in these previous studies can be multifactorial. One of them may be due to a comparison of the effects of ventilation strategies within the different surgical procedures. It is also unknown whether ventilation itself is the main cause or it exacerbates the production of inflammatory mediators.
Among the various methods adopted to eliminate residual CO2, active aspiration of CO2 at the end of the procedure or leaving a sub hepatic drain to expel the residual CO2 has been tried, but with varying degrees of success [9]. Carbon dioxide diffuses to the body more during extraperitoneal than intraperitoneal insufflation, and its diffusion is not influenced by the duration of intraperitoneal insufflation [10]. Furthermore, extraperitoneal carbon dioxide insufflation leads to higher Pa CO2 (tension of carbon dioxide in arterial blood) values in the postoperative period [11]. Intraperitoneal carbon dioxide was shown to be affected by raising the intraperitoneal pressure above the venous vessels pressure, which prevents carbon dioxide resorption leading to hypercapnia. Hypercapnia by itself increases minute ventilation by as much as 60% to normalize the end-tidal carbon dioxide (etco2) and activates the sympathetic nervous system leading to an increase in blood pressure, heart rate, myocardial contractility, and arrhythmias. It also sensitizes the myocardium to catecholamines, particularly when volatile anesthetic agents are used [12].
A 10% to 15% increase in intraoperative minute ventilation is usually sufficient to compensate the increased flow of CO2, thus avoiding an increase in end-tidal CO2 [13,14]. Knowing the contribution of pneumoperitoneum CO2 to the postoperative pain, we realized in this study a hyperventilation at the end of surgery in group VP. We found that postoperative analgesia was better in this group. Better is the postoperative analgesia, faster will be the postoperative rehabilitation.
Conclusion
Protective ventilation with hyperventilation at the end of surgery can reduce the post operative pain and better the resulting rehabilitation profile. However, a larger sample size is needed to have more representative results. We may also realize the dosage of the inflammation’s mediators to focus on the role of our ventilation strategy on the part of inflammation in postoperative pain.
Abdul Hakeem, Asif Iqbal, Mohammad Dawood, Basharat Saleem, Abdul Hameed (2016) Effects of co2 pneumoperitoneum on arterial partial pressure of carbon dioxide ph, end tidal carbon dioxide and bicarbonate in patients during laparoscopic cholecystectomy. J. Evolution Med. Dent. 5: 2299-2302. [ Ref ]
Maharjan SK, Shrestha BR (2007) Do we have to hyperventilate during laparoscopic surgery? Kathmandu University Medical Journal. 5: 307- 311. [ Ref ]
Fredman B, Jedeikin R, Olsfanger D, Flor P, Gruzman A (1994) Residual pneumoperitoneum: A cause of postoperative pain after laparoscopic cholecystectomy. Anesth Analg.79: 152-154. [ Ref ]
Ekstein P, Szold A, Sagie B, Werbin N, Klausner JM et al. (2006) Laparoscopic surgery may be associated with severe pain and high analgesia requirements in the immediate postoperative period. Ann Surg. 243: 41-46. [ Ref ]
Woolf CJ, Chong MS (1993) Préemptive analgesia- treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 77: 362-379. [ Ref ]
Aran T, MA unsal, S guven, C Kart, EC cetin, et al. (2012) carbon dioxide pneumoperitoneum induces systemic oxidative stress: a clinical study. Eur J Obstet Gynecol Reprod Biol 161: 80-83. [ Ref ]
Han C, Z Ding, J Fan, J Sun, Y Qian (2012) Comparison of the stress response in patients undergoing gynecological laparoscopic surgery using carbon dioxide pneumoperitoneum or abdominal walllifting methods. J Laparoendosc Adv Surg Tech A. 22: 330-335 [ Ref ]
Kilpatrick B, P Slinger (2010) Lung protective strategies in anaesthesia. Br J Anaesth 105: 108-116. [ Ref ]
Alexander JF, Hull MGR (1987) Abdominal pain after laparoscopy: The value of gas drain. Br J Obstet Gynaecol. 94: 267-269. [ Ref ]
Mullett CE, Viale JP, Sagnard PE (1993) Pulmonary CO2 elimination during surgical procedures using intra- or extra-peritoneal CO2 insufflation. Anesth Analg. 76: 622-666. [ Ref ]
Demiroluk S (2004) Effects of intraperitoneal and extraperitoneal carbon dioxide insufflation on blood gases during the perioperative period. J Laparoendosc Adv Surg Tech A. 14: 219-222. [ Ref ]
Gutt CN, Oniu T, Mehrabi A (2004) Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 21: 95-105. [ Ref ]
McMahon AJ, Baxter JN, Kenny G (1993) Ventilatory and blood gas changes during laparoscopic and open cholecystectomy. Br J Surg 80: 1252-1254. [ Ref ]
Kenefick JP, Leader A, Maltby JR, et al. (1987) Laparoscopy: Bloodgas values and minor sequale associated with three techniques based on isoflurane. Br J Anaesth 59: 189-194. [ Ref ]