Journal Name: International Journal of Cancer and Treatment
Article Type:Research Article
Received date:30 November, 2021
Accepted date:17 December, 2021
Published date:24 December, 2021
Citation: Murguia-Perez M, Leal-Tapia KA, Ramírez-Balderrama LA, García-Mendoza YI, Mendoza-Ramírez S, et al. (2021) Determination of Mutations in EGFR, ALK and PDL-1 Expression in Patients with Non-Small Cell Lung Carcinoma in Latino Population. Int J Cancer Treat Vol: 4, Issu: 2 (30-37).
Copyright: © 2021 Murguia-Perez M et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:Recently the presence of some mutations such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) translocations have been found in lung cancer, as well as the aberrant expression of PD-L1, whose therapeutic targets prolong the survival rate in patients with this neoplasm. OBJETIVE. Identification of mutations in the EGFR and ALK genes, in addition to determining the expression of PD-L1 in cases of NSCLC in patients from UMAE No 1 Bajío.
Methods: Observational, cross-sectional and retrospective study, by searching for the determination of ALK, PD-L1 (IHC) and EGFR (PCR) in patients with NSCLC in a period of 4 years using descriptive statistics, and search for associations of these detections with clinical-pathological variables using Pearson’s correlation.
Results: 43 cases of NSCLC were found, among adenocarcinomas and lung squamous cell carcinomas, most of them men. PD-L1 was positive in 67.4%, EGFR mutated in 13.9% and ALK positive in 9.3%. No statistical significance in the study of association of variables.
Conclusions: It is important to screen for the presence of mutations in EGFR, ALK and PD-L1 expression, due to the possibility of improving the prognosis of patients through the early use of target treatments.
Keywords: Non small-cell lung carcinoma; EGFR; ALK; PD-L1.
Abbreviations: : NSCLC: non-small cell lung carcinoma; SCLC: small cell lung carcinoma; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; AdCa: adenocarcinoma; SqCC: squamous cell carcinoma; LCC: large cell carcinoma; TPS: total positive score; TKR: tyrosine kinase receptors; TKI: tyrosine kinase inhibitor; IHC: immunohistochemistry; PCR: polymerase chain reaction; rt-PCR: real time-polimerase chain reaction; WHO: World Health Organization; UMAE N° 1: High Specialty Medical Unit No. 1 Bajio; IMSS: Social Security Mexican Institute.
Abstract
Background:Recently the presence of some mutations such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) translocations have been found in lung cancer, as well as the aberrant expression of PD-L1, whose therapeutic targets prolong the survival rate in patients with this neoplasm. OBJETIVE. Identification of mutations in the EGFR and ALK genes, in addition to determining the expression of PD-L1 in cases of NSCLC in patients from UMAE No 1 Bajío.
Methods: Observational, cross-sectional and retrospective study, by searching for the determination of ALK, PD-L1 (IHC) and EGFR (PCR) in patients with NSCLC in a period of 4 years using descriptive statistics, and search for associations of these detections with clinical-pathological variables using Pearson’s correlation.
Results: 43 cases of NSCLC were found, among adenocarcinomas and lung squamous cell carcinomas, most of them men. PD-L1 was positive in 67.4%, EGFR mutated in 13.9% and ALK positive in 9.3%. No statistical significance in the study of association of variables.
Conclusions: It is important to screen for the presence of mutations in EGFR, ALK and PD-L1 expression, due to the possibility of improving the prognosis of patients through the early use of target treatments.
Keywords: Non small-cell lung carcinoma; EGFR; ALK; PD-L1.
Abbreviations: : NSCLC: non-small cell lung carcinoma; SCLC: small cell lung carcinoma; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; AdCa: adenocarcinoma; SqCC: squamous cell carcinoma; LCC: large cell carcinoma; TPS: total positive score; TKR: tyrosine kinase receptors; TKI: tyrosine kinase inhibitor; IHC: immunohistochemistry; PCR: polymerase chain reaction; rt-PCR: real time-polimerase chain reaction; WHO: World Health Organization; UMAE N° 1: High Specialty Medical Unit No. 1 Bajio; IMSS: Social Security Mexican Institute.
