Journal Name: International Journal of Cancer and Treatment
Article Type: Research
Received date: 10 April, 2023
Accepted date: 15 May, 2023
Published date: 22 May, 2023
Citation: Shiha G, Soliman R, Mikhail NNH, Hassan AA, Eslam M et al. (2023) A Prospective Study for Validation of General Evaluation Score for Hepatocellular Carcinoma Risk Stratification in Chronic Hepatitis C Patients. Int J Cancer Treat Vol: 6, Issu: 1 (18-26).
Copyright: © 2023 Shiha G et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: We developed and both internally and externally validated a simple scoring system called General Evaluation Score (GES) for HCC risk stratification.
Aim:To ascertain the validity of this score in a large prospective cohort of cured hepatitis C patients with compensated advanced chronic liver disease who achieved a sustained virological response following direct acting antivirals.
Patients and methods:This single-center prospective study included 463 consecutive patients, with advanced fibrosis (≥F3) who achieved SVR. The patients were recruited from the outpatient clinics at the Egyptian Liver Research Institute and Hospital between January 2018 and October 2019. All patients underwent abdominal ultrasound and multislice computed tomography for surveillance of HCC before starting antiviral therapy. Patients were followed up every 6 months after the end of treatment using ultrasonography and alpha-fetoprotein in addition to MSCT every 12 months.
Results:A total of 463 patients were included, of which 197 (42.5%), 114 (24.6%), and 152 (32.8%) had low, intermediate, and high-risk scores calculated before treatment, respectively. HCC incidence rate was 2.61/100 py (95% CI = 1.73–3.80); 25 cases developed HCC during follow-up. The incidence of HCC was 0.97% (95% CI: 0.31–2.34), 1.68% (95% CI: 0.53–4.05), and 5.57% (95% CI: 3.35–8.74) in the low, intermediate, and high-risk groups, respectively. The HCC incidence increased significantly with higher scores (p < 0.001). Harrell’s c-statistic for this model was 0.728.
Conclusion:This prospective study demonstrated the ability of GES to predict HCC occurrence and accurately stratify patients into low-, intermediate-, and high-risk groups.
Keywords
CHC, HCC risk, GES score, Prospective study.
Abstract
Introduction: We developed and both internally and externally validated a simple scoring system called General Evaluation Score (GES) for HCC risk stratification.
Aim:To ascertain the validity of this score in a large prospective cohort of cured hepatitis C patients with compensated advanced chronic liver disease who achieved a sustained virological response following direct acting antivirals.
Patients and methods:This single-center prospective study included 463 consecutive patients, with advanced fibrosis (≥F3) who achieved SVR. The patients were recruited from the outpatient clinics at the Egyptian Liver Research Institute and Hospital between January 2018 and October 2019. All patients underwent abdominal ultrasound and multislice computed tomography for surveillance of HCC before starting antiviral therapy. Patients were followed up every 6 months after the end of treatment using ultrasonography and alpha-fetoprotein in addition to MSCT every 12 months.
Results:A total of 463 patients were included, of which 197 (42.5%), 114 (24.6%), and 152 (32.8%) had low, intermediate, and high-risk scores calculated before treatment, respectively. HCC incidence rate was 2.61/100 py (95% CI = 1.73–3.80); 25 cases developed HCC during follow-up. The incidence of HCC was 0.97% (95% CI: 0.31–2.34), 1.68% (95% CI: 0.53–4.05), and 5.57% (95% CI: 3.35–8.74) in the low, intermediate, and high-risk groups, respectively. The HCC incidence increased significantly with higher scores (p < 0.001). Harrell’s c-statistic for this model was 0.728.
Conclusion:This prospective study demonstrated the ability of GES to predict HCC occurrence and accurately stratify patients into low-, intermediate-, and high-risk groups.
Keywords
CHC, HCC risk, GES score, Prospective study.
Introduction
With an estimated 71 million people infected with the hepatitis C virus (HCV) worldwide and being the most common cause of hepatocellular carcinoma (HCC) in cirrhotic patients, HCV represents a major public health problem [1,2].
Various studies have provided unequivocal evidence that viral clearance after direct acting antivirals (DAAs) reduces, but does not eliminate, the risk of HCC occurrence in post sustained virological response (SVR) cirrhotic patients [3- 6]. In individuals with cirrhosis, current guidelines urge biannual HCC surveillance by ultrasound with or without alphafetoprotein (AFP) [7,8]. Data showing increased longevity, a higher rate of early tumor identification, and more successful curative therapies among individuals undergoing HCC screening back up these observations [9]. However, with the growing number of cured HCV individuals, more individualized approaches on HCC screening based on efficient predictive tools are urgently required.
A number of HCC risk prediction scores have recently been developed; however, none of them turned out to provide an ideal scoring method; therefore, the current study was conducted. Despite their flaws, the existing scores enable HCC risk stratification and aid focusing screening efforts on patients at high risk of HCC development, with the aim of reducing the burden on the already overwhelmed HCC screening resources [10-14].
