Journal Name: International Journal of Cancer and Treatment
Article Type: Research
Received date: 20 August, 2021
Accepted date: 06 September, 2021
Published date: 13 September, 2021
Citation: Murguia-Perez M, Enríquez-Brena SZ, Ramírez- Balderrama L, García-Mendoza YI, Mendoza-Ramírez S, E et al (2021) Androgen Receptors Expression in Patients with Triple-Negative Breast Cancer in Latino Population. Int J Cancer Treat Vol: 4, Issu: 2 (11-18).
Copyright: © 2021 Murguia-Perez M et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Breast cancer is a pathology with repercussions on public health, which is histologically classified into 2 large groups, ductal and lobular; Although there is no variability in the treatment therapy based on these characteristics, with the help of immunohistochemical techniques it is sub classified according to the molecular classification recognized by the WHO into 5 categories, including triple-negative breast cancer, which presents a worse prognosis than that of the rest of the groups. Some of these groups are those with low expression of claudins, and others with apocrine differentiation that may have reactivity to Her2/neu or be properly triple negative. In these, adjuvant therapy is only with chemotherapy. In the subgroup of apocrine type tumors, when studying androgen receptors it was noted that they have an important role in tumorigenesis and that there may be an expression in part of triple-negative tumors, creating a therapeutic target that can benefit these patients, with benefit in clinical trials that have been conducted.
Methods: This study is an ambilective, descriptive, cross-sectional study that includes paraffin embedded tissue samples in triple-negative breast malignant tumors of patients detected in in the database of the Department of Surgical Pathology of High Specialty Medical Unit No. 1 Bajío who meet inclusion criteria. Immunohistochemistry technique was performed by manual method in three steps, using monoclonal antibody against androgen receptors (androgen receptor, Zeta Diagnostic brand, EP120 clone), and evaluated by peer review made on a Leica DM1000 light microscope. The histological variables were reviewed and the positivity or negativity of androgen receptors and their percentage of expression were assessed, replicating the H-Score system. Also, the correlation of androgen receptor expression with histological variables was performed.
Results: Of 76 cases that met the inclusion criteria, 18.42% had androgen receptors by immunohistochemistry. This means that about 2 out of every 10 patients with triple-negative breast cancer have tumors with presence of androgen receptors; In the cases that presented them, a search was carried out for correlations between the morphological histological variables and concerning the presence of these receptors,without finding significant associations and we corroborated that their presence is independent of the morphology, as previously described in the literature.
Conclusions: In triple-negative cases, the existence or not of androgen receptors can be added to suggest offering target treatment, blocking them. Our results propose that the systematization in the search for androgen receptors in triple-negative breast cancer offers the possibility of targeted therapy. Equally important is having been able to corroborate that the presence of androgen receptors is an independent parameter to tumor histological types.
Keywords:Triple-negative breast cancer; Androgen receptors, Immunohistochemistry
Abbreviations:NOS-IDC: Non-special type invasive ductal carcinoma; AR: androgen receptors; ER: estrogen receptors; PR: progesterone receptors; TNBC: triple-negative breast carcinoma; MBC: metaplastic breast carcinoma; LA: luminal A; LB: luminal B; UMAE N° 1: High Specialty Medical Unit No. 1 Bajio; WHO: World Health Organization; BL: basallike; IM: immunomodulatory; M: mesenchymal; LAR: luminal androgen receptor.
Abstract
Background: Breast cancer is a pathology with repercussions on public health, which is histologically classified into 2 large groups, ductal and lobular; Although there is no variability in the treatment therapy based on these characteristics, with the help of immunohistochemical techniques it is sub classified according to the molecular classification recognized by the WHO into 5 categories, including triple-negative breast cancer, which presents a worse prognosis than that of the rest of the groups. Some of these groups are those with low expression of claudins, and others with apocrine differentiation that may have reactivity to Her2/neu or be properly triple negative. In these, adjuvant therapy is only with chemotherapy. In the subgroup of apocrine type tumors, when studying androgen receptors it was noted that they have an important role in tumorigenesis and that there may be an expression in part of triple-negative tumors, creating a therapeutic target that can benefit these patients, with benefit in clinical trials that have been conducted.