Background
Lung cancer is the leading cause of death from malignant neoplasms worldwide and in Mexico, this due to late diagnoses, nature of the tumor, and limited interventions in treatment [1]. In our country, the incidence rate of lung cancer is estimated at 7.5 new cases per 100,000 inhabitants, according to the International Agency for Research on Cancer (IARC) [2]. In addition, it is the 5th malignant neoplasm with the highest incidence rate, after breast, prostate, cervix, and colorectal cáncer [3]. Generally, and for ease of clinical management, it is classified into two main histological groups: small cell lung carcinoma (SCLC) which represents 15% of all lung cancers, and non-small cell lung carcinoma (NSCLC) covering 85%; in turn, the World Health Organization (WHO) has classified NSCLC into 3 main types: adenocarcinoma, squamous cell carcinoma and large cell carcinoma [4].
Previously, the treatment of NSCLC was limited to radiation therapy, chemotherapy, or a combination of both; for a few years now, the treatment of lung carcinoma has entered a new era with the discovery of mutations in tyrosine kinase receptors (TKR), more specifically epidermal growth factor receptor (EGFR) and kinase translocations of anaplastic lymphoma kinase (ALK), along with other molecular abnormalities, since the presence of these mutations or translocations confer a remarkable sensitivity to first, second, and last line therapies directed to these abnormalities [5,6]. These advances have considerably prolonged the survival of patients with NSCLC, in contrast to those patients who do not harbor EGFR or ALK molecular alterations, and present a limited response to chemotherapy [7]. Although much progress has been achieved recently in the treatment of lung cancer, these patients still face a relatively low survival rate. Therefore, immunotherapy has been considered a very promising therapeutic strategy for different types of tumors. One of the most widely used immunotherapies is monoclonal antibodies against programmed death-1 (PD-1) or programmed death-ligand-1 (PD-L1). Aberrant PD-L1 expression has been found to range from 19% to 100% of NSCLC patients and is associated with a poor prognosis [8,9]. However, immune checkpoint inhibitors targeting PD-1 or PD-L1 increase survival for patients with advanced NSCLC [10].
Lung cancer is one of the malignant neoplasms that occur most frequently in our country, however, the management of advanced NSCLC has improved, by discovering the presence of mutations in EGFR and ALK, which helps to guide the therapy and which have been shown to have a better response to oral TKI inhibitors, are associated with longer-lasting results, lower toxicity, and an improvement in quality of life compared to conventional chemotherapy. Furthermore, immunotherapy also plays an important role since PD-1 inhibitors or its PD-L1 ligand also confer a benefit in the survival of patients with advanced NSCLC; however, few studies in our country show the incidence these mutations, which is of an utmost importance to offer a targeted treatment that provides a better response to patients with NSCLC; in addition to not knowing such data, target therapies are not authorized in all hospital centers in our country. The reason for our study is to know the frequency of molecular alterations in the NSCLC of our medical center and to identify possible associations with various clinical pathological variables representative of the Latino population.