Broadly, the ideal risk score should be a simple one comprising of routinely measured parameters and being developed using a large cohort of patients and both internally and externally validated, including with respect to prospective cohorts. To date, none of the proposed risk scores fulfill these criteria in a sufficient manner in order to be recommended for incorporation into routine clinical practice.
Recently, we developed and both internally and externally validated a simple scoring system called General Evaluation Score (GES) to accurately stratify the risk of HCC among chronic hepatitis C (CHC) patients with compensated advanced chronic liver disease (cACLD) (F3 and F4) who achieved SVR after DAA therapy [15]. GES was developed and validated using three large cohorts of 4400 Egyptian CHC patients and was further internationally validated in a large, independent cohort of multiethnic population of 12038 patients from more than 10 countries, with a robust performance across all these cohorts [16].
To ascertain the validity of this score in order to prime it for recommendation in clinical practice, in this study, we investigated the diagnostic performance of GES in a large prospective cohort of cured HCV patients with compensated advanced chronic liver disease (cACLD) who achieved SVR following DAAs with over 2 years of follow-up. Consistently, GES exhibits high ability in HCC risk stratification, which supports our claim that it is ready for recommendation of incorporation in clinical practice.
Patients and Methods
Cohort
This single center prospective study included 463 consecutive patients, with cACLD (F3 and F4) who achieved a SVR after DAA therapy. The patients were consecutively recruited from the outpatient clinics of the Egyptian Liver Research Institute and Hospital (ELRIAH) between January 2018 and October 2019.
Patients were included if they met the following inclusion criteria: they were 18 years or older with HCV/cACLD according to Baveno VI consensus and were willing to be treated with DAA. Patients with either hepatitis B virus (HBV) or human immunodeficiency virus (HIV) coinfection, or with history of previous IFN-treatment, liver transplantation, renal impairment, liver cell failure, and having no history of or current HCC as well as patients with history or existing other malignancies were excluded [17].
All participants received a 12 or 24-weeks course of one of several DAAs regimens in accordance with Egyptian national treatment protocol, American Association for the Study of Liver Diseases (AASLD) 2014 and World Health Organization (WHO) 2014 guidelines for treatment of genotype 4 chronic hepatitis C infection [18,19].
The study protocol was approved by the Research and Ethical Committee of ELRIAH. The protocol and conduct of the study complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects and its amendments [20]. Written informed consent was obtained from all patients.
Patients’ evaluation
Clinical and laboratory data were collected before the initiation of the antiviral treatment and on a regular basis at 6-month intervals of follow-up, according to a standardized protocol. All patients underwent abdominal ultrasounds and multislice computed tomography (MSCT) for HCC detection before the initiation of the antiviral therapy.
Patients’ follow-up
Patients were followed up on every 6 months after completion of treatment. The assessment included virological, hematological, and biochemical laboratory testing, abdominal ultrasound examination, FibroScan, and triphasic MSCT [21]. Collected biochemical parameters included Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), alkaline phosphastase, prothrombin time, international normalized ratio, total bilirubin, albumin, platelets, and alphafetoprotein (AFP). HCV‑RNA testing was performed by means of a real-time HCV-RNA PCR (Cobas Ampliprep, Cobas Taqman 48, Roche, Rotkreuz, Switzerland) according to manufacturer’s instructions. For the majority of patients, a yearly MSCT was scheduled in order to early detect HCC cases.
Diagnosis of fibrosis and HCC
The diagnosis of fibrosis and HCC was made according to the standard guidelines [17,21]. Patients were diagnosed as having advanced liver fibrosis (F3) or cirrhosis (F4) based on transient elastography (>9.6 and >14.6 kPa, respectively) [22].
Transient elastography was considered reliable when the following criteria had been met: (a) 10 successful measurements; (b) an interquartile range (IQR) lower than 30% of the median value; and (c) a success rate of more than 60%. Liver stiffness was considered as the median of all valid measurements [23]. For high BMI (≥30 kg/m2), an examination with the XL probe by two experienced operators was performed [24]. Transient elastography was done using FibroScan 502 (Echosens, Paris, France). Moreover, four hepatologists, who are experts in the FibroScan technique, performed the Transient elastography.
and FIB-6, which is a machine learning algorithm that can be determined by using the web site: http://fib6.elriah.info [27].
The diagnosis of HCC was made in accordance with The European Association for the Study of the Liver (EASL) and AASLD guidelines [18,21]. Multiphase CT (MSCT) or MRI was done to the patient if there were any focal hepatic lesions diagnosed by abdominal ultrasound and /or an AFP value >20 ng/ml. The MSCT diagnosis of HCC was based on the characteristic arterial enhancement and early washout in the delayed phase [28,29]. HCC was staged by means of the Barcelona Clinic Liver Cancer (BCLC) staging system, as recommended by AASLD 2018 [30]. BCLC staging uses a set of criteria to guide the management of patients with HCC. The classification takes the following variables into account:
performance status, Child–Pugh score, and radiologic tumor extent (tumor size, multiple tumors, vascular invasion, nodal spread and extrahepatic metastases) [31,32].