Methods: This study is an ambilective, descriptive, cross-sectional study that includes paraffin embedded tissue samples in triple-negative breast malignant tumors of patients detected in in the database of the Department of Surgical Pathology of High Specialty Medical Unit No. 1 Bajío who meet inclusion criteria. Immunohistochemistry technique was performed by manual method in three steps, using monoclonal antibody against androgen receptors (androgen receptor, Zeta Diagnostic brand, EP120 clone), and evaluated by peer review made on a Leica DM1000 light microscope. The histological variables were reviewed and the positivity or negativity of androgen receptors and their percentage of expression were assessed, replicating the H-Score system. Also, the correlation of androgen receptor expression with histological variables was performed.
Results: Of 76 cases that met the inclusion criteria, 18.42% had androgen receptors by immunohistochemistry. This means that about 2 out of every 10 patients with triple-negative breast cancer have tumors with presence of androgen receptors; In the cases that presented them, a search was carried out for correlations between the morphological histological variables and concerning the presence of these receptors,without finding significant associations and we corroborated that their presence is independent of the morphology, as previously described in the literature.
Conclusions: In triple-negative cases, the existence or not of androgen receptors can be added to suggest offering target treatment, blocking them. Our results propose that the systematization in the search for androgen receptors in triple-negative breast cancer offers the possibility of targeted therapy. Equally important is having been able to corroborate that the presence of androgen receptors is an independent parameter to tumor histological types.
Keywords:Triple-negative breast cancer; Androgen receptors, Immunohistochemistry
Abbreviations:NOS-IDC: Non-special type invasive ductal carcinoma; AR: androgen receptors; ER: estrogen receptors; PR: progesterone receptors; TNBC: triple-negative breast carcinoma; MBC: metaplastic breast carcinoma; LA: luminal A; LB: luminal B; UMAE N° 1: High Specialty Medical Unit No. 1 Bajio; WHO: World Health Organization; BL: basallike; IM: immunomodulatory; M: mesenchymal; LAR: luminal androgen receptor.
Background
Breast cancer has an impact on public health, worldwide in 2018, there were 2,093,876 new cases, 11.6% of the total malignancies detected [1]. In Mexico, 27,283 new cases were detected, 14.3% of the total malignancies detected in 2018, causing 6,884 deaths [2]. In the Latino population, a high incidence of this type of neoplasm has been described, with a frequency of 21.3% compared to all malignant breast neoplasms [3]. They can have different cellular origins (OMM): epithelial, myoepithelial, mesenchymal, fibroepithelial, nipple, hematolymphoid, and metastatic4.The main histological types are ductal and lobular carcinoma [4]. Non-special type invasive ductal carcinoma (NOS-IDC) is the prevalent morphology in Mexico and globally [5].
According to the expression of the immunohistochemical markers that are performed to determine prognostic factor and treatment, they are separated into 5 molecular types: luminal A (LA), luminal B (LB), HER2/neu, basallike, normal breast type. LA has the highest expression for estrogen receptors; LB expresses estrogen receptors to a lesser extent, and in some references, it depends on the cell proliferation index (Ki67 with a cut-off point of 20%); the HER2/neu group that depends on the overexpression or amplification of this membrane receptor; basal-like expresses genes from myoepithelial cells of the breast. The normal breast type expresses genes from adipocytes and epithelial cells. The basal-like molecular type and HER2/neu have the worst prognosis, while LA has the best prognosis and LB has an intermediate prognosis [6]. Molecular characterization allows opting for therapies directed against a specific molecular type and predicts response to treatment and prognosis. There is a “triple-negative” tumor group, which constitutes 10 to 20% of all cases of breast cancer [7]; prevails in African-American pre-menopausal women, although more than 40% of affected patients are older than 65 years of age, generally present in younger women than other immunophenotypes, it does not express estrogen, progesterone or HER2/neu u receptors and represents 15% of all malignant neoplasms in the breast [8-10]. Clinically, they have a poor long-term prognosis, with a specific pattern of relapse; it has higher mortality and risk of liver, lung, and bone metastases; it is highly invasive, associated with a high risk of local recurrence and low disease-free survival [11,12]. Histologically they are well defined, large, with a high mitotic index, tumor necrosis, vigorous margins, abundant intratumoral lymphocytes, syncytia organization, high nucleus-cytoplasm index; Because of this, they are classified as high-grade tumors. They present the appearance of mostly infiltrating ductal carcinoma, although it may vary in morphology [13]. The standardized treatment is neoadjuvant with chemotherapy at the beginning of treatment, it presents high response rates, but with short-lasting results, due to resistance mechanisms that develop [7,13].
