Journal Name: International Journal of Cancer and Treatment
Article Type: Research Methods
Received date: 12 March, 2021
Accepted date: 19 April, 2021
Published date: 26 April, 2021
Citation: Suganyadevi P, Saravanakumar M, Mohandas S (2021) Effects of two Isolated Compounds from Red Sorghum Bran on p53 and Bcl-2 Gene Expression in Human Breast Cancer Cell Line (MCF-7). Int J Cancer Treat Vol: 4, Issu: 1 (01-07).
Copyright: © 2021 Suganyadevi P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aims: The aim of our research was to isolate anthocyanin from red sorghum bran and to study its effects on p53 and bcl2 gene expressions in Human Breast cancer cell line (MCF-7).
Main methods: Two compounds were isolated from red sorghum bran by using Sephadex LH-20 column chromatography. The antiproliferative activity of isolated compounds were analysed by MTT assay and the expression studies were performed by RT-PCR analysis.
Key findings: Our research focusing on apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside another compound as a potention anticancer agent have demonstrated that the compounds isolated from red sorghum bran inhibited breast cancer cell lines by up regulation of P53 gene expression and down regulation of Bcl-2 gene expression.
Significance: In conclusion, the two compounds isolated from red sorghum bran, were effective in up regulation of P53 and down regulation of Bcl-2 gene expression.
Keywords: Apigenin-5-β-D-glucopyranoside, MCF – 7 cell line, Red sorghum bran.
Abstract
Aims: The aim of our research was to isolate anthocyanin from red sorghum bran and to study its effects on p53 and bcl2 gene expressions in Human Breast cancer cell line (MCF-7).
Main methods: Two compounds were isolated from red sorghum bran by using Sephadex LH-20 column chromatography. The antiproliferative activity of isolated compounds were analysed by MTT assay and the expression studies were performed by RT-PCR analysis.
Key findings: Our research focusing on apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside another compound as a potention anticancer agent have demonstrated that the compounds isolated from red sorghum bran inhibited breast cancer cell lines by up regulation of P53 gene expression and down regulation of Bcl-2 gene expression.
Significance: In conclusion, the two compounds isolated from red sorghum bran, were effective in up regulation of P53 and down regulation of Bcl-2 gene expression.
Keywords: Apigenin-5-β-D-glucopyranoside, MCF – 7 cell line, Red sorghum bran.
Introduction
Consumption of fruits and vegetables that are adundant in flavonoids has been associated with reduced incidence of many form of cancer [1,2]. These agents are universally present in fruits, vegetables, grains, nuts, tea and fruits. Anthocyanins occur ubiquitously in the plant kingdom and confer the bright red, blue and purple colors to fruits and vegatables. Pure anthocyanins and anthocyanin - rich extracts from fruits and vegatables have exhibited anti-proliferative activity towards multiple cancer cell types in vitro. One of these promising properties is the perturbation of the cell – cycle progression. Research in our laboratory has been focused on apigenin-5-β-D-glucopyranoside [3]. Apigenin-5-β-Dglucopyranoside is a flavones that is widely distributed in many fruits, vegetables and grains. The mechanism(s) for the selective effect of the anthocyanins on the growth of cancer cell against normal cells is/ are not known. Although anthocyanins have attracted much attention for their possible benefits, little is known regarding their anti - cancer action.
In the present study, the antiproliferative activity of purified anthocyanin compounds isolated from red sorghum bran and its effects on p53 and bcl-2 gene expressions in Human Breast cancer cell line (MCF-7) was determined.
Methods Section
Samples
The Sorghum bicolor (L.), red sorghum bran was collected from the village area (Coimbatore district, Tamil Nadu, India). The plant was identified and authenticated (No. BSI/SRC/5/23/2011-12/Tech.1486) by Botanical survey of India (BSI), Tamil Nadu Agricultural University (TNAU), Coimbatore, India. The bran was collected and was stored at-20°C.
Sample extraction
The bran of red sorghum (200g) was extracted by incubation with 1% Hydrochloric acid in methanol. Overnight at room temperature, followed by a filtration through whatman filter paper no.4. Methanol was removed by a rotary evaporation under 35°C and the pigmented fraction extracts were stored for a further study.
