Journal Name: International Journal of Cancer and Treatment
Article Type: Research
Received date: 21 May 2019
Accepted date: 11 June 2019
Published date: 14 June 2019
Citation: Darwish A, latif AA, Abdallah D (2019) Biological Subtypes of Breast Cancer and Pattern of Locoregional Relapse. Int J Cancer Tremnt Vol: 2, Issu: 1 (47-53)
Copyright: © 2019 Darwish A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To evaluate the rate of locoregional recurrence (LRR) of breast cancer according to its biological subtype.
Methods: we retrospectively reviewed the clinicopathological data of 821 patients with stage I-III breast cancer, who undergone MRM or BCS ± adjuvant radiotherapy in a single institution. We investigated the effect of biological subtypes, determined by Estrogen receptor (ER) receptor, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) status and Ki 67, on locoregional recurrence (LRR).
Results: Luminal A subtype represented 46.7% of our patients, luminal B 26.3%, luminal B/HER2 10.7 %, the HER2 positive 7.2 % and the triple negative (TN) 9.6%. Patients with Her2 positive and TN subtypes were younger than other groups (<0.001), presenting with more advanced (T3) tumors (<0.001), more nodal involvement (0.009) and higher grade (<0.001) compared to other groups. After a median follow up of 62 months, the rate of LRR was 4.9% (41/821). Mean LRR-FS was 88 months (95% CI 84.7–90.5). The incidence of LRR differed significantly according to the biological subtype. Patients with luminal A subtype showed the lowest rate of LRR (1.6%) compared to other suptypes; 5.6% in luminal B, 6.8% in luminal B/Her2, 10.2% in Her2 positive. TNBC had the highest LRR (13.9%). In univariate analysis, younger age, increasing tumor size, nodal involvement and tumor subtype significantly predict LRR. In multivariate analysis, independent factors associated with increased LRR were tumor size and subtype.
Conclusion: According to our results, Her2 positive and TN subtypes are associated with higher LRR rate. These subtypes may need more aggressive local treatment.
Keywords:
Breast cancer; Locoregional; Recurrence; Biological; Subtype
Abstract
Purpose: To evaluate the rate of locoregional recurrence (LRR) of breast cancer according to its biological subtype.
Methods: we retrospectively reviewed the clinicopathological data of 821 patients with stage I-III breast cancer, who undergone MRM or BCS ± adjuvant radiotherapy in a single institution. We investigated the effect of biological subtypes, determined by Estrogen receptor (ER) receptor, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) status and Ki 67, on locoregional recurrence (LRR).
Results: Luminal A subtype represented 46.7% of our patients, luminal B 26.3%, luminal B/HER2 10.7 %, the HER2 positive 7.2 % and the triple negative (TN) 9.6%. Patients with Her2 positive and TN subtypes were younger than other groups (<0.001), presenting with more advanced (T3) tumors (<0.001), more nodal involvement (0.009) and higher grade (<0.001) compared to other groups. After a median follow up of 62 months, the rate of LRR was 4.9% (41/821). Mean LRR-FS was 88 months (95% CI 84.7–90.5). The incidence of LRR differed significantly according to the biological subtype. Patients with luminal A subtype showed the lowest rate of LRR (1.6%) compared to other suptypes; 5.6% in luminal B, 6.8% in luminal B/Her2, 10.2% in Her2 positive. TNBC had the highest LRR (13.9%). In univariate analysis, younger age, increasing tumor size, nodal involvement and tumor subtype significantly predict LRR. In multivariate analysis, independent factors associated with increased LRR were tumor size and subtype.
Conclusion: According to our results, Her2 positive and TN subtypes are associated with higher LRR rate. These subtypes may need more aggressive local treatment.
Keywords:
Breast cancer; Locoregional; Recurrence; Biological; Subtype
Abbreviations and Acronyms
BCT: Breast conservative treatment; DCIS: Ductal carcinoma in situ; HER2: Human epidermal growth factor 2; HR: Hazard ratio; LVI: Lymphovascular invasion; LRR: Locoregional recurrence; LRR-FS: Locoregional recurrence free survival; TNBC: Triple negative breast cancer.