Calculation of GES
GES method was developed and validated by Shiha et al. in 2020 [15]. Point scores are assigned to each covariate (Table 1), and the total score is calculated. Patients are classified as having a low GES risk (≤6 points), an intermediate-risk (>6–7.5 points), or a high-risk score (>7.5 points).
Hypothesis to be tested
If this score is valid; the majority of HCC cases fall in the category of high-risk patients and the least number of HCC cases would occur among patients with low risk. Moreover, HCC cases developed during follow-up would be BCLC stages 0, A, and B. Accordingly, the null hypothesis is that there is no statistical difference between HC incidences in the three risk groups.
Sample size calculation
The sample size was calculated using Open Epi Software as described previously [33]. It was calculated based on power of 80% and an alpha-error of 5%. Incidence in different risk categories together with the distribution of patients into different risk groups were obtained from Shiha et al. with low-risk patients representing 57.7%, high-risk patients representing 17.5%, whereas the incidence in the low-risk group was 1.9/100 person year (py) and the one in the high-risk group 9.5/100 py [15].
Statistical calculations were performed following Kelsey et al. using the Fleiss correction [34,35]. A sample of 411 cases was found to be sufficient for a statistically powerful study. However, considering that an expected loss of cases is estimated during follow-up, a larger sample should have been recruited at baseline.
Statistical analysis
Statistical analyses were performed using version 26, SPSS (Statistical Package for Social Sciences) (IBM Corp., USA). Continuous variables were reported as median (IQR). Categorical variables were reported as frequency (%). Nonparametric tests, Mann–Whitney test for quantitative and Chi-square or Fisher’s exact test for qualitative comparisons were used. Times to events and cumulative incidences were calculated with the Kaplan–Meier method and compared using the log-rank (Mantel–Cox) test. The follow-up duration was calculated as the time between the end of treatment and last follow-up, or the date of event development (HCC occurrence), whichever occurred first.
The performance of GES was evaluated using directions as follows:
Overall performance evaluation by the Brier score. A Brier score can take on any value between 0 and 1, with 0 being the best score achievable and 1 being the worst score achievable. The lower the Brier score, the more accurate the prediction(s) [36].
Discrimination by Harrell’s C-statistic. A rough rule for interpretation is that values above 0.80 indicate very good models; between 0.70 and 0.80, good models; and between 0.50 and 0.70, fair models [37].
Calibration using the Hosmer–Lemeshow test. The output returns a chi-square value (a Hosmer–Lemeshow chi-squared) and a p-value. Small p-values (under 5%) mean that the model is a poor fit [38].
Evaluating the performance of the risk stratification as a screening procedure against HCC development as the gold standard. Using the risk stratification results, patients were classified into risky group (intermediate and highrisk score) and less-risky group (low-risk score) and then performance statistics (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy) were calculated.
Results
Patients’ characteristics
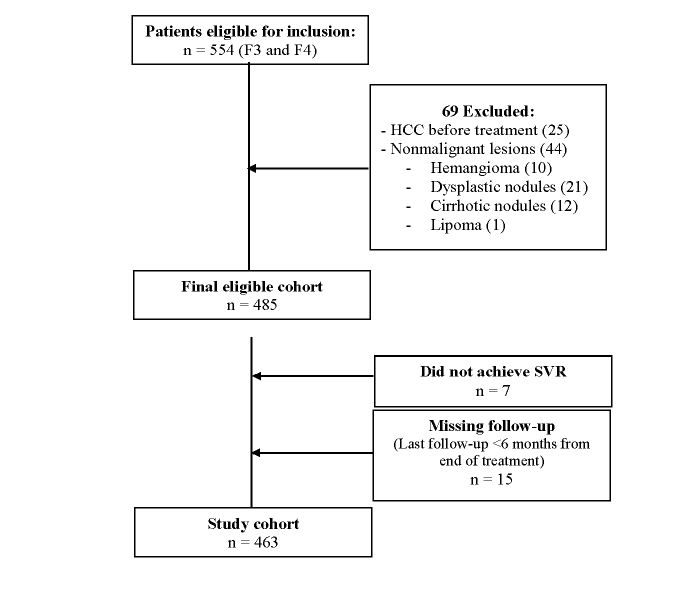
A total of 554 patients were enrolled in the study. Examination before initiation of DAA treatment indicated that 69 patients should be excluded (25 cases with lesions suspicious of early HCC and 44 cases with nonmalignant liver lesions: 10 liver hemangioma, 21 with liver dysplastic nodules, 12 with liver cirrhotic nodules, and 1 with liver lipoma). Accordingly, only 485 patients remained enrolled in this study. However, 15 patients did not attend the regular follow-up after the end of treatment or their last follow-up was less than 6 months from the end of treatment; and 7 patients did not achieve SVR after the first course of DAAs (Figure 1). Consequently, our current analysis included 463 patients. Clinical characteristics at baseline for these patients are presented in table 2. We observed that 78 of them were stage F3/LSM ranging from >9.6.1 to 14.6 kPa, and 385 were stage F4/LSM ranging from >14.6 to 75 kPa.