Anti-androgen receptor therapy has been evaluated with an overall beneficial response of 19 to 29% in monotherapy [3]. They represent a therapeutic target as they are present in triple-negative tumors. Currently we detect and quantify the expression of androgen receptors (AR) in patients with triple-negative breast cancer (TNBC) in our unit, in the available collection. This is the first study in the Latino population conducted to determine the expression of androgen receptors in TNBC samples.
Methods
An ambilective, descriptive, cross-sectional study was carried out, in search of cases with a histopathological report of invasive ductal or lobular carcinoma of the breast in the database of the Department of Surgical Pathology of High Specialty Medical Unit No. 1 Bajío (UMAE N° 1), which ran from January 1, 2015, to December 31, 2019. All cases that presented the following criteria were included: Cases of breast tumors with a histological report of ductal and/or lobular carcinoma of the breast, with negative immunohistochemistry for hormonal receptors (estrogens and progesterone), and HER2/neu, and that the corresponding paraffin blocks and lamellae existed for their evaluation. All cases that did not meet these criteria were discarded from the study. It was verified that there were no duplicate samples. Slides stained with hematoxylin and eosin, immunohistochemical reactions (ER, PR, HER2/neu), and paraffin blocks corresponding to each case were collected. They were reviewed in pairs to confirm the diagnosis, the diagnosis was corroborated according to the WHO 2019 Classification of Breast Tumours, and the histological parameters issued in the report were corroborated, which include morphological variables considered in this study. (age of the patient, tumor size, histological type (previously mentioned), histological grade according to the Nottingham scale, lymphatic and vascular invasion). Also, an intentional search was made for posterior surgical specimens in the case of the cutting needle samples, considering these as material for this study only as there was no material from the same tumor from subsequent interventions (quadrantectomies, mastectomies, among others). Subsequently, the cases with triple-negative results were selected, and from them, a paraffin block was selected under appropriate conditions per case to search for androgen receptors utilizing immunohistochemical reactions.
Histological sections were made at 3 micrometters of each selected paraffin block on positively charged slides; they were deparaffinized and hydrated, and tissue antigenic recovery was carried out using a pressure cooker, Diagnostic Biosystem brand, with EDTA buffer for heat induced epitope recovery, pH 9.0. Subsequently, it was washed with hydrogen peroxide for 5 minutes, rinsed with PBS, and the immunoperoxidase technique was carried out by capillary action, with the mounting of all the lamellae on cover plates. Primary antibody (androgen receptor, Zeta Diagnostic brand, EP120 clone) was applied and left to incubate for 1 hour. Subsequently, it was rinsed with PBS and a second biotinylated antibody was added for 10 minutes, rinsing was performed, diaminobenzidine (DAB) was applied and developed for 1 minute, rinsed with distilled water, and contrasted with hematoxylin for 5 to 10 seconds. Each slide was passed through carbonated or ammonia water, dehydrating with 96 proof alcohol, clearing with xylol, and mounting with coverslips. The observation was made on a Leica DM1000 light microscope. The histological variables were reviewed and the positivity or negativity of androgen receptors and their percentage of expression were assessed, replicating the H-Score system. Also, the correlation of androgen receptor expression with histological variables was performed. The statistical analysis was carried out in statistical software IBM SPSS Statics v23.0 and Microsoft Excel 2016, to determine descriptive statistics, measures of central tendency will be included, such as: mean, median and mode, as well as dispersion measures: range, mean deviation, variance and standard deviation. Subsequently, the results were analyzed for associations of the variables with the expression of androgens and correlations in the same way, using the Pearson test, with alpha < 0.05 and beta of 80%.