Isolation of anthocyanin
The concentrated filtrates were then applied onto an amperlite XAD -7 resin column (Sigma Aldrich, Missouri, USA) (1.5 x 40 cm), and washed with distilled water, followed by an elution with 1% Trifluoroacetic acid in methanol [4,5]. The elution was evaporated again after being collected by a fraction collector. Isolated anthocyanins were analysed on a UV-Visible spectrophotometer (Genesys 5 Technical trade links pvt.ltd). The fractions with the highest absorbance at 480nm were pooled and evaporated to remove methanol and dried under vaccum at 35°C.
Purification of anthocyanin
5 ml of concentrated isolated methanolic pigments were fractioned by Sephadex LH-20 column (100 cm x 2.6 cm, sigma, USA) using water(0.1% TFA)- Methanol(40:60, v/v) as eluent (4,5). The flow rate was 0.3 mL/min. Fractions were collected by a fraction collector. The fractions were evaporated on a rotary evaporator to remove methanol to facilitate the removal of water remaining in the sample. The evaporation temperature was less than 38⁰C. The homogeneity of individual fractions was checked by HPLC analysis. The lyophilized sample was used for expression studies.
High – performance liquid chromatography
The anthocyanin samples was diluted accordingly with methanol and HPLC analysis was carried out using a liquid chromatography system (Shimadzu, Kyoto, Japan) with a Photodidode array detector. The Detection was carried at 480 nm. The flow rate was maintained at 1.0ml/min. A Reverse Phase C18 column having a particle size of 5.0 μm was used for HPLC analysis of anthocyanins. The sample injection was 20 μl. A gradient mobile phase was used for elution : elution A was 10% Formic acid in water and B was Acetonitrile/ water/formic acid (5:4:1). Apigenin-5-β-D-glucopyranoside was used as a standard.
Cell lines and culture medium
MCF-7 (Human, Breast cancer cell line) was cultured in DMEM supplemented with 10% heat inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/ml), streptomycin (100 µg/ml) and amphotericin B (5 µg/ml) in an humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25cm² culture flasks and all experiments were carried out in 96 microtitre plates (Tarsons India Pvt. Ltd., Kolkata, India).
Preparation of test solutions: For cytotoxicity studies, each weighed test drugs were separately dissolved in distilled DMSO and volume was made up with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 1 mg/ ml concentration and sterilized by filtration. Serial two fold dilutions were prepared from this for carrying out cytotoxic studies.
Determination of cell viability by MTT Assay: Principle: The ability of the cells to survive a toxic insult has been the basis of most cytotoxicity assays. This assay is based on the assumption that dead cells or their products do not reduce tetrazolium. The assay depends both on the number of cells present and on the mitochondrial activity per cell. The principle involved is the cleavage of tetrazolium salt 3-(4, 5 dimethyl thiazole-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) into a blue coloured product (formazan) by mitochondrial enzyme succinate dehydrogenase. The number of cells was found to be proportional to the extent of formazan production by the cells used Francis and Rita [6]. Procedure:The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 x 105 cells/ml using DMEM containing 10% FBS. To each well of the 96 well microtitre plate, 0.1 ml of the diluted cell suspension (approximately 10,000 cells) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100 µl of different test concentrations of test drugs were added on to the partial monolayer in microtitre plates. The plates were then incubated at 37°C for 3 days in 5% CO2 atmosphere, and microscopic examination was carried out and observations were noted every 24 h interval. After 72 h, the drug solutions in the wells were discarded and 50 µl of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100 µl of propanol was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (CTC50) values is generated from the dose-response curves for each cell line.
RT-PCR for p53 and Bcl-2 gene expression.
1 X 106 MCF-7 cells were plated per well of 6 well tissue culture plate and incubated at 37°C/5% CO2 overnight. These cells were treated with 20 mg/ ml of the test sample dissolved in DMEM containing 10% FBS. As a negative control DMEM containing 10% FBS was used and as a positive control cells were treated with 5µg/ml Doxorubicin hydrochloride. Plates were incubated for further 48 hours. Total RNA was isolated from these cells and mRNA expression levels of p53 and BCl-2 were carried out using semi quantitative reverse transcriptase polymerase chain reaction (RT-PCR).
Reverse transcription (RT)
Reverse transcription of total RNA from MCF-7 control, MCF-7 cells treated with 20 mg/ml samples A (Luteolin-5- β-D-glucopyranoside), B (apigenin-5-β-D-glucopyranoside), and C (Control with out samples, Negative control) and MCF7 cells treated with 5µg/ml Doxorubicin (Positive control) were performed separately using 200 units of RevertAid™ M-MuLV Reverse Transcriptase (Fermentas Life Sciences).