Methods
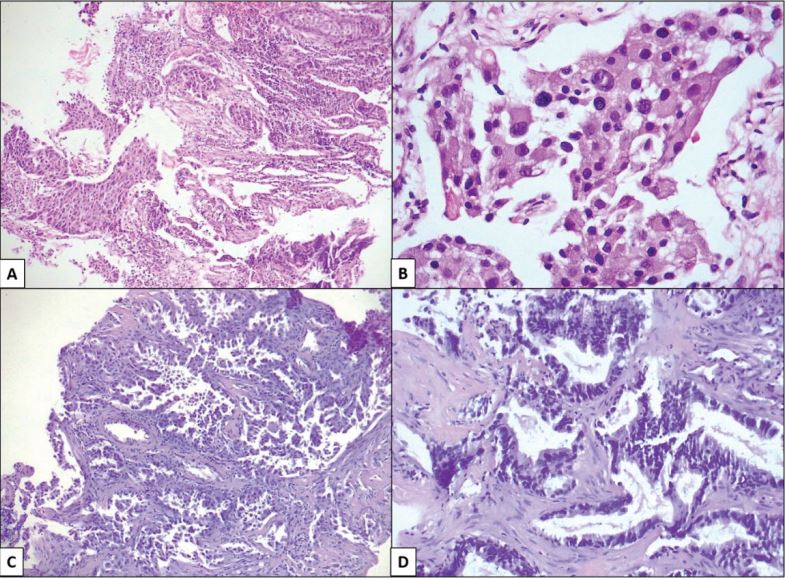
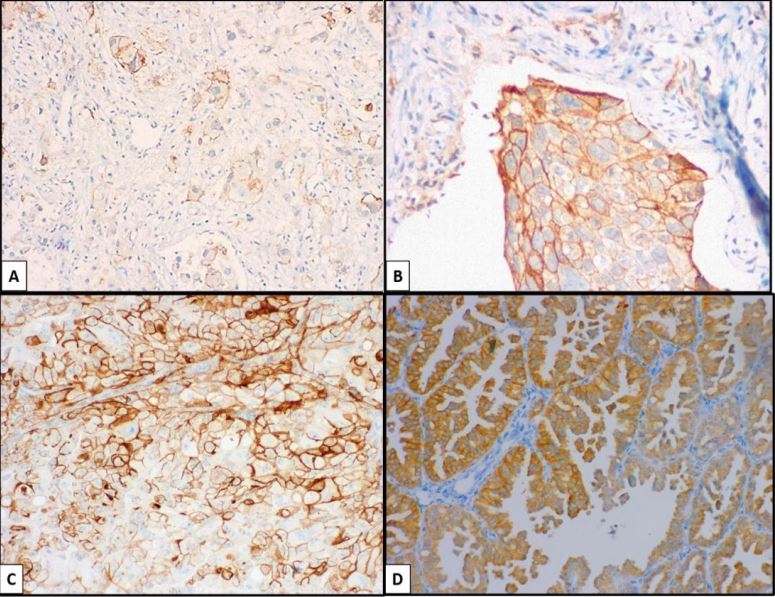
An observational, cross-sectional, and ambilective study was carried out by searching for results in the archives from the Department of Pathology of UMAE N°1, IMSS, with a diagnosis of NSCLC in a period of 4 years, corresponding from January 1, 2015, to August 31, 2019. Pathological results corresponding to patients older than 18 years with a complete clinical record and with a diagnosis of NSCLC, whose histological types correspond to adenocarcinoma (AdCa), squamous cell carcinoma (SqCC), and large cell carcinoma (LCC); Subsequently, the slides and paraffin blocks corresponding to each case were collected, they were evaluated in pairs to confirm the established diagnosis, and to determine if the material was adequate to perform immunohistochemical (IHC) and polymerase chain reaction (PCR) techniques; All cases with a diagnosis of SCLC, those with insufficient sample quantity for IHC and CRP, and cases in which no paraffin blocks were found were excluded. From the selected cases, a paraffin block was chosen to carry out the previously determined studies (Figures 1,2).
For the IHC technique, each selected block was cut at 3 microns on electrocharged slides, deparaffinization and hydration were performed, followed by tissue antigen recovery by pressure with a Diagnostic Biosystem pressure cooker, with buffered EDTA pH 9.0 recovery. Subsequently, it was washed with hydrogen peroxide for 5 minutes, rinsed with PBS, all the slides were mounted on cover plates and racks, and primary antibody was applied against PD-L1, (clone SP263, Ventana®) and ALK (clone D5F3, Ventana®) was incubated for one hour, with subsequent rinsing with PBS, a second biotinylated antibody was added for 10 minutes, rinsing with PBS, diaminobenzidine (DAB) was applied and subsequent development for 1 minute, it was passed through distilled water and contrasted with hematoxylin for 5 to 10 seconds. Each slide was bathed in carbonated or ammonia water, dehydrating with 96° alcohol, clearing with xylol, and mounting with coverslips. The preparations were observed in a light microscope (Carl Zeiss® Axio Imager A2) and were evaluated in an image analyzer (MoticEasyScan®), subsequently archiving 2 images. PD-L1 status was determined by the percentage of tumor cells with any membrane staining on the background. The percentages of the tumor area occupied by any tumor cell were used to determine the tumor positive score (TPS), being considered negative (0%) or positive from 1%; low-positive expression of 1-50%; high-positive expresión > 50% were considered. For the interpretation of ALK, an average of 500 cells was analyzed by 2 observers, and it was reported as positive or negative. EGFR gene mutation detection was performed by real-time PCR (rt-PCR) with the Rotor-Gene Q instrument (Qiagen®). Scorpions® and Arms® technologies were used with the EGFR RGQ PCR kit. The following somatic mutations were identified: most frequent mutations of exons 18, 19, 20, 21, and resistance mutation T790. The final reporting the status of the gene was stated as mutated or not mutated.
Statistical analysis was performed using IBM SPSS Statics v23.0 Statistical Software to identify each of the variables. Descriptive statistics were used where mean, median, mode, and standard deviation were used for quantitative variables; and for the qualitative proportions. For the inferential statistical analysis, the Pearson study was used to search for possible associations and correlations between the different variables, those with a correlation of p <0.05 being statistically significant.
Figure 1:A; B) Squamous cell carcinoma (magnification 10X and 40X). C; D) Adenocarcinoma (magnification 10X and 40X).
Figure 2:A; B) PD-L1. C; D) ALK.
Results
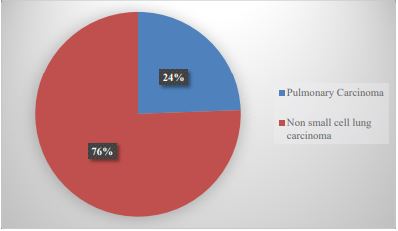
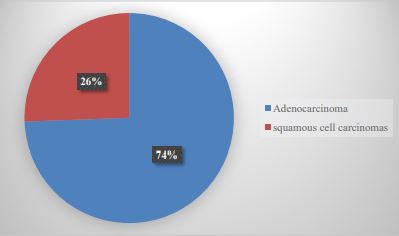
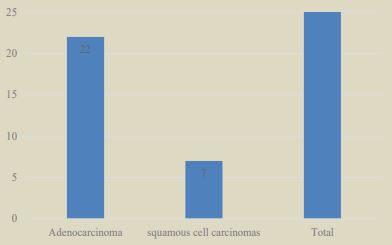
During the study period, 67,522 cases registered in our service were identified. The cases diagnosed as lung carcinoma were 86 (0.127%), of which 21 cases (24.4%) were diagnosed as SCLC and 65 cases (75.5%) as NSCLC (Graph 1); Of the NSCLCs, only 43 cases could be included in the study, of which 23 were men (53.4%) and 20 were women (46.5%). The mean age of the patients was 61.9 + 11.9 years (Table 1). The histopathological varieties of the analyzed cases corresponded to AdCa (32 cases, 74.4%) and SqCC (11 cases, 25.5%) (Graph 2).
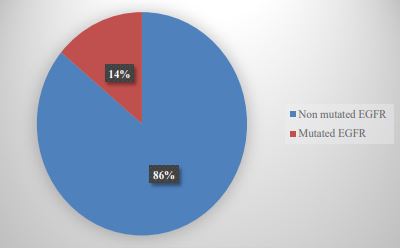
In 29 patients, PD-L1 was positive (67.4%) of which 19 had low expression TPS (65.5%) and 10 had high expression TPS (34.5%) (Table 2); in terms of intensity; 15 graduated with mild intensity (51.7%), 13 moderate (44.8%) and 1 high intensity (3.4%). Of these 29 positive cases for PDL-1, 22 corresponded to AdCa (75.8%) and 7 to SqCC (24.2%) (Graph 3). Of the 43 cases studied, we found mutations in the EGFR gene in only 6 cases (13.9%) (Graph 4). Regarding ALK, we found mutations in only 4 patients (9.3%) (Graph 5).
In addition, some histological parameters were analyzed, such as the presence of necrosis, vascular permeation, inflammatory infiltrate, and number of mitoses in 10 high-power fields; 25 cases (58.1%) presented different percentages of necrosis; vascular permeation in 16 cases (37.2%); Lymphoid-type inflammatory infiltrate was observed in 26 cases with mild inflammatory infiltrate (60.5%), 6 with moderate (14%) and 5 with intense (11.6%), in one of the cases the inflammatory infiltrate, was absent (2.3%); finally, the mitosis index presented a mean of 14.6 mitosis.
Associations were made through Pearson’s study between expression of PD-L1, ALK, and EGFR, as well as the presence of necrosis, vascular permeation, inflammatory, infiltrate, and mitotic index, without finding statistical significance in any of them.
Graph 1:Reported cases of lung carcinoma in patients from UMAE N°1.
Graph 2:Histological varieties of non-small cell lung carcinomas in UMAE N°1.
Graph 3:PD-L1 expression in the different histological varieties.
Graph 4: Incidence of EGFR mutation in NSCLC cases.
| Variable | Number | Percentage |
|---|---|---|
| Men | 23 | 53.4% |
| Women | 20 | 46.5% |
| Ages | 61.9 ± 11.9 anos |
Table 1: NSCLC Demographic characteristics.
| AdCa | SqCC | Total | |
|---|---|---|---|
| TPS <50% | 15 (51.7%) | 4(13.7%) | 19 (65.4%) |
| tps >50% | 7 (24.2%) | 3 (10.4%) | 10 (34.6%) |
Table 2: Percentaje of PD-L1 positive NSCLC cases.
Discussion
Lung cancer in Mexico represents one of the main causes of death, and the main etiological factor in lung carcinogenesis is tobacco consumption, in addition to other factors, such as genetic susceptibility, poor diet, occupational exposure, and air pollution can act independently or in conjunction with smoking to configure the descriptive epidemiology of lung cancer [11,12].
NSCLC is classified into 3 main types: AdCa, SqCC, and LCC. Before the WHO classification of 2016, AdCa was defined by the presence of acinar/tubular structures or mucin production, and SqCC as carcinoma with keratinization or intercellular bridges, however with the current classification if the immunohistochemistry shows that poorly differentiated carcinoma that lacks microscopic evidence or slight glandular differentiation expresses “adenocarcinoma markers”, such as TTF-1 and/or Napsin A, is diagnosed as a solid adenocarcinoma and if immunohistochemistry shows that poorly differentiated carcinoma lacks or presents slight squamous differentiation expressing “SqCC markers”, such as p40, CK5 / 6 and p63, it is diagnosed as non-keratinizing KSqCC [13]. SqCCs are classified into keratinizing SqCC, non-keratinizing SqCC, and basaloid SqCC [14]. LCC is the least common of all lung carcinomas (15%). It is a malignant epithelial neoplasm that does not meet the criteria for adenocarcinoma, squamous cell carcinoma, or small cell carcinoma. Histologically, it is characterized by large polygonal cells, with a vesicular nucleus and a large nucleolus [15]. The most frequent histological subtype of lung cancer is AdCa, which coincides with the results of our study since we found a total of 32 cases that correspond to 74.4% of the total of NSCLC cases found [16].
As mentioned at the beginning, currently the therapy against NSCLC depends on finding or detecting alterations in various proteins, especially transmembrane proteins, among them the best known and studied are mutations in the EGFR gene and ALK translocations, together with other less frequent molecular abnormalities; and also with the determination of the expression of PD-L1 [5].
The EGFR gene is located on the short arm of chromosome 7 and codes for a transmembrane protein, with a molecular size of 170 kDa, and belongs to a family of four membrane receptors, with tyrosine kinase (TK) activity: ErbB1 (EGFR, HER1), ErbB2 (HER2 / NEU), ErbB3 (HER3) and ErbB4 (HER4). All members of this family have a similar structure consisting of three regions: an extracellular region, where the ligand-binding domain is located; a transmembrane region, where it anchors to the cell membrane; and an intracellular region, where the TK5 domain is found. EGFR mutations are observed in approximately 15% of NSCLCs, a large proportion have mutations in exon 19 or 21 (approximately 45 and 40% of patients, respectively) that activate the tyrosine kinase domain in EGFR. The discovery of TKI was considered a landmark finding in the treatment of lung cancer [17]. EGFR-TKI is the first-line treatment targeting EGFR sensitizing mutations which results in longer progression-free survival, improved quality of life, and decreased treatment-related serious side effects compared to those who received standard chemotherapy [18]. Therefore, many clinical guidelines recommend that all patients with EGFR sensitizing mutations receive firstline treatment with these drugs, in addition to all patients with advanced or metastatic NSCLC [19,20]. In our study we found a relatively low incidence compared to the consulted bibliography regarding the presence of EGFR mutations (13.9%), finding that 33.3% presented mutations in exon 21 and in the same way in exons 19 and 21.
Another recent advance in the treatment of NSCLC is the incorporation of TKIs of the ALK protein in patients with positivity for this antibody; This subgroup of patients represents between 2-5% of the total NSCLC; Furthermore, the clinical characteristics associated with this mutation are very similar to patients with EGFR mutations; these patients appear to be younger than the average lung cancer patient (mean age 52 years) and with a histological predominance of adenocarcinomas ( mainly in adenocarcinoma with signet ring cells) and they are frequently non-smokers [21]. The response rate, quality of life, and survival are better in these patients when they receive treatment with an ALK TKI such as Crizotinib compared to when only receiving regular chemotherapy [22]. In our study we found an incidence for the presence of ALK mutations of 9.3%, which represents a relatively high incidence compared to other studies carried out, since, for example, in the study by Martelli et al, where a total of 120 were included and the overall frequency of ALKpositive NSCLC was 5.8%; as in another study carried out by Perner et al, where NSCLC samples from 603 patients in total were analyzed, finding by FISH a frequency of positive NSCLC ALK of 2.7% [23,24].
One of the most widely used immunotherapies is monoclonal antibodies against PD-1 or PD-L1. Its action is based on the ability of some tumors to evade the immune system through the expression of PD-L1, a ligand for a protein called PD-1. When the union between PD-1 and PDL1 occurs, the activation of the T lymphocyte is inhibited and therefore the normal immune response against tumor cells is inhibited. Some of these therapies are already approved for the treatment of lung cancer, with overall survival rates greater than those obtained with traditional chemotherapy [25].
Numerous primary monoclonal antibodies directed against PD-1 or its ligand PD-L1 have been developed, which are associated with the use of drugs such as nivolumab and pembrolizumab for PD-1 and atezolizumab, durvalumab, and avelumab for PDL-1. The drugs atezolizumab and durvalumab bind to PD-1 without activating it, blocking with their binding the interaction with the PD-L1 ligand, and consequently demonstrating its beneficial effect in different types of tumors [26]. According to Yu et al, the prevalence of PD-L1 expression in the population of patients with NSCLC ranges between 24% and 60%, very similar to that found by the group of Ishii et al, who reported a percentage of positivity of 71.6% of the total of 102 patients in the series when considering positive staining for PD-L1 that present in the membrane and/or cytoplasm of tumor cells and in the study carried out by Skov et al, they found a positivity for PD-L1 in 499 patients out of a group of 791 in total, corresponding to 63% [27-29]. Small variations in these percentages that could be related to: 1) the type of PD-L1 clone used in the immunohistochemical study; 2) the wide range in the minimum percentage value stipulated to consider a sample as positive, which according to the authors varies between 1% and 50%; however, there is little scientific evidence available in this regard. In our study, the expression was considered positive from 1% of the tumor cells [30]. Furthermore, we found significant differences in PD-L1 expression between adenocarcinoma and SqCC, the first one with the highest expression. Other studies have reported similar findings, while some have shown increased expression in SqCC or no difference [31-33].
In this study, a search was also carried out for some histological variables such as the percentage of necrosis, the presence of inflammatory infiltrate, the mitotic index and the presence or the absence of vascular permeation, and associations between them and with the presence of EGFR mutations were intentionally sought out of ALK and PD-L1 expression, without finding any association of statistical importance; There was also not enough evidence from studies looking for these histopathological variables. However, in a study by Usui et al, it was found that vascular invasion was observed in a high percentage of NSCLC cases and that, although the demonstration of vascular invasion by tumor cells does not always necessarily imply a fatal prognosis, it is considered a serious prognosis due to the higher incidence of distant metastases in these cases and the presence of metastases in some studies has been associated with greater positivity for PD-L1 [34]. Wang et al showed a statistically significant prevalence, 2.3 times higher, of PDL1 positivity in patients with metastatic disease compared to early and locally advanced disease [35]. Evans et al found that tumor samples taken from the primary lung tumor showed significantly lower rates of PD-L1 positivity than pleural and lymph node samples. Based on the above, the presence of vascular permeation could be related to a more advanced stage of the disease and therefore greater positivity for PD-L1, however in this study no relationship was found between the presence of vascular permeation and the percentage of PD-L1 expression.
Conclusion
Although the prevalence of EGFR mutations is generally relatively low, screening should continue due to the limited efficacy of regular chemotherapy for NSCLC patients and universal EGFR mutation testing may improve the overall prognosis with the early use of first-line EGFR-TKI treatment. The positivity for ALK represents a considerable percentage to carry out its detection in a systematic way, since the response rate, quality of life, and survival are better in these patients when they receive treatment with an ALK-TKI than when they receive treatment with regular chemotherapy.
The incidence of PD-L1 expression represents a considerable number of patients who could benefit from the systematic detection by immunohistochemistry of this marker since its expression helps to determine if the patient is a candidate for immunotherapy, which is associated with a better response to treatment, as well as a reduction in the adverse effects associated with it, as well as a greater global survival rate. Finally, no statistically significant association was found between the immunohistochemical expression of PD-L1, with the presence of ALK, EGFR mutations, or with the histopathological variables studied, which means that the behavior of the disease does not depend on the presence or absence of said molecular mutations or the presence of PD-L1.
Declarations
Ethical considerations
This study followed the Declaration of Helsinki. Following Mexican laws with less risk to the minimum. Informed consent was exempted by the Local Research and Ethics Committee of High Specialty Medical Unit No. 1 Bajio of the Mexican Institute of Social Security. All experimental protocols were approved by Local Research and Ethics Committee of High Specialty Medical Unit No. 1 Bajio of the Mexican Institute of Social Security, with register CONBIOETICA 11 CEI 003 20 18080 and register COFEPRIS 17 CI 11 020 146. The number of institutional register of this research is R-2020-1001-024.
Study limitations
Due to their ambilective nature, to a large extent, the variables of the cases and the material used were dependent on the availability in the previous file of the unit, not allowing a strict follow-up of the same and limiting the results; consequently, biasing its interpretation and the search for correlations.
Conflict of interests
The authors clarify that this study was carried out with the institution’s resources and that they do not present any conflict of interest with any private research group or with the pharmaceutical industry.
Acknowledgements
None apply.
Authors’ contributions
MM conceived the study, participated in its design, coordination, evaluation of histopathology simples and immunohistochemistry results, and edited the draft of the manuscript. KL participated in the data collection, histopathology study and statistical analysis. YG and MO participated in data collection and English traslation of the manuscript. LR and YG performed the histopathology studies of biopsy tissue samples. BM participated in the study coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Funding
None apply.
Availability of data and materials
The data sets generated and / or analyzed during the current study are not publicly available due to [consent to publication of identifying images or other personal or clinical details of participants who compromise anonymity] but are available at the corresponding author at a reasonable request.
Consent for publication
None apply.
Competing interests
The authors declare that they have no competing interests in the elaboration of this investigation.
None to declare.
Bylicki O, Paleiron N, Margery J, Guisier F, Vergnenegre A, et al. (2017) Targeting the PD-1/PD-L1 Immune Checkpoint in EGFR-Mutated or ALK-Translocated Non-Small-Cell Lung Cancer. Target Oncol 12: 563- 569.[ Ref ]
International Agency for Research on Cancer. (2012) GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Incidence/Mortality> Rates: Cancers by population. [ Ref ]
Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD (2005) Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ 83: 171-177.[ Ref ]
Duma N, Santana-Davila R, Molina JR (2019) Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 94: 1623-1640.[ Ref ]
Sweis RF, Thomas S, Bank B, Fishkin P, Mooney C, et al. (2016) Concurrent EGFR Mutation and ALK Translocation in Non-Small Cell Lung Cancer. Cureus 8: e513. [ Ref ]
Bylicki O, Paleiron N, Margery J, Guisier F, Vergnenegre A, et al. (2017) Targeting the PD-1/PD-L1 Immune Checkpoint in EGFR-Mutated or ALKTranslocated Non-Small-Cell Lung Cancer. Target Oncol 12: 563-569.[ Ref ]
Banna GL, Passiglia F, Colonese F, Canova S, Menis J, et al. (2018) Immune-checkpoint inhibitors in non-small cell lung cancer: A tool to improve patients’ selection. Crit Rev Oncol Hematol 129: 27-39.[ Ref ]
Didkowska J, Wojciechowska U, Mańczuk M, Lobaszewski J (2016) Lung cancer epidemiology: Contemporary and future challenges worldwide. Ann Transl Med 4: 1-11.[ Ref ]
Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P (2016) Risk factors for lung cancer worldwide. Eur Respir J 48: 889-902.[ Ref ]
Vargas-Rojas MI (2008) Pd-1 y sus ligandos como reguladores de la respuesta inmune. Rev inst nal enf resp mex 21: 272-279.[ Ref ]
Barta JA, Powell CA, Wisnivesky JP (2019) Global epidemiology of lung cancer. Ann Glob Heal 85: 1-16.[ Ref ]
Bravo-Iñiguez CE, Fox SW, De Leon LE, Tarascio JN, Jaklitsch MT, et al. (2019) Cumulative nonsmoking risk factors increase the probability of developing lung cancer. J Thorac Cardiovasc Surg 158: 1248-1254.e1. [ Ref ]
Inamura K (2017) Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol 7: 1-7.[ Ref ]
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, et al. (2015) The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J Thorac Oncol 10: 1243-1260. [ Ref ]
Ángel M, Valdés S, Fernández B, Mariana G, Gago M (2017) Non–SmallCell Lung Cancer. Unusual Small Bowel Metastasis with Invagination. Finlay Rev Enfermedades no Transm 7: 219-224.[ Ref ]
Kuhn E, Morbini P, Cancellieri A, Damiani S, Cavazza A, et al. (2018) Adenocarcinoma classification: Patterns and prognosis. Pathologica 110: 5-11.[ Ref ]
Han JY, Park K, Kim SW, Lee DH, Kim HY, et al. (2012) First-SIGNAL: firstline single-agent iressa versus gemcitabine and cisplatin trial in neversmokers with adenocarcinoma of the lung. Journal of Clinical Oncology 30: 1122-1128.[ Ref ]
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-smallcell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology 13: 239-246.[ Ref ]
Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R (2005) Resumen de aprobación de medicamentos de la FDA: erlotinib (Tarceva®) tabletas. El oncólogo 10: 461-466.[ Ref ]
Azzoli CG, Temin S, Aliff T, Baker S, Brahmer J, et al. (2011) 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non–small-cell lung cancer. Journal of Clinical Oncology 29: 3825-3831.[ Ref ]
Shaw AT (2009) Clinical features and outcome of patients with nonsmall cell lung cancer WHO harbor EML4-ALK. J Clin Oncol 27: 4247- 4253.[ Ref ]
Shaw AT (2013) Crizotinib versus chemotherapy in advanced ALK positive lung cancer. N Engl J Med 368: 2385-2394.[ Ref ]
Martelli MP, Sozzi G, Hernandez L, Pettirossi V, Navarro A, et al. (2009) EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 174: 661-670.[ Ref ]
Perner S, Wagner PL, Demichelis F, Mehra R, LaFargue CJ, et al. (2008) EML4-ALK fusion lung cancer: A rare acquired event. Neoplasia 10: 298- 302.[ Ref ]
Yang LL, Wu YL (2014) Recent advances of immunotherapy in lung cancer: antiprogrammed cell death-1/programmed death ligand-1 antibodies. Lung Cancer Management 3: 175-190.[ Ref ]
Rebelatto MC, Mistry A, Sabalos C (2015) Development of a PD-L1 companion diagnostic assay for treatment with MEDI4736 in NSCLC and SCCHN patients (abstract). J Clin Oncol 33(suppl): 8033.[ Ref ]
Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR (2016) PD-L1 expression in lung cancer. J Thorac Oncol 11: 964-975.[ Ref ]
Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, et al. (2015) Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 10: 426-430.[ Ref ]
Skov BG, Rorvig SB, Jensen THL, Skov T (2020) The prevalence of programmed death ligand-1 (PD-L1) expression in non-small cell lung cancer in an unselected, consecutive population. Mod Pathol 33: 109-117.[ Ref ]
Antonia Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, et al. (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17: 883-895.[ Ref ]
Kitazono S, Fujiwara Y, Tsuta K, Utsumi H, Kanda S, et al. (2015) Reliability of small biopsy samples compared with resected specimens for the determination of programmed deathligand 1 expression in non– small-cell lung cancer. Clin Lung Cancer 16: 385-390.[ Ref ]
Ye L, Leslie C, Jacques A, Mesbah Ardakani N, Amanuel B, et al. (2018) Programmed death ligand-1 expression in non-small cell lung cancer in a Western Australian population and correlation with clinicopathologic features. Mod Pathol 32: 524.[ Ref ]
Evans M, O’Sullivan B, Smith M, Taniere P (2018) Predictive markers for anti-PD-1/PD-L1 therapy in non-small cell lung cancer-where are we? Transl Lung Cancer Res 7: 682-690.[ Ref ]
Usui S, Minami Y, Shiozawa T, Iyama S, Satomi K, et al. (2013) Differences in the prognostic implications of vascular invasion between lung adenocarcinoma and squamous cell carcinoma. Lung Cancer 82: 407- 412.[ Ref ]
Wang H, Agulnik J, Kasymjanova G, Wang A, Jimenez P, et al. (2018) Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol 29: 1417-1422.[ Ref ]