Results
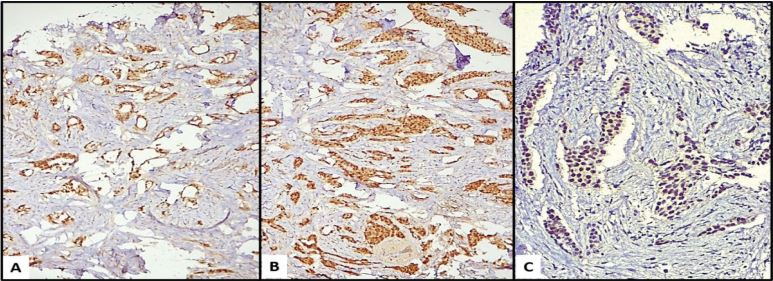
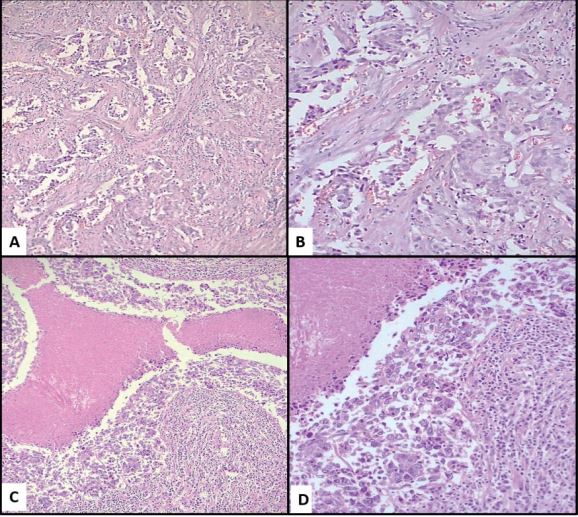
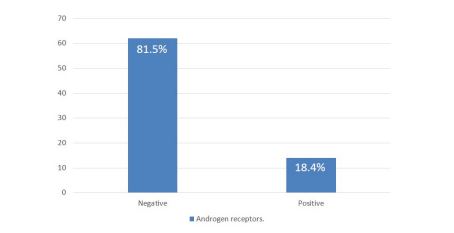
In the period studied, 67,522 cases of surgical pathology were identified, of which 1,612 correspond to histological reports of breast carcinoma, corresponding to 2.36% of all cases; of these, 197 were TNBC (12.22% of breast cancer cases); applying the inclusion and exclusion criteria, 76 cases were finally examined, and an intentional search for AR was carried out by immunohistochemistry; 14 of these cases were positive for AR (Figure 1), which represents 18.42% of the cases of TNBC considered; 62 cases were negative, corresponding to 81.58% of the total cases (Table 1 and graphic 1). Regarding the clinical variables considered, it was observed that the positive cases for AR, 9 of them (64.29%) were found in the group over 50 years of age, while 5 of them (35.71%) were under 50 years of age and the average age of these patients was 54 years. In the ARnegative group, 37 patients (59.68%) were found in the group over 50 years old and 24 of them (38.71%) in those under 50 years old, with an average age of 52 years. One of the patients could not be categorized because her age was not included in the histopathological report. Older age was observed in the AR-positive group, although only by a 2-year difference. Regarding tumor size, we only found 2 ARpositive cases (14.29%) and they were smaller than 5cm, the rest of the AR-positive cases (12 cases, 85.71%) could not be assessed due to the type of material (cases of revision, incisional biopsies). In the group of tumors negative to AR, 1 case was greater than 5 cm (1.61%), 14 cases were smaller than 5 cm (22.58%) and the remaining 47 cases (75.81%) were not able to be evaluated. The predominant histological type in the group of AR-positive tumors was NOS-IDC with 13 cases (92.86%, Figure 2A,2B), one case corresponded to metaplastic breast carcinoma (MBC) (7.14%, Figure 2C,2D). Regarding the group of negative AR tumors, the prevalence of histological type was the same (NOS-IDC) with 52 cases (83.87%), followed by MBC of which there were 6 cases (9.68%); the remaining 4 cases (6.45%) corresponded to invasive or mixed lobular carcinomas. According to the histological grade assessed by the Nottingham scale, in the AR-positive group, they mostly corresponded to grade 2 (7 cases, 50%) and grade 3 (6 cases, 42.86%); in one of the cases the scale was not applicable (7.14%); In the AR-negative group, we found that the majority were grade 1 tumors (5 cases, 8.06%), followed by grade 2 (30 cases, 48.39%) and grade 3 (23 cases, 37.10%); In 4 cases (6.45%) it was not possible to perform the Nottingham scale due to histological type, cutting needle biopsies, and scarce neoplastic tissue. Surgical margins in the AR-positive group were positive in 1 case (7.14%); negative in 2 cases (14.29%) and due to the type of biopsy (incisional) or revision cases in 11 cases (78.57%) it was not possible to assess them. In the ARnegative group, there were 5 cases (8.06%) with a positive margin, 10 cases (16.13%) with a negative margin, and 47 cases (75.81%) that could not be classified for the reasons previously