Polymerase Chain reaction (PCR)
PCR For β-actin: The sequences of the primers used for β-actin are as follows: Forward: 5’-GTGGGGCGCCCCCAGGCACCA -3’; Reverse: 5’- CTCCTTAATGTCACGCACGATTTC-3’. The amplification was performed in a Master cycler® Thermocycler (Eppendorf, Germany) using the following program. Initial denaturation of 94°C for 2 minutes followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 64°C for 1 min and extension at 72°C for 1.2 min and terminated after final extension of 72°C for 8 min. Expected size of product: 540 bp.
The sequences of the primers used for p53 are as follows: Forward: 5 ’ - C TG AG GT TG G C TC TG AC TGTAC C AC C ATC C - 3 ’ ; Reverse: 5’-CTCATTCAGCTCGGAACATCTCGAAGCG -3’. The amplification was performed in a Master cycler® Thermocycler (Eppendorf, Germany) using the following program. Initial denaturation of 94°C for 2 minutes followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 69°C for 1 min and extension at 72°C for 1.2 min and terminated after final extension of 7°C for 8 min. Expected size of product: 371 bp.
The sequences of the primers used for Bcl-2 are as follows: Forward: 5’- GTTCGGTGGGGTCATGTGTGTGGAGA-3’; Reverse: 5’- GCTGATTCGACGTTTTGCCTGAAGAC-3’. The amplification was performed in a Master cycler® Thermocycler (Eppendorf, Germany) using the following program. Initial denaturation of 94°C for 2 minutes followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 64°C for 1 min and extension at 72°C for 1.2 min and terminated after final extension of 72°for 8 min. Expected size of product: 462 bp.
Results
Isolation and purification
In red sorghum bran, many components such as sugar, acid and salts occur except for anthocyanins. To better isolate anthocyanins from the red sorghum bran, the crude extract generally was passed through Amberlite XAD – 7 resin column exhibited a high spectral characteristics. The isolated anthocyanins were further purified through sephadex LH 20 column and two compounds were isolated from red sorghum bran anthocyanin. The isolated two compounds luteolin-5-βD-glucopyranoside and apigenin-5-β-D-glucopyranoside-5- β-D-glucopyranoside was taken for invitro cytotoxicity assay and expression studies in MCF – 7 cell line.
HPLC analysis
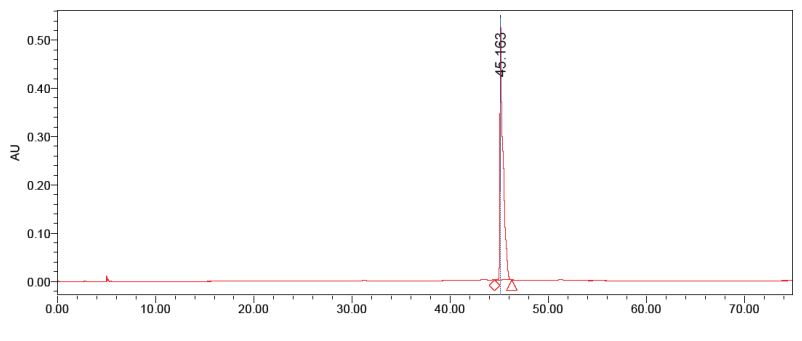
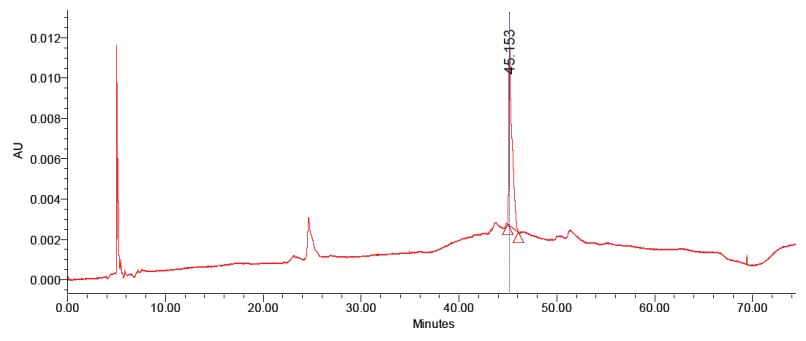
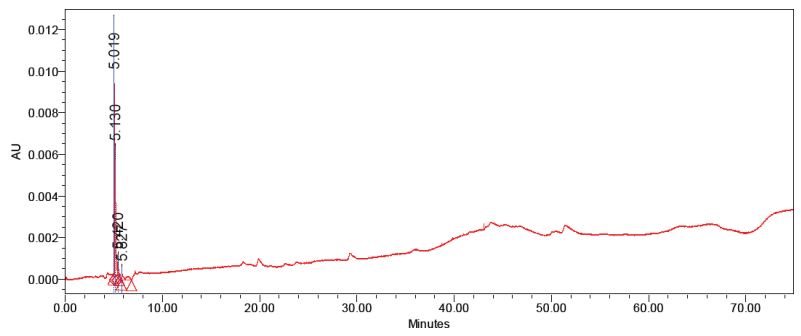