The main given DAA treatment included Sofosbuvir 400 mg /Daclatasvir 60 mg with weight-based ribavirin for 12 (316 patients) or 24 weeks (2 patients), followed by Sofosbuvir/Daklinza without Ribavirin for 12 (77 patients) or 24 weeks (50 patients) duration. Sofosbuvir/Ledipasivir was given to 10 patients for 12 weeks and 2 patients for 24 weeks while Sofosbuvir/Ledipasivir/Ribavirin for 12 weeks was given to 6 patients [18,19].
HCC occurrence
The mean follow-up duration was 24.79 ± 6.21 months after the end of DAA treatment (range 6–40 months). HCC developed in 25 cases during the study period, with HCC incidence rate estimated to be 2.61 / 100 py (95% CI = 1.73– 3.80).
The characteristics of the patients according to the development of HCC are depicted in table 2.
Patients who developed HCC were identified as being older (65.0 (57.0–67.5) vs. 57.0 (51.0–64.0), p < 0.001) males (72% vs. 49.8%, p = 0.031) compared to those who did not develop HCC during the duration of the follow-up. Analyzing the fibrosis scores (LSM, FIB‑4, APRI, and FIB‑6), we found that only FIB-6 showed a significant difference between patients that developed HCC and those who did not (Table 3).
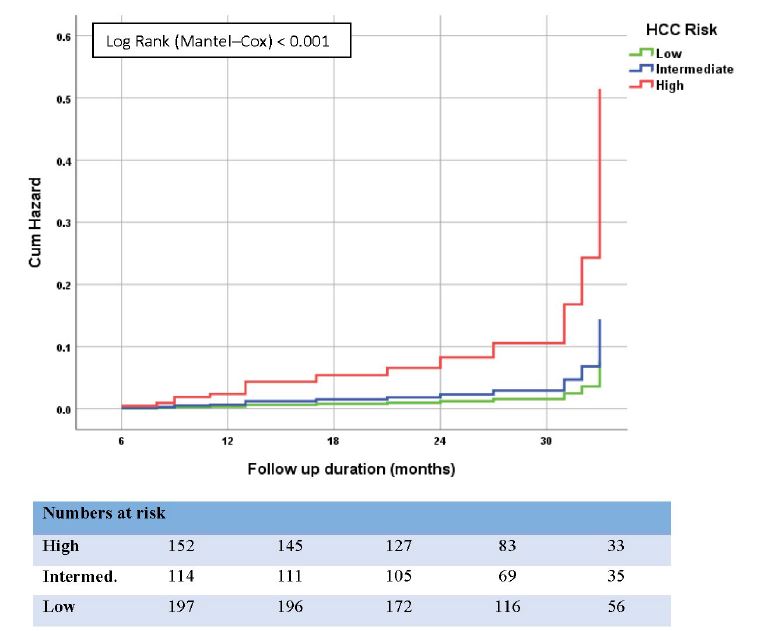
The cumulative incidence curve of HCC development according to GES is depicted in figure 2. Of the 463 study patients, 197 (42.5%), 114 (24.6%), and 152 (32.8%) had low, intermediate, and high-risk scores calculated before treatment, respectively.
Figure 1:Flow chart of patients in the study.
The incidences of HCC were 0.97% (95% CI: 0.31–2.34), 1.68% (95% CI: 0.53–4.05), and 5.57% (95% CI: 3.35–8.74) in the low-, medium-, and high-risk groups, respectively. The HCC incidence increased significantly with higher scores (p < 0.001, Figure 3).
Harrell’s c-statistic for this model was 0.728. Brier score was 0.309 and Hosmer–Lemeshow test p-value was 0.578. NPV to rule out the patients at low risk of HCC development was 97.4% (95% CI: 95.0-98.7) (Table 4).
GES performance in cirrhotic patients
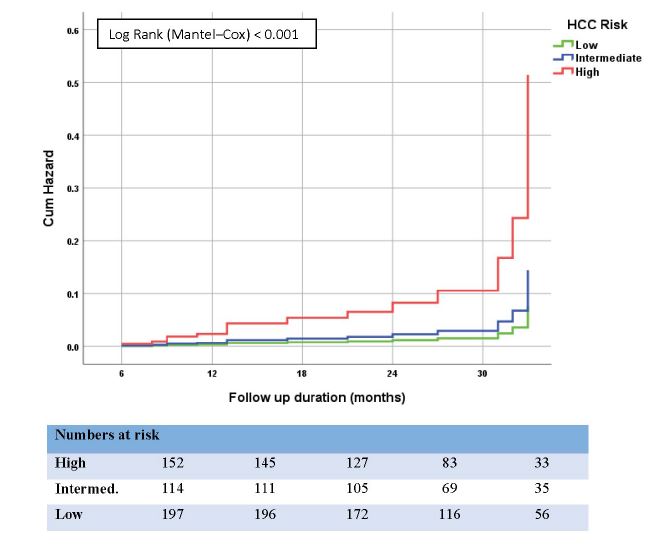
To ascertain the GES performance in patients with cirrhosis, a similar analysis was performed for the 395 cirrhotic patients separately. We found that 142 (36.9%), 105 (27.3%), and 138 (35.8%) of them had low, intermediate, and high-risk scores calculated before treatment, respectively. Figure 3 shows the cumulative incidence curve of HCC development for each group. The incidences of HCC were 1.37% (95% CI: 0.43–3.30), 1.35% (95% CI: 0.28–3.69) and 3.95% (95% CI: 2.07–6.85) in the low-, medium-, and high-risk groups, respectively. The HCC incidence increased significantly with higher scores (p = 0.044). Harrell’s c-statistic for this model was 0.692. Brier score was 0.348 and Hosmer–Lemeshow test p-value was 0.412. NPV to rule out the patients at low risk of HCC development was 97.1% (95% CI: 94.3–98.6) (Table 4).
Discussion
In this prospective study, we proved that GES accurately
Figure 2:Cumulative Hazard (%) of HCC in all patients (cACLD) with HCV after end of DAA therapy, shown by Kaplan–Meier curves comparing different risk groups.
Figure 3:Cumulative Hazard (%) of HCC in cirrhotic patients with HCV after end of DAA therapy, shown by Kaplan–Meier curves comparing different risk groups.
| Variable | Score | |
|---|---|---|
| Sex | Female | 0 |
| Male | 3.5 | |
| Age | ≤54 years | 0 |
| >54 years | 1 | |
| Fibrosis stage | F0-2 | 0 |
| F3 | 1.5 | |
| F4 | 3 | |
| Albumin | ≥ 3.8g/dL | 0 |
| <3.8 g/dL | 2 | |
| Alphafetoprotein | ≤20 ng/ml | 0 |
| >20 ng/ml | 3 | |
| Total | 0–12.5 | |
Table 1:CSomponents of the General Evaluation Score (GES).
| Variable | All patients | Non-HCC Patients | HCC patients | p-value |
|---|---|---|---|---|
| Patient number | 463 | 438 | 25 | |
| Age (years) | 57.0 (51.0–64.0) | 57.0 (51.0–64.0) | 65.0 (57.0-67.5) | <0.001 |
| Sex | ||||
| Males | 236 (51.0) | 218 (49.8) | 18 (72.0) | 0.031 |
| Females | 227 (49.0) | 220 (50.2) | 7 (28.0) | |
| ALT (U/L) | 58.0 (40.0–85.0) | 58.0 (39.8–86.0) | 48.0 (37.5–84.0) | 0.272 |
| AST (U/L) | 63.0 (43.0–93.0) | 62.5 (44.0–91.0) | 72.0 (40.5–102.0) | 0.593 |
| Total Bilirubin (mg/dL) | 0.90 (0.70–1.20) | 0.90 (0.70–1.20) | 0.90 (0.70–1.45) | 0.159 |
| Albumin (g/dL) | 3.8 (3.4–4.2) | 3.8 (3.4–4.2) | 3.7 (3.0–4.0) | 0.123 |
| Platelets count (/cmm3) | 115.0 (84.0–156.0) | 114.5 (83.8–151.3) | 138.0 (87.5–181.5) | 0.577 |
| AFP (ng/ml) | 8.9 (4.8–21.7) | 8.8 (4.8–21.6) | 16.3 (4.5–30.6) | 0.544 |
| Fibrosis stage | ||||
| F3 | 78 (16.8) | 71 (16.2) | 7 (28.0) | 0.126 |
| F4 | 385 (83.2) | 367 (83.8) | 18 (72.0) | |
| LSM (kPa) | 21.8 (17.0–31.2) | 21.8 (17.1–29.9) | 27.0 (14.5–35.3) | 0.654 |
| FIB4 | 4.30 (2.74–6.95) | 4.30 (2.76–6.89) | 4.41 (2.62–7.78) | 0.352 |
| APRI | 1.44 (0.91–2.44) | 1.43 (0.91–2.47) | 1.69 (0.71–2.38) | 0.831 |
| FIB6 | 3.50 (2.89–4.25) | 3.46 (2.84–4.25) | 4.00 (3.82–4.89) | 0.009 |
| Comorbidities | ||||
| DM | 117 (25.3) | 111 (25.3) | 6 (24.0) | 0.881 |
| HTN | 92 (19.9) | 87 (19.9) | 5 (20.0) | 0.987 |
| Obesity # | 274 (59.2) | 264 (60.3) | 10 (40.0) | 0.045 |
| Data are presented as frequency (%) or median (IQR). #Obesity (BMI ≥ 30 kg/m²) DM = Diabetes mellitus, HTN = Hypertension, AST = Aspartate aminotransferase, ALT = Alanine aminotransferase, AFP = Alphafetoprotein, LSM = Liver Stiffness Measurement by FibroScan | ||||
Table 2:Baseline characteristics according to HCC development.