Introduction
The incidence of local-regional recurrences after surgery for breast cancer is generally low,but it adversely affect the disease free survival and overall survival [1]. It has been proved that the prevention of four local-regional recurrences should prevent one breast cancer death [2].
Multiple factors are considered as important predictors of local-regional failure as tumor size, nodal status, high histologic grade, lymphovascular invasion and positive margins [3,4].
However, breast cancer with similar standard clinicopathological characteristics can still show widely variable clinical behaviors [5]. This diversity in natural history may reflect the underlying molecular biology of the disease [6,7].
Molecular subtyping confirms that breast cancer is not a single entity, but comprises at least four genetically distinct diseases based on the expression of a 496-gene ‘‘intrinsic’’[8,9].
The major intrinsic breast cancer subtypes include: luminal A (HR positive, HER2 negative, low proliferative activity), luminal B (HR positive, higher proliferative activity or HER2 positive), HER2 enriched (HR negative and HER2 overexpressing tumors), and triple-negative breast cancer (TNBC) (HR and HER2 negative) [10]. Gene expression profiling suggested 14 to be the best cutoff point of KI67 to discriminate luminal A from luminal B breast cancer subtype [11].
Molecular subtypes of breast cancer have correlated with variations in recurrence rates and survival, and have been used for prognostication and tailoring of systemic therapy [12,13]. However, their role in deciding the optimal locoregional management is still controversial.
Better and deeper understanding of the pattern of localregional recurrences across the different breast cancer molecular subtypes may improve prediction of locoregional recurrence (LRR) and may be an effective modality in tailoring the optimal local-regional treatment of each subtype.
The aim of this work was to assess the pattern of localregional recurrence across the different molecular subtypes in patients with newly diagnosed breast cancer treated with either breast-conserving therapy or mastectomy and adjuvant radiotherapy if indicated.
Patients and Methods
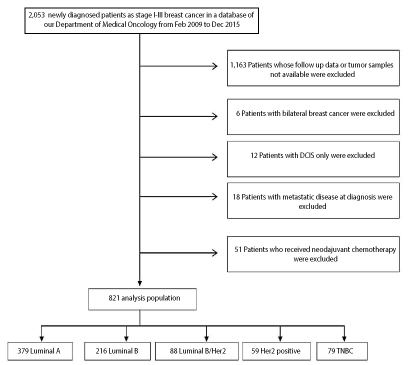
We retrospectively reviewed the medical records of histologically-confirmed operable breast cancer patients treated with breast conserving therapy or mastectomy with curative intent in our institution between 2009 and 2015. We collected all the clinicopathological characteristics, treatment information and patient follow up data. We found 2,053 patients with stages I to III breast cancer. We excluded patients with metastatic breast cancer, bilateral breast cancer, carcinoma in situ and patients who received neoadjuvant chemotherapy. Patients whose follow up data or tumor samples not available, were excluded too. Finally, 821 patients met the eligibity criteria of our study (Figure 1). We collected all the clinicopathological characteristics, treatment information and patient follow up data for the eligible patients.
Figure 1: Flow diagram of the patients through the study. DCIS, ductal carcinoma in situ. TNBC, triple negative breast cancer.
Molecular subtyping is done using immunohistochemical assessment of ER & PR &Her 2 &Ki 67. For the quantitative measurement, ER- and PR-positivity was defined as ≥1% of tumor cells showing positive nuclear staining of any intensity; negative staining was reported if the percentage of tumor cells showing staining of any intensity was <1%. A minimum of 100 tumor cells were assessed, and the percentage of tumor cell nuclei in was recorded.
Tumors were considered HER2-positive if they had a score of 3+ or 2+ on IHC and this score was confirmed with FISH. For Ki 67 nuclear positive staining was assessed, and then classified into two groups (<14% and >14%)
For molecular typing, patients were classified into four subtypes: luminal A (ER+ or PR+, HER2−, and Ki-67 <14%); luminal B ([ER+ or PR+, HER2−, and Ki-67 ≥14%] or [ER+ or PR+ and HER2+]); HER2-enriched (ER− and PR- and HER2+); and basal-like (ER- and PR- and HER2-). This study was approved by the local Ethics Review Board.