The purified anthocyanins were checked for homogeneity with the standard apigenin-5-β-D-glucopyranoside. The second fraction matches with the standard apigenin-5-βD-glucopyranoside with the same retention time (Figure 1,2). The first fraction doesn’t match with the standard (Figure 3). Thus the data indicate that two compounds was able to isolate from red sorghum bran by the combination of Amberlite XAD-7 and Sephadex LH – 20 column chromatography.
Figure 1: HPLC analysis of standard apigenin.
Figure 2: HPLC analysis of purified apigenin-5-β-D-glucopyranoside isolated from red sorghum bran by sephadex LH 20 column.
Figure 3: HPLC analysis of purified luteolin-5-β-D-glucopyranoside isolated from red sorghum bran by sephadex LH 20 column.
Invitro cytotoxity assay
There was no significant cytotoxicity observed even at 20 mg/ml concentration by MTT assay. This could be due to the presence of color to the sample which interferes with the reading at 570 nm.
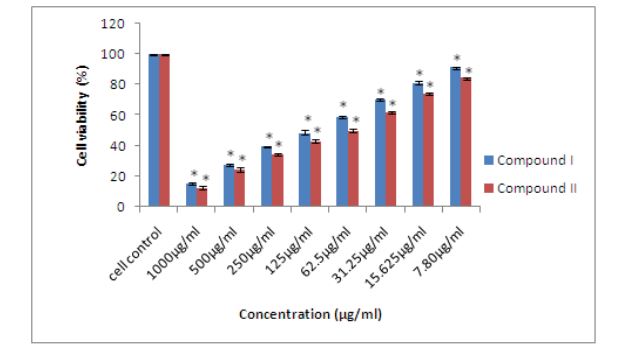
Effect of compound I and II isolated from red sorghum bran anthocyanins on MCF-7 cells proliferation evaluated by MTT assay: The effect of compound I (apigenin5-β-D-glucopyranoside) and compound II (luteolin-5- β-D-glucopyranoside) isolated from red sorghum bran anthocyanins on MCF-7 cells proliferation evaluated by MTT assay. The compound I (apigenin-5-β-D-glucopyranoside) showed 90% inhibition and compound II (luteolin-5-β-Dglucopyranoside) showed 93% inhibition at 1000 µg/ml (Figure 4).
The DNA fragmentation analysis of oligonuceosomalsized fragments in the MCF-7 cells treated with red sorghum bran anthocyanins: The apoptotic studies of anthocyanin on MCF – 7 cell showed oligonuceosomalsized fragments in the MCF-7 cells even very less concentration of 62.5 µg/ml.
Effects of purified compounds on p53 and bcl-2 gene expression in human breast cancer cell line (MCF-7)
Expression of p53 gene in MCF-7 cell line: p53 protein is essential for check point control that arrests Human cells with damaged DNA in G1 phase of cell cycle. p53 also leads to induction of proteins that promotes apoptosis.
In this study of P53 gene expression, the MCF-7 cells were treated with 20mg/ml of apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside isolated from red sorghum bran. Our results indicated that the expression of p53 increased after one hr incubation, and were maintained at a high level over 48 hr of treatment with 20 mg/ml of apigenin-5-β-D-glucopyranoside and luteolin-5-β-Dglucopyranoside isolated from red sorghum bran.