stated. Lymphatic invasion in the AR positive group was documented in 7 cases (50%), absent in 2 cases (14.29%) and due to the set of factors previously listed in 5 cases (35.71%), this parameter was not evaluated. In the AR-negative group, 31 tumors (50%) presented lymphatic invasion, 17 cases (27.42%) lacked it, and the remaining 14 (22.58%) were not assessable in this parameter. Angioinvasion was observed in the AR-positive group in 3 cases (21.43%), 4 of these (28.57%) were negative and 7 cases (50%) were not assessable. At the same time, in the AR-negative group, 22 cases (35.48%) had angioinvasion, 17 tumors (27.42%) did not present it, and it was not evaluated in 23 (37.10%) (Table 2).
An intentional search was made for associations between the variables presented, associating them against the rest of the variables and themselves, without finding significant correlations.
Figure 1: (A–C) Nuclear expression of androgen receptor in three examples of invasive ductal carcinoma. 40X, 100X, 400X.
Figure 2: (A–B) NOS-IDC with desmoplasia, tubular formation and intermediated nuclear grade, 100X and 400X. (C- D) MBC, with desmoplasia, geographic necrosis and high nuclear grade, 100X and 400X.
Graphic 1: Expression of androgen receptors in TNBC specimens
| AR positivos (N=14) | AR negatives (N= 62) | |
|---|---|---|
| Patient No. (%) | 14 (100%) | 62 (100%) |
| Mean age (years) | 54 (46, 62) | 52 (44, 60) |
Table 1: Age distribution of cases in patients with triple negative breast cancer with AR positive and negative.
| Variables | With AR N=14 | Without AR N=62 | p | |
|---|---|---|---|---|
| Age > 50 y No. (%) | 9 (64.29%) | 37(59.68%) | 0.4007 a | |
| Size (cm) <5 cm (%) | 2 (14.2%) | 14 (22.58%) | 0.3899 b | |
| Histological Type | NOS-IDC No. | 13 (92.8%) | 52 (83.8%) | 0.3516 b |
| Lobular No. | 0 | 0 | 0 | |
| Special No. | 1 (7.1%) | 6 (9.6%) | 0.6186 b | |
| Other No. | 0 | 4 (6.45%) | 0.6212 b | |
| Histological Grade | I No. % | 0 | 5 (8.0%) | 0.6860 b |
| II No. % | 7 (50%) | 30 (48.3%) | 0.4566 a | |
| III No. % | 6 (42.8%) | 23 (37.1%) | 0.3443 a | |
| Lymphatic system invasion | 7 (50%) | 31 (50%) | 0.3613 b | |
| Vascular invasion | 3 (21.4%) | 22 (35.4%) | 0.3988 b | |
Table 2: Distribution of cases among variables in patients with triple negative breast cancer with and without AR from 2015 to 2019. a < 0.05. b 80%.
Discussion
Breast cancer is a public health problem; In the Latino population, a high incidence of TNBC has been described, 21.3%, concerning all breast cancers11. Once this group has been identified, it has been proposed to search for the expression of AR in tumor tissue because it can provide the opportunity for targeted treatment to these patients, since, in the absence of these, the standardized treatment is neoadjuvant chemotherapy at the beginning of treatment with high response rates, but with short-lasting results, due to resistance mechanisms that develop [12,13], At UMAE N° 1, Guanajuato, Mexico, this group received treatment with chemotherapy based on platinum antineoplastic agent and taxanes, as well as the addition of anthracyclines in advanced stages, with excellent responses at the beginning, but with early relapses at 2 years.
Our study identified 12.2% of TNBC cases, documenting Lehmann et al that this molecular type constitutes 10 to 20% of all breast cancer cases [14], the result of this protocol follows the international bibliography. Regarding the prevalence of AR expression in TNBC, it varies from 13.7% to 75%, finding our result in this range, where the presence of AR was confirmed in 18.42% of the tumors studied. It is very important to emphasize that although this study describes the expression of AR in primary samples from TNBC patients, and several similar studies have been previously performed, and the data from this study substantiates those former studies, the surgical specimens evaluated correspond exclusively to Latino population, which is unique.
In the bivariate analysis, we did not find statistical significance in the correlation of the expression of AR with any of the variables evaluated. The mean age of the patients in the cases studied with positive AR was 54 years; Breast cancer, in general, is considered in women of childbearing age with an increase directly proportional to the increase in age, McGuire et al mentioned that more than 40% of affected patients are over 65 years of age, and the cases with triplenegative immunophenotype occur in younger women than those with another immunophenotype [8]. Most authors consider it to be a non-associated factor. Tumor size has been described as not associated with the presence of AR, while other studies have mentioned that the tumors that present them tend to be smaller.
In our study, it was observed that NOS-IDC was the predominant morphology, however, this characteristic was not generally found associated with the presence of these receptors; On the other hand, it has been observed that some of the special types of carcinomas (metaplastic, mucinous and medullar) express AR to a lower percentage [15,16], finding in the case series of this study a small but existing percentage of tumors with this histology: 6 cases (9.68% of all positive cases). It has been reported that breast carcinomas with histological grade 1 mostly express AR, however, we did not find any AR-positive case histological grade 1 of the Nottingham scale, rather there was an expression in grades 2 and 3 [17]. The lymphovascular invasion is a predictor of greater aggressiveness of the disease, regardless of the expression of AR, we found 7 cases with lymphatic invasion (50%) and 3 cases with angioinvasion (21.43%) [18].
Numerous molecular sub-classifications have been made in the group of TNBC, one of the frequently used classifications is the one proposed by Lehmann et al, which includes 6 sub-groups: two of the basal-like (BL-1 and 2), immunomodulatory (IM), mesenchymal (M) and luminal androgen receptor (LAR) subtype [14]. This same classification was reduced to 4 categories (BL1, BL2, M, and LAR) after a subsequent review. Low-claudin tumors are another less common subtype [19]. Also, this molecular classification includes tumors with apocrine differentiation that comprise neoplasms with apocrine morphology and that are positive for AR. It can be found: negative ER and PR with positive HER2/neu + and AR or as triple-negative with AR expression [20].
Current therapy can be effective, but with significant unwanted effects: decreased immunity to other diseases, changes in liver enzymes, hair loss, loss of appetite, nausea, vomiting (temporary or permanent), frigidity or impotence, as well such as anxiety and depression; it also carries a high cost. Patients with a complete response have a good prognosis [21,22]. The ARs are physiologically found in healthy breast tissue, they are expressed in up to 90% of primary breast tumors and 75% of metastatic lesions [23]. Together with ERs and PRs, they are the main members of the steroid superfamily that induce mammary cell growth by binding to their corresponding receptors (ligands), resulting in the clonal proliferation of non-neoplastic and neoplastic cells [24]. The fundamental role of estrogen and progesterone receptors regarding the prognosis and treatment of these neoplasms is widely known. In contrast, little is known about the exact role of the androgen receptor in breast cancer tumorigenesis.
The ligands of these receptors, androgens, influence the risk of contracting breast cancer through direct binding to their receptors or indirectly through their transformation to estradiol, or by competing for steroid-binding proteins [25]. During postmenopause, there is a decrease in estrogen levels, so adrenal androgens are responsible for an estrogenic replacement for the cells. This supplemental metabolic pathway represents the main source of circulating estrogens and androgens in this age group [26].