End points and Statistical methods
The primary endpoints were LRR and LRR-free survival (LRFS)
LRR was defined as first site of failure being ipsilateral; inbreast recurrence after lumpectomy, chest wall recurrence after mastectomy, or recurrence in the ipsilateral axillary, supraclavicular, internal mammary or infraclavicular lymph nodes that is confirmed by pathological biopsy and without any evidence of distant disease.
LRR-Free-Survival (LRR-FS) was defined as the time from surgery to the date of LRR, death due to any cause, or the last follow-up.
`and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov- Smirnov test was used to verify the normality of distribution of variables, Comparisons between groups for categorical variables were assessed using Chi-square test (Fisher or MonteCarlo). ANOVA was used to compare more than two groups for normally distributed quantitative variables and followed by Post Hoc test (Tukey) for pairwise comparison. Kruskal Wallis test was used to compare different groups for not-normally distributed quantitative variables and followed by Post Hoc test (Dunn’s) for pairwise comparison. Rates of LRR free survival was calculated by Kaplan-Meier method. For the multivariate analysis, Cox Regression were used. Significance of the obtained results was judged at the 5% level.
Results
Distribution of clinicopathologic characteristics between different molecular subtypes
Of 2,053 patients of the overall cohort, 821 newly diagnosed BC patients were eligible to be included in this study. The baseline clinicopathological characteristics of the eligible patients, stratified by their biological subtypes, are listed in table 1. Luminal A subtype consisted of 379 patients (46.7%), luminal B subtype consisted of 216 patients (26.3 %), luminal B/HER2 subtype consisted of 88 patients (10.7 %), the HER2 subtype consisted of 59 patients (7.2 %) and the triple negative subtype consisted of 79 patients (9.6%).
Table 1: Distribution of clinicopathologic characteristics among molecular subtypes.
| Luminal A | Luminal B | Luminal B/Her2 | Her2 positive | Triple negative | P value | |
|---|---|---|---|---|---|---|
| (n = 379) | (n=216) | (n=88) | (n=59) | (n=79) | ||
| Age | 56 (26-80) | 59 (35-75) | 55 (32-82) | 47 (35-76) | 51 (40-68) | <0.001 |
| Surgery | 0.09 | |||||
| BCS | 95 (25.1%) | 68 (31.5%) | 29 (33%) | 41 (69.5%) | 27 (34.2%) | |
| MRM | 284 (74.9%) | 148 (68.5%) | 59 (67%) | 18 (30.5%) | 52 (65.8%) | |
| Histology | 0.001 | |||||
| IDC | 341 (90%) | 192 (88.9%) | 78 (88.6%) | 59 (100%) | 78 (98.7%) | |
| ILC | 38 (10%) | 24 (11.1%) | 10 ( 11.3%) | 0 | 1 (1.3%) | |
| Grade | <0.001 | |||||
| I | 12 (3.2%) | 4 (1.9%) | 1 (1.1%) | 0 | 0 | |
| II | 355 (93.7%) | 201 (93%) | 70 (79.5%) | 41 (69.5%) | 62 (78.5%) | |
| III | 12 (3.2%) | 11 (5.1%) | 17 (19.3%) | 18 (30.5%) | 17 (21.5%) | |
| T | <0.001 | |||||
| 1 | 112 (29.6%) | 59 (27.3%) | 11 (12.5%) | 5 (8.5%) | 8 (10.1%) | |
| 2 | 208 (54.9%) | 126 (58%) | 64 (72.6%) | 39 (66.1%) | 48 (60.7%) | |
| 3 | 35 (9.2%) | 25 (11.6%) | 13 (14.7%) | 15 (25.4%) | 17 (21.5%) | |
| Unknown | 24 (6.3%) | 6 (2.8%) | 0 | 0 | 6 (6.7%) | |
| N | 0.009 | |||||
| 0 | 166 (43.8%) | 81 (37.5%) | 9 (10.2%) | 5 (8.5%) | 5 (6.3%) | |
| 1 | 124 (32.7%) | 67 (31%) | 24 (27.3%) | 23 (38.9%) | 30 (38%) | |
| 2 | 65 (17.2%) | 42 (19.4%) | 41 (46.6%) | 24 (40.6%) | 33 (41.8%) | |
| 3 | 6 (1.6%) | 7 (3.2%) | 8 (9.1%) | 7 (11.6%) | 11 (13.9%) | |
| Unknown | 18 (4.7%) | 19 (8.8%) | 6 (6.8 %) | 0 | 0 | |
| Extranodal invasion | 0.475 | |||||
| No | 323 (85.2%) | 179 (82.9%) | 72 (81.1%) | 50 (84.7%) | 64 (81%) | |
| Yes | 56 (14.8%) | 37 (17.1%) | 16 (18.2%) | 9 (15.3%) | 15 (19%) | |
| LVI | 36 (40.9%) | 20 (33.9%) | 27 (34.1%) | 0.652 | ||
| Negative | 159 (42%) | 78 (36%) | 52 (59%) | 39 (66.1%) | 52 (65.8%) | |
| positive | 220 (58%) | 138 (64%) | ||||
| Chemotherapy | <0.001 | |||||
| No | 87 (23%) | 44 (20.4%) | 12 (13.6%) | 0 (0.0%) | 0 (0.0%) | |
| Anthracycline-based | 194 (51.2%) | 109 (50.4%) | 41 (46.6%) | 25 (42.4%) | 43 (54.4%) | |
| Anthracycline/Taxanes | 76 (20%) | 47 (22.7%) | 35 (39.8%) | 29 (49.2%) | 30 (38%) | |
| Unknown | 22 (5.8%) | 16 (7.4%) | 0 (0.0%) | 5 (8.5%) | 6 (7.6%) | |
| Trastuzumab | <0.001 | |||||
| No | 379 (100.0%)B | 216 (100.0%) | 57 (64.8%) | 36 (61%) | 79 (100.0%) | |
| Yes | 0 (0.0%) | 0 (0.0%) | 19 (21.6%) | 17 (28.9%) | 0 (0.0%) | |
| Unkown | 0 (0.0%) | 0 (0.0%) | 12 (13.6%) | 6 (10.1%) | 0 (0.0%) | |
| Hormonal therapy | 0 (0.0%) | 0 (0.0%) | <0.001 | |||
| No | 303 (79.9%) | 158 (73.1%) | 0 (0.0%) | 59 (100.0%) | 79 (100.0%) | |
| Tamoxifen | 18 (4.7%) | 18 (8.3%) | 53 (60.2%) | 0 (0.0%) | 0 (0.0%) | |
| Aromatase inhibitor | 53 (14.0%) | 29 (13.4%) | 23 (26.1%) | 0 (0.0%) | 0 (0.0%) | |
| Both | 5 (1.3%) | 11(5.1%) | 12 (13.6%) | 0 (0.0%) | 0 (0.0%) | |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0(0.0%) | |||
| Follow up in months | 77(8–102) | 66 (9–89) | 43 (20–55) | 43 (20–55) | 40 (6–50) | <0.001 |
BCS: Breast Conserving Surgery
MRM: Modified Radical Mastectomy
LVI: Lymphovascular Invasion
Patients with luminal A, luminal B and luminal B/HER2 subtypes were significantly older than patients in the Her2 positive and TNBC subtypes (p<0.001).
Most of the T1 tumors were seen in patients with luminal A (29.6%) and luminal B (27.3%) subtypes compared to other groups, while T3 tumors were more frequently seen in patients with Her2+ (25.4%) and TN (21.5%) subtypes, the difference was statistically significant (p<0.001).
Infiltrating ductal carcinoma was the commnest histologic type in all tumor subtypes, whereas lobular cancer was more frequently identified in luminal A, luminal B and luminal B/HER2 groups.
Most of the patients in all groups had grade 2 tumors. Grade 3 tumors were significantly seen in luminal B/ HER2, HER2 positive and TN tumors compared to luminal A and luminal B (p < 0.0001). Luminal A and luminal B had significantly lower rate of lymph nodes involvement compared to other subtypes (0.009).
Modified radical mastectomy was more frequent than breast conserving surgery, in all groups. Systemic chemotherapy was given to 76.6% of the patients. Patients with luminal A and luminal B subtypes were the least to receive systemic chemotherapy (71.2% in luminal A and 73.1% in luminal B compared with 86.4% in luminal B/Her2, 91.6% in Her2 positive, and 92.4 % in TNBC [p <0.001]). In the subset of patients who received chemotherapy, Anthracycline based chemotherapy was the most to be given (65%). Trastuzumab was received by 46.6% of the patients in the Luminal B/Her2 group and 49.6% in the Her2 positive group.
Hormonal therapy was given almost to all patients with ER and or PR positive tumors. Hormonal therapy was in the form of tamoxifen alone, aromatase inhibitors alone or sequential tamoxifen and aromatase inhibitors. Tamoxifen alone was given to 79.9% of patients with luminal A, 73.1% of patients with luminal B compared to 60.2% of patients with Luminal B/Her2. While aromatase inhibitors either alone or sequential with tamoxifen was given to 39.7% of patients with luminal B/HER2 compared to 18.7% of patients in luminal A and 21.7% of patients with luminal B (P <0.001).
The period of follow up differs among the five groups, with the longest follow up identified in patients with luminal A tumors followed by luminal B, luminal B/Her2, HER2 positive and TNBC (P <0.001).
Patterns of LRR by biological subtypes
After a median follow up of 62 months, the rate of locoregional recurrence was 4.9% (41/821). Mean LRR-FS was 88 months (95% CI 84.7-90.5).
The incidence of LRR differed significantly according to the biological subtype. Patients with luminal A subtype showed the lowest rate of LRR (1.6%) compared to other subtypes; 5.6% in luminal B, 6.8% in luminal B/Her2, 10.2% in Her2 positive subtype. TNBC had the highest LRR (13.9%).
The most common sites of LRR were breast and chest wall followed by regional lymph nodes. In luminal A the rate of local and regional recurrence was 1.6 % and 0.3% respectively, and this difference was statistically significant. Whereas the rate of local and regional relapse in luminal B was 4.2% and 1.4% respectively, in luminal B/Her2 was 6.8% and 3.4% respectively. HER2 positive and TNBC subtypes were associated with rate of local and regional recurrence of 6.8% and 3.4% for HER2 positive and 11.4% and 2.5% for TNBC, respectively (Table 2).
Table 2: Locoregional recurrence in tumor subtypes.
| Recurrence | Luminal A | Luminal B | Luminal B/Her2 | Her2 positive | Triple negative | P value |
|---|---|---|---|---|---|---|
| (n = 379) | (n=216) | (n=88) | (n=59 | (n=79) | ||
| Locoregional reccurence | 6 (1.6%) | 12 (5.6%) | 6 (6.8%) | 6 (10.2%) | 11 (13.9%) | 0.001 |
| Local | 5 (1.3%) | 9 (4.2%) | 5 (5.6%) | 4 (6.8%) | 9 (11.4%) | |
| Regional | 1 (0.3%) | 3 (1.4%) | 1 (1.1%) | 2 (3.4%) | 2 (2.5%) |
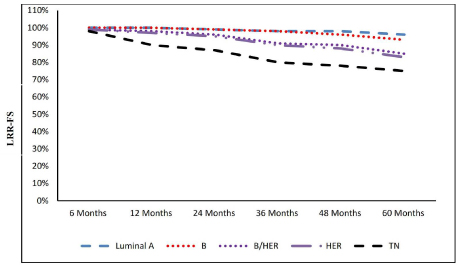
The mean LRR-FS varied across the tumor subtypes, in luminal A the mean LRR-FS was 97.1 months (95% CI 93.4– 99.2), in luminal B 85.9 (95% CI 84.4-87.5), in Luminal B/ Her2 82.1 (CI44.62-88.3), in Her2 positive 40.4 (95% CI 31.1-46.2) and in TN 38.5 (95% CI 29.5- 44.3) (Figure. 2).
Figure 2:Molecular subtypes and locoregional-free survival.
In univariate analysis, factors significantly predicting LRR were younger age, increasing tumor size, nodal involvement and tumor subtype. In multivariate analysis, independent factors associated with increased LRR were tumor size (p:0.018) and tumor subtypes. Luminal B (p:0.002), luminal B/Her2 (p: 0.001), Her2 positive (p <0.001) and TNBC (p<0.001) were significantly associated with increased risk of LRR, when compared to luminal A subtype (Table 3).
Table 3: Multivariate analysis for the parameters affecting relapse.
| #Multivariate | ||||
|---|---|---|---|---|
| p | HR | 95% CI | ||
| LL | UL | |||
| Age | 0.252 | 0.982 | 0.953 | 1.013 |
| T | 0.018 | 4.445 | 1.291 | 15.307 |
| N | 0.916 | 161309.4 | 0 | 3.3´10102 |
| Subtype | ||||
| Luminal A | ||||
| Luminal B | 0.002 | 4.994 | 1.849 | 13.489 |
| Luminal b, her2 | <0.001 | 28.188 | 7.391 | 107.507 |
| Triple negative | <0.001 | 315.749 | 49.324 | 2021.28 |
| Her2 positive | <0.001 | 196.807 | 27.927 | 1386.93 |
Discussion
Tailoring the systemic therapy and targeted therapy for patients with early breast cancer according to their molecular subtypes, has achieved significant progress and resulted in improvement in clinical outcomes [14-16]. On the contrary, locoregional treatment decisions still depend only on the clinicopathological criteria as tumor size, nodal status, high histologic grade, lymphovascular invasion and positive margins [17,18].
The potential of consideration of BC molecular subtypes during decision making may improve tailoring locoregional treatment as well as systemic treatment [19]. Quantifying the LRR rate across the different molecular subtypes could be a key factor in optimizing the locoregional control.
Gene expression analysis of BC distinguish distinct molecular subtypes. In this study we did not use these molecular signatures because of unavailability and high cost, instead, we used less expensive immunohistochemistry (IHC) surrogates for major intrinsic biologic Subtypes that have been validated in several trials [11,15]. we investigated the correlation of biological subtype with LRR.
Some studies have reported that the tumor subtype may influence the risk of locoregional recurrences [20-23]. However, other studies found no significant differences between molecular subtypes and locoregional recurrence [24,25].
In this report, the overall risk for locoregional failure was generally low (4.9%) yet it differed significantly across the five biological subtypes.
Higher rates of locoregional- recurrence have been reported in older studies. Voduc et al. identified 10-year rates of locoregional recurrence ranging from 8 % for the luminal A tumors to 13–20 % for other subtypes, in breast cancer patients treated in the period between 1986 and 1992 [7].
The decline in the incidence of LRR reflects the progress in management of breast cancer that have been witnessed over the past two decades whether in the local or systemic therapy.
In our study, luminal A tumors had the lowest rate of LRR compared to other subtypes and the difference was significant. Our result was in accordance with that of Dominici et al. who reported LRR of 1% in luminal A, compared with 6.5% in luminal B, 2% in Her2 positive and 10.9% in TNBC; this difference was significant [26].
Our results compare also with Gabos et al. who found that HER2 positive and TNBC subtypes were associated with the highest rate of loco-regional recurrence [27].
In univariate analysis, we identified larger tumor size, nodal involvement, younger age and tumor subtype as significant predictors of LRR, while in multivariate analysis only tumor size and subtype were the only independent factors.
Braunstein et al. [28] found in a study of 2233 early breast cancer patients that tumor subtypes, age ≤ 50 years old and involved axillary LN as predictive factors in univariate analysis.
Most of our patients (76.6%) received systemic chemotherapy; luminal A group was the least to receive chemotherapy (71.2%) while triple negative group had highest rate, where 92.4% received chemotherapy. Adjuvant trastuzumab was given to 24.5 % of Her2 positive patients. Adjuvant hormonal therapy (tamoxifen or aromatase inhibitor or both) was received by 97.7% of the HR positive tumors.
Our study revealed that LRR depends not only on conventional clinicopathologic parameters, but also on tumor biological subtypes. Incorporation of biological subtypes, with clinicopathological factors, into treatment decision making could allow better tailoring of adjuvant radiotherapy treatment based on the risk of LRR [28,29].
Our study has some limitations; being retrospective, classification of tumor subtypes based on IHC-surrogates and not on molecular signature and the low percentage of Her2 positive patients receiving adjuvant trastuzumab (24.5%).
In conclusion, we found that biological subtypes can predict LRR in early breast cancer patients treated by MRM or BCS, this information can aid in deciding the locoregional treatment whether surgery or adjuvant radiation treatment. Tumor subtypes with higher LRR rate may benefit from more aggressive local therapy and closer follow up.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding sources
None.
Acknowledgements
None.
There are no references