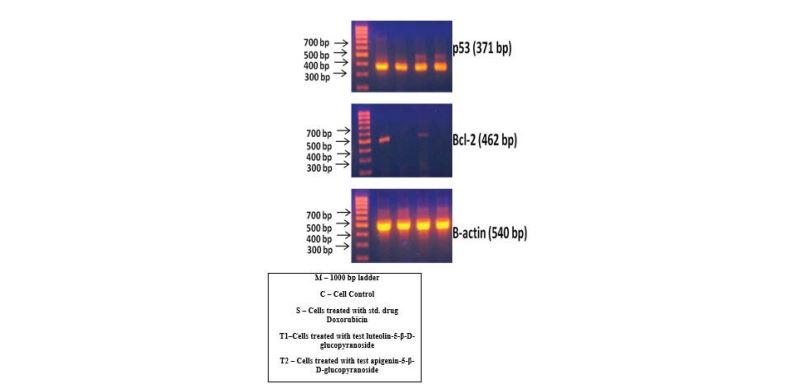
Expression of p53 gene was investigated by RT-PCR in untreated and treated MCF-7 cells. Quantitation of each band was performed by densitometry analysis software and the results expressed as the ratio of p53/β – actin in percent to the medium (control). Doxorudicin hydrochloride (DOX) was used positive control. As shown in figure 5 and table 1 in comparision to the medium control, the p53 gene expression is increased in the presence of apigenin-5-β-Dglucopyranoside and luteolin-5-β-D-glucopyranoside.
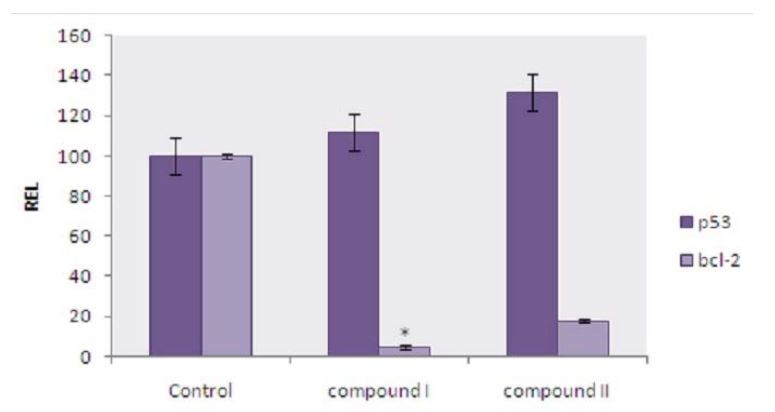
In table 1, the % to medium in p53 (medium)( -ve control) is 100, in p53 (DOX) (+ve control) is 112.32, in p53 (sample A) is luteolin-5-β-D-glucopyranoside the % to medium is 111.7, but in p53 (sample B) Apigenin-5-β-D-glucopyranoside, the % to medium is 131.36. In such cases the apigenin-5-β-Dglucopyranoside value was higher than the + ve control Doxorubicin hydrochloride, which shows the p53 gene expression was upregulated when treated with Apigenin5-β-D-glucopyranoside in MCF-7 cells than luteolin-5-β-Dglucopyranoside. So, Apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside significantly upregulates the p53 gene expression.
Expression of Bcl2 gene in MCF-7 cell line: Bcl-2 protein function is to suppress apoptosis or promotes cell survival. In our study Bcl-2 gene expression, MCF – 7 cells were treated with 20 mg/ml.
In this study of Bcl-2gene expression, the MCF-7 cells were treated with 20mg/ml of apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside isolated from red sorghum bran. Our results indicated that the expression of Bcl-2 markedly increased after one hr incubation, and were maintained at a high level over 48 hr of treatment with 20 mg/ml of apigenin-5-β-D-glucopyranoside and luteolin-5-βD-glucopyranoside isolated from red sorghum bran.
In table 1, the % to medium in Bcl 2 (medium)( -ve control) is 100, in Bcl-2 (DOX) (+ve control) is 9.15, in Bcl-2 (sample A) is luteolin-5-β-D-glucopyranoside the % to medium is 17.64, but in Bcl 2 (sample B) Apigenin-5-β-D-glucopyranoside, the % to medium is 4.7. In such cases the apigenin-5-β-Dglucopyranoside value was lower than the + ve control Doxorubicin hydrochloride, which shows the Bcl-2 gene expression was downregulated when treated with apigenin5-β-D-glucopyranoside in MCF-7 cells than luteolin-5-β-Dglucopyranoside. So apigenin-5-β-D-glucopyranoside and luteolin-5-β-D-glucopyranoside significantly down regulates the Bcl-2 gene expression by at least 50 -70% .