There is evidence to suggest that androgen receptors can inhibit or promote tumorigenesis of the breast. In some reports, it antagonizes the union of DNA with the alpha estrogen receptor, this prevents the transcription of proliferative genes; other authors have shown that they assume the role of alpha estrogen “pseudoreceptors”, particularly in triple-negative breast cancer. There has also been reports of breast cancer resistant to endocrine treatment associated with high expressions of AR. The first clinical trial on the therapeutic possibility of ARs was published by Gucalp et al in 2013, with an AR antagonist, where a clinical benefit rate of 19% was found with a median disease-free survival of 12 weeks, corroborating the therapeutic potential of these [27].
Among the drugs mentioned in case series reports are bicalutamide and enzalutamide, which belong to the first and second generation of androgen receptor antagonists; by proposing the standardized search for androgen receptors to triple-negative tumors, is an option for adjuvant targeted therapy that could be applied in patients with this type of tumor. Other therapeutic targets are currently under study, including PARP1, receptor and non-receptor tyrosine kinases, immune checkpoints, epigenetic proteins, and STAT3 [28,29].
Conclusions
Our study on the expression of androgen receptors in a patient with TNBC is the first carried out in Latino population, which is highly relevant, we show that a percentage of the TNBC cases that we evaluated present AR expression and could be susceptible to targeted therapy with anti-androgens, opening the possibility that in studies with longer series of subsequent cases help standardize the offer of this type of treatment. The presence of androgen receptors is a parameter independent of tumor histological types. To obtain significant results, it is necessary to expand the number of cases and take it to a prospective plan where the variables can be closely monitored. Finally, it is important to mention that this study served as the basis for carrying out another prospective study in our hospital, which is currently under development.
Declarations
Ethical considerations
This study followed the Declaration of Helsinki. Following Mexican laws with less risk to the minimum. Informed consent was exempted by the Local Research and Ethics Committee of High Specialty Medical Unit No. 1 Bajío of the Mexican Institute of Social Security. All experimental protocols were approved by Local Research and Ethics Committee of High Specialty Medical Unit No. 1 Bajío of the Mexican Institute of Social Security, with register CONBIOETICA 11 CEI 003 20 18080 and register COFEPRIS 17 CI 11 020 145. The number of institutional register of this research is R-2020-1001-004.
Study limitations
Due to their ambilective nature, to a large extent, the variables of the cases and the material used were dependent on the availability in the previous file of the unit, not allowing a strict follow-up of the same and limiting the results; consequently, biasing its interpretation and the search for correlations.
Conflict of interests
The authors clarify that this study was carried out with the institution’s resources and that they do not present any conflict of interest with any private research group or with the pharmaceutical industry.
Acknowledgements
None apply.
Authors’ contributions
MM conceived the study, participated in its design, coordination, evaluation of histopathology simples and immunohistochemistry results, and edited the draft of the manuscript. SE participated in the data collection, histopathology study and statistical analysis. SM and MO participated in data collection and english traslation of the manuscript. LR and YG performed the histopathology studies of biopsy tissue samples. MH and BM participated in the study coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Funding
None apply.
Availability of data and materials
The data sets generated and / or analyzed during the current study are not publicly available due to [consent to publication of identifying images or other personal or clinical details of participants who compromise anonymity] but are available at the corresponding author at a reasonable request.
Consent for publication
None apply.
Competing interests
The authors declare that they have no competing interests in the elaboration of this investigation. None to declare.
International Agency for Research on Cancer, WHO, Breast Source: Globocan 2018.[ Ref ]
International Agency for Research on Cancer, WHO, Mexico Source: Globocan 2018.[ Ref ]
Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR (2000) Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. The Journal of steroid biochemistry and molecular biology 72: 23-27.[ Ref ]
Allison K (2019) WHO Classification of Tumours.[ Ref ]
Seely J, Alhassan T (2018) Screening for breast cancer in 2018—what should we be doing today? Current Oncology 25: S115-S124[ Ref ]
Dabbs JD (2016) Breast pathology. Elsevier.[ Ref ]
Lebert J, Lester R, Powell E, Seal M, McCarthy J (2018) Advances in the systemic treatment of triple-negative breast cancer. Current Oncology 25: S142-S150.[ Ref ]
McGuire A, Brown J, Malone C, McLaughlin R, Kerin M (2015) Effects of Age on the Detection and Management of Breast Cancer. Cancers 7: 908- 929.[ Ref ]
Meligy B (2018) Androgen Receptor Expression in Estrogen Receptor Negative Breast Cancer. Manuscrito original.[ Ref ]
Mehanna J, Haddad F, Eid R, Lambertini M, Kourie H (2019) Triplenegative breast cancer: current perspective on the evolving therapeutic landscape. International Journal of Women’s Health 11: 431-437.[ Ref ]
Chan J, Tan T, Dent R (2018) Are There Any Clinically Relevant Subgroups of Triple-Negative Breast Cancer in 2018? Journal of Oncology Practice 14: 281-289.[ Ref ]
Satish S (2018) Triple Negative Breast Cancer (TNBC): A Challenge for Current Cancer Therapy. Journal of Human Virology & Retrovirology 6.[ Ref ]
Schmid P, Adams S, Rugo H, Schneeweiss A, Barrios C, et al. (2018) Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. New England Journal of Medicine 379: 2108-2121[ Ref ]
Lehmann BD (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation 121: 2750-2767.[ Ref ]
Jam S, Abdollahi A, Zand S, Khazaeipour Z, Omranipour R, et al. (2019) Androgen Receptor Expression in Triple-Negative Breast Cancer. Archives of Breast Cancer 6: 90-94.[ Ref ]
Ghannam A, Galal S, Ellity M (2016) Expression of androgen receptors in primary breast cancer. European Society for Medical Oncology 27:43-67.[ Ref ]
Park S, Koo J, Park H, Kim JH, Choi SY, et al. (2009) Expression of androgen receptors in primary breast cancer. Annals of oncology 21: 488-492.[ Ref ]
Pistelli M, Caramanti M, Biscotti T, Santinelli A, Pagliacci A, et al. (2014) Androgen receptor expression in early triple-negative breast cancer: clinical significance and prognostic associations. Cancers 6: 1351-1362.[ Ref ]
Zavala EV (2008) Alteración de la integridad de las uniones estrechas en el desarrollo del cáncer. El papel de las claudinas, Artículo de revisión Bioquímica. Bioquimia, Medigraphic 33: 19-29.[ Ref ]
Geyer F, Pareja F, Weigelt B, Rakha E, Ellis I, et al. (2017) The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. The American Journal of Pathology 187: 2139-2151.[ Ref ]
Yoo HJ (2005) Efficacy of progressive muscle relaxation training and guided imagery in reducing chemotherapy side effects in patients with breast cancer and in improving their quality of life. Supportive Care in Cancer 13: 826-833.[ Ref ]
Liu Ya-Xuan (2018) Clinical significance of androgen receptor expression in triple negative breast cancer‑an immunohistochemistry study. Oncol Lett 15: 10008-10016.[ Ref ]
Bleach R, McIlroy M (2018) The Divergent Function of Androgen Receptor in Breast Cancer; Analysis of Steroid Mediators and Tumor Intracrinology. Front Endocrinol 9: 594.[ Ref ]
Nour El Hoda S (2019) Ismael, Immunohistochemical Expression of Androgen Receptors (AR) in Various Breast Cancer Subtypes. Open Access Macedonian Journal of Medical Sciences 7: 1259-1265.[ Ref ]
Ashwani KM, Usha A, Shivani N (2012) Expression of androgen receptor in breast cancer & its correlation with other steroid receptors & growth factors. Indian J Med Res 135: 843-852.[ Ref ]
Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, et al. (2018) Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treatment Reviews 68: 102-110.[ Ref ]
Lee A, Djamgoz M (2018) Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treatment Reviews 62: 110-122.[ Ref ]
Qin J, Yan L, Zhang J, Zhang W (2019) STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. Journal of Experimental & Clinical Cancer Research 38: 195.[ Ref ]
Jones P (2009) Discovery of 2-{4-[(3S)-piperidin-3-y[phenyl}-2Hindazole-7-carboxamide (MK-4827): a novel oral poly (ADP-ribose) polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem 52: 7170-7185.[ Ref ]