| Variable | All patients | Low risk | Intermediate risk | High risk |
|---|---|---|---|---|
| Patient number | 25 | 4 | 4 | 17 |
| BCLC score | ||||
| O | 4 (16.0) | 2 (50.0) | 0 (0.0) | 2 (11.8) |
| A | 9 (36.0) | 0 (0.0) | 1 (25.0) | 8 (47.1) |
| B | 4 (16.0) | 0 (0.0) | 0 (0.0) | 4 (23.5) |
| C | 4 (16.0) | 2 (50.0) | 0 (0.0) | 2 (11.8) |
| D | 4 (16.0) | 0 (0.0) | 3 (45.0) | 1 (5.9) |
| Duration after EOT | ||||
| 1st year | 5 (20.0) | 0 (0.0) | 2 (50.0) | 3 (17.6) |
| 2nd year | 8 (32.0) | 0 (0.0) | 2 (50.0) | 6 (35.3) |
| 3rd year | 12 (48.0) | 4 (100.0) | 0 (0.0) | 8 (47.1) |
| Data are presented as frequency (%) EOT = End of treatment, BCLC = Barcelona Clinic Liver Cancer staging system | ||||
Table 3:Characteristics of HCC tumors according to GES risk categorization.
| All patients | Cirrhotic patients | |
|---|---|---|
| All patients | 463 | 385 |
| Follow-up period, month (range) | 24.79 ± 6.21 (6–40) | 24.72 ± 6.11 (6–40) |
| HCC cases | 25 | 18 |
| GES risk groups | ||
| Low | 197 (42.5%) | 142 (36.9%) |
| Intermediate | 114 (24.6%) | 105 (27.3%) |
| High | 152 (32.8%) | 138 (35.8%) |
| HCC in the 3 risk groups | ||
| Low | 4/197 (2.0%) | 4/142 (2.8%) |
| Intermediate | 4/114 (3.5%) | 3/105 (2.9%) |
| High | 17/152 (11.2%) | 11/138 (8.0%) |
| Incidence, % | ||
| Low | 0.97 (0.31-2.34) | 1.37 (0.43-3.30) |
| Intermediate | 1.68 (0.53-4.05) | 1.35 (0.28-3.69) |
| High | 5.57 (3.35-8.74) | 3.95 (2.07-6.85) |
| Log-rank test | <0.001 | 0.044 |
| Harrell’s C-statistic | 0.728 | 0.692 |
| Brier score | 0.309# | 0.348# |
| Hosmer–Lemeshow test sig. | 0.578 | 0.412 |
| Performance statistics# | ||
| Sensitivity | 68.0 (48.4–82.8) | 61.1 (38.6–79.7) |
| Specificity | 69.2 (64.7–73.3) | 65.4 (60.4–70.1) |
| PPV | 11.2 (7.1–17.2) | 8.0 (4.5–13.7) |
| NPV | 97.4 (95.0–98.7) | 97.1 (94.3–98.6) |
| Accuracy | 69.1 (64.8–73.2) | 65.2 (60.3–69.8) |
| #Comparing risky patients (high-risk groups) with less-risky patients (intermediate- + low-risk group) | ||
Table 4:Evaluation of GES in all patients.
stratifies the risk of HCC occurrence after DAA therapy in a cohort of CHC patients with advanced fibrosis and liver cirrhosis. The HCC incidence increased significantly with higher scores (p < 0.001). Harrell’s c-statistic for this model was 0.728. During follow-up, 25 patients developed HCC; 17 (68%) of them were stratified as high risk in addition to 4 patients (16 %) as intermediate risk at the baseline. Thus, GES was able to predict about 84% of the observed patients with HCC. Notably, we found that up to 70% of the detected HCC were diagnosed in an early stage (BCLC 0-B), which is more amenable to curative therapies and improved overall survival [39].
Moreover, this study is the first prospective confirmation of our previous reports about the accuracy of GES that has been validated among various geographically and ethnically distinct cohorts in health care settings [15, 40-42]. A prospective design to evaluate the diagnostic accuracy of risk scores has vital advantages over a retrospective one and is highly recommended in the literature [43,44]. These include a patient sample that is better defined in terms of the patients’ clinical and laboratory characteristics, standardized methods for performing and interpreting the test(s) and a gold standard procedure, in addition to minimizing the risk of influencing the results by selection bias [44,45]. Therefore, a validation of the GES in the prospective prediction of HCC occurrence in advanced fibrosis and liver cirrhosis patients (independent cohort) would provide the strongest assessment of the utility of the score. Before putting this novel score to clinical use, studies are necessary to evaluate its cost-effectiveness.
These results are in agreement with the conclusion of a recent meta-analysis by Lockart et al, [46] about HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis where they concluded that a more precise identification of patients at risk of HCC after HCV cure would clearly have significant cost-effectiveness and resource use implications. Interestingly, the results of this meta-analysis suggested that surveillance should not be offered to all patients with fibrosis F3, although some patients with fibrosis F3 would benefit from surveillance. Hence, they encouraged the development of validated predictive models to better identify individuals with F3 fibrosis who should be offered surveillance. Considering results of our study and the conclusion of this meta-analysis; patients who are identified as high-risk using GES should be offered surveillance
Moreover, with the incidence of HCC being 0.97% in the patients assigned to the low-risk group (42.5%) using GES, GES indicates that these patients can be screened at longer intervals, e.g., 1–2 yrs, or they could even be safely excluded from the HCC screening program. On the other hand, the high-risk group includes about 32.8% of the patients (HCC incidence 5.57%) for whom more intense screening may be required. Accordingly, this contributes to the potential cost effectiveness of the score.
Our study demonstrates several points of clinical design strength. This is the first study to our knowledge that incorporates a prospective validation of a risk score in a precalculated sample size cohort. The study duration is more than two years of follow-up after applying strict criteria to verify the absence of any HCC cases before inclusion in the study.
The study had a few limitations as well. All patients were assigned to a single center and had previous infection with the HCV-4 genotype. However, retrospective studies have demonstrated that GES has equally high diagnostic accuracy across other HCV genotypes, ethnicities and centres [16,40]. In the current cohort, cACLD was characterized and classified into F3 and F4 stages using FibroScan. There is still a possibility of misclassification, as some F3 individuals are genuinely cirrhotic. Though, the diagnostic accuracy of GES was virtually the same in the overall cohort and in those with cirrhosis, ruling out the possibility of any impact of potential misclassification on the assessment of diagnostic accuracy of the score.
Conclusion
This current prospective study demonstrated the ability of GES to predict HCC occurrence and accurately stratify patients into low-, intermediate-, and high-risk groups.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. It was performed as part of the employment of the authors in the Egyptian Liver Research Institute and Hospital (ELRIAH).
Conflict of Interest
The authors declare no conflicts of interest
Authors’ Contribution
GS designed the study. GS & RS supervised clinical work. NM performed the statistical analyses. GS, NM, RS, AH, ME and IW interpreted the data. AH supervised the laboratory work. All authors drafted the paper provided input into the manuscript and approved the final version.
WHO (2017) Global hepatitis report. World Health Organization, Geneva.[ Ref ]
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69: 182-236.[ Ref ]
Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, et al. (2018) The shortterm incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 67: 2244-2253.[ Ref ]
Carrat F, Fontaine H, Dorival C, Mélanie S, Alpha D, et al. (2019) Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 393: 1453-1464.[ Ref ]
Ide T, Koga H, Nakano M, Hashimoto S, Yatsuhashi H, et al. (2019) Directacting antiviral agents do not increase the incidence of hepatocellular carcinoma development: A prospective, multicenter study. Hepatol Int 13: 293-301.[ Ref ]
Shiha G, Mousa N, Soliman R, Nnh Mikhail N, Adel Elbasiony M, et al. (2020) Incidence of HCC in chronic hepatitis C patients with advanced hepatic fibrosis who achieved SVR following DAAs: A prospective study. J Viral Hepat 27: 671-679.[ Ref ]
European association for the study of the liver (2018) EASL recommendations on treatment of hepatitis C 2018. J Hepatol 69: 461- 511.[ Ref ]
Ghany MG, Morgan TR, AASLD-IDSA Hepatitis C Guidance Panel (2020) Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 71: 686-721.[ Ref ]
Mittal S, Kanwal F, Ying J, Chung R, Sada YH, et al. (2016) Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol 65: 1148-1154.[ Ref ]
Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, et al. (2017) Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol 2017: 32248-32251.[ Ref ]
Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, et al. (2019) Predictors of hepatocellular carcinoma occurrence after direct-acting antiviral therapy in patients with hepatitis C virus infection. Hepatol Res 49: 136-146.[ Ref ]
Hu CC, Weng CH, Hua MC, Chang PH, Lin CL, et al. (2020) New scoring method to predict risk of hepatocellular carcinoma in patients with chronic hepatitis C after pegylated interferon and ribavirin therapy. J Interferon Cytokine Res 40: 82-91.[ Ref ]
Tani J, Morishita A, Sakamoto T, Takuma K, Nakahara M, et al. (2020) Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol Lett 19: 2205- 2212.[ Ref ]
Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, et al. (2020) aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020: 30478-30485.[ Ref ]
Shiha G, Waked I, Soliman R, Elbasiony M, Gomaa A, et al. (2020) GES: A validated simple score to predict the risk of HCC in patients with HCVGT4- associated advanced liver fibrosis after oral antivirals. Liver Int 40: 2828-2833.[ Ref ]
Shiha G, Soliman R, Mikhail NNH, Carrat F, Azzi J, et al. (2022) International multicenter validation of GES score for HCC risk stratification in CHC patients. EASL: The international liver congress.[ Ref ]
de Franchis R, Baveno VI Faculty (2015) Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 63: 743- 752.[ Ref ]
AASLD/IDSA HCV Guidance Panel (2015) Hepatitis C guidance: AASLDIDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62: 932-954.[ Ref ]
World Health Organization (2014) Guidelines for the screening, care and treatment of persons with hepatitis C infection.[ Ref ]
CIOMS/WHO (1993) International Ethical Guidelines for Biomedical Research Involving Human Subjects. CIOMS, Geneva.[ Ref ]
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 69: 182-236.[ Ref ]
Bonder A, Afdhal N (2014) Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep 16: 372.[ Ref ]
Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, et al. (2007) Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 56: 968-973.[ Ref ]
Shiha GE, El-Etreby S, Bahgat M, Hamed M, El Sherbini M, et al. (2018) Chronic Hepatitis C Patients with Obesity: Do we Need two Operators for Accurate Evaluation of Liver Stiffness? Ann Hepatol 17: 795-801.[ Ref ]
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, et al. (2011) Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 53: 726-736.[ Ref ]
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, et al. (2006) APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43: 1317-1325.[ Ref ]
Shiha G, Soliman R, Mikhail NNH, Alswat K, Abdo A, et al. (2022) Development and multicenter validation of FIB-6; a novel, machinelearning, simple bedside score to rule out liver cirrhosis and compensated advanced chronic liver disease in CHC patients. Hepatol Res 52: 165-175.[ Ref ]
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, et al. (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67: 358-380.[ Ref ]
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, et al. (2017) Asia- Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int 11: 317-370.[ Ref ]
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, et al. (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68: 723-750.[ Ref ]
Llovet JM, Brú C, Bruix J (1999) Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis 19: 329-338.[ Ref ]
Forner A, Reig ME, de Lope CR, Bruix J (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis 30: 61-74.[ Ref ]
Dean AG, Sullivan KM, Soe MM (2013) OpenEpi: Open source epidemiologic statistics for public health.[ Ref ]
Kelsey JL, Whittemore AS, Evans AS (1996) Methods in Observational Epidemiology. Oxford Univ Press, Oxford, New York.[ Ref ]
Fleiss JL, Levin B, Paik MC (2003) Statistical Methods for Rates and Proportions. John Wiley & Sons, Inc.[ Ref ]
Brier GW (1950) Verification of Forecasts Expressed in Terms of Probability. Monthly Weather Review 78: 1-3.[ Ref ]
Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. (1982) Evaluating the yield of medical tests. JAMA 247: 2543-2546.[ Ref ]
Hosmer Jr DW, Lemeshow S, Sturdivant RX (2013) Applied logistic regression: John Wiley & Sons.[ Ref ]
Singal AG, Pillai A, Tiro J (2014) Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med 11: e1001624.[ Ref ]
Abe K, Fujita M, Hayashi M, Takahashi A, Ohira H, et al. (2021) Muticentre external validation of the GES score for predicting HCC risk in Japanese HCV patients who achieved SVR following DAAs. Liver Cancer Int 2: 102- 109.[ Ref ]
Shiha G, Soliman R, Mikhail N, Hassan A, Eslam M (2021) Development of a simple dynamic algorithm for individualized hepatocellular carcinoma risk-based surveillance using pre- and post-treatment general evaluation score. Liver Int 41: 2768-2776.[ Ref ]
Shiha G, Mikhail NNH, Soliman R, Hassan A, Eslam M, et al. (2022) Predictive performance and clinical utility of HCC risk scores in chronic hepatitis C: a comparative study. Hepatol Int 16: 159-170.[ Ref ]
Obuchowski NA, Zhou XH (2002) Prospective studies of diagnostic test accuracy when disease prevalence is low. Biostatistics 3: 477-492.[ Ref ]
Semmler G, Meyer EL, Kozbial K, Schwabl P, Hametner-Schreil S, et al. (2022) HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol 76: 812-821.[ Ref ]
Yip TC, Hui VW, Tse Y, Wong GH (2021) Statistical strategies for HCC risk prediction models in patients with chronic hepatitis B. Hepatoma Res 7: 7.[ Ref ]
Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M (2022) HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology 76: 139-154.[ Ref ]