The expression of p53 and bcl-2 gene in MCF-7 cells was first determined by RT-PCR after incubation with luteolin-5- β-D-glucopyranoside and apigenin-5-β-D-glucopyranoside. An increase in p53 gene expression was observed after incubation with luteolin-5-β-D-glucopyranoside and apigenin-5-β-D-glucopyranoside when compared with the control. In the case of effect of bcl-2 gene expression on MCF-7 cells reduced bcl-2 gene expression was observed after incubation with luteolin-5-β-D-glucopyranoside and apigenin-5-β-D-glucopyranoside. The relative gene expression level; REL was normalized and compared to the expression of β-actin (Figure 6).
From the results it was found that the effect of Apigenin5-β-D-glucopyranoside on the p53 & Bcl-2 seem to be even more active than the doxorubicin (Figure 5,6) and the MTT assay also shows that at 1000 µg/ml (Figure 4) 90% inhibition was observed.
Figure 4: Effect of compound I and II isolated from red sorghum bran anthocyanins on MCF-7 cells proliferation evaluated by MTT assay. Error bars represent as Mean ± standard error (n=3). * indicates significantly different at (p < 0.05) from the control analyzed by Duncan’s multiple range test.
Figure 5:Agarose gel electrophoresis of RT-PCR products of Luteolin-5-β-D-glucopyranoside and Apigenin-5-β-D-glucopyranoside isolated from red sorghum bran on p53 and bcl-2 gene expression on breast cancer (MCF -7) cell line.The effects of Sorghum anthocyanins on the expression of p53, bcl-2 and β-actin in MCF-7 cells. The cells were incubated with sorghum anthocyanins for 48 hrs and the total RNA was isolated and reverse transcribed. The resulting cDNAs were subjected to PCR with the indicated primers and the reaction products were subjected to electrophoresis in 1% agarose gel and visualized by EtBr staining. Doxorubicin was used as the internal control.
| S.No | Gene (treatment) | Ratio to β-actin | % to Medium |
|---|---|---|---|
| 1 | p53 (Medium) | 0.8526 | 100 |
| 2 | p53 (Dox) | 0.9576 | 112.32 |
| 3 | p53 (Luteolin-5-β-D-glucopranoside) | 0.9524 | 111.7 |
| 4 | p53 (Apigenin-5-β-D-glucopyranoside) | 1.112 | 131.36 |
| 5 | bcl-2 (Medium) | 0.306 | 100 |
| 6 | bcl-2 (Dox) | 0.028 | 9.15 |
| 7 | bcl-2 (Luteolin-5-β-D-glucopyranoside) | 0.054 | 17.64 |
| 8 | bcl-2 (Apigenin-5-β-D-glucopyranoside) | 0.0144 | 4.7 |
Table 1: Showing the quantitative analysis of RT PCR analysis of Luteolin5-β-D-glucopyranoside and Apigenin-5-β-D-glucopyranoside isolated from red sorghum bran on p53 and bcl-2 gene expression on breast cancer cell line. Showing the quantitative analysis of RT PCR analysis of Luteolin-5-βD-glucopyranoside and Apigenin-5-β-D-glucopyranoside isolated from red sorghum bran on p53 and bcl-2 gene expression on breast cancer cell line.
Figure 6:showing the graphical representation of Apigenin-5-β-D-glucopyranoside (compound I) and Luteolin-5-β-D-glucopyranoside (compound II) isolated from red sorghum bran on p53 and bcl-2 gene expression on breast cancer (MCF -7) cell line.Error bars represent as Mean ± standard error (n=3). * indicates significantly different at (p < 0.05) from the control analyzed by Duncan’s multiple range test.
Discussion
Suganyadevi et.al., [7], already reported the presence of apigenin and leutolin in crude sorghum bran anthocyanin extract. Apigenin was an antimutagenic against nitropyrene – induced genetoxicity in Chinese hamster ovary cells and inhibited mitogen – activated protein kinase and the down stream oncogenes in √ - H – ras – transformed NIH 3T3 cells [8]. Suganyadevi et al., [9], reported that the crude red sorghum bran anthocyanin upregulates the expression of p53 and also down regulates the expression of bcl-2 gene in MCF-7 cell lines. Dietary apigenin inhibited tumour necrosis factor – induced intercellular adhesion molecule -1 upregulation [10].
Suganyadevi et al., [9], reported the antiproliferative activity of crude 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran showed 90% inhibition in breast cancer cell lines.
The mechanisms by which apigenin-5-β-Dglucopyranoside protected against skin carcinogensis in animal models seem to be related to the induction of cell cycle arrest at the G2/M and /or Go/G1 phase [11,12]. Recently we demonstrated that apigenin-5-β-D-glucopyranoside inhibited the growth of three Human colon carcinoma cell lines in association with a reversible G2/M cell – cycle arrest [13]. That arrest was associated with decreased activity of p34 cdc2 kinase and reduced accumulation of p34 cdc2 and cyclin B1 proteins [14].
Conclusion
Flavonoids are a major class of non nutritive components of plant – derived diets, and they are widely distributed in plant – based foods and have been suggested to play a role in cancer prevention. Current studies in our laboratory focusing on apigenin-5-β-D-glucopyranoside and luteolin5-β-D-glucopyranoside as a potention anticancer agent have demonstrated that the compounds isolated from red sorghum bran inhibited breast cancer cell lines by upregulation of p53 gene expression and down regulation of Bcl-2 gene expression. Among the two compounds isolated from red sorghum bran, the apigenin-5-β-D-glucopyranoside was more effective in upregualtion of p53 and down regulation of Bcl-2 gene expression.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Supporting Data
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Funding
This work was supported by Department of science and technology, New Delhi, India (Grant No: SR/WOS-A/LS265/2010) under Women Scientist Scheme.
Authors’ Contribution
All authors read and approved the final manuscript.
Acknowledgment
I acknowledge DST (Department of Science and Technology, New Delhi, India) for supporting the grant (Grant No: SR/WOS-A/LS-265/2010) under Women Scientist Scheme
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90: 157 -177.[ Ref ]
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: Chemistry, metabolism and structure – activity relationships. J Nutr Biochem 13: 572-574.[ Ref ]
Birt DF, Shull JS, Yaktine AL (1998) Chemoprevention of cancer. Modern Nutrition in Health and Disease. Williams & Wilkins, Baltimore. [ Ref ]
Fossen T, Slimestad R, Andersen OM (2003) Anthocyanins with 4’-glucosidation from red onion, Allium cepa. Phytochemistry 64: 1367-1374.[ Ref ]
Fossen T, Anderson OM (1998) Cyanidin 3-O-(6″-Succinyl-βglucopyranoside) and other anthocyanins from phragmites australis. Phytochemistry 49: 1065-1068.[ Ref ]
Francis D, Rita L (1986) Rapid colorometric assay for cell growth and survival modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods 89: 271-277.[ Ref ]
Suganyadevi P, Saravanakumar M, Mohandas S (2011) Identification of 3-deoxyanthocyanin from red sorghum (Sorghum bicolor) bran and its biological properties. African Journal of Pure and Applied Chemistry 5:181-193.[ Ref ]
Kuo ML, Yang NC (1995) Reversion of v-H-RAS- transformed NIH 3T3 cells by apigenin-5-β-D-glucopyranoside through inhibiting mitogen active protein kinase and its downstream oncogenes. Biochem Biophys Res Commun 212: 767-775.[ Ref ]
Suganyadevi P, Saravanakumar M, Mohandas S (2013) The antiproliferative activity of 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran through p53 – dependent and Bcl-2 gene expression in breast cancer cell lines. Life Sciences 92: 379-382.[ Ref ]
Kuo M, Lee K, Lin J (1992) Genotoxicities of nitropyrenes and their modulation by apigenin-5-β-D-glucopyranoside, tannic acid, ellagic acid and indole – 3- carbinol in the Salmonella and CHO systems. Mutat Res 270: 87-95[ Ref ]
Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ et al. (2000) Cell cycle arrest at G2/M and growth inhibition by apigenin-5-β-D-glucopyranoside in human colon carcinoma cell lines. Mol Carcinog 28: 102-110.[ Ref ]
Panes J, Gerritsen ME, Anderson DC, Miyasaka M, Granger DN (1996) Apigenin-5-β-D-glucopyranoside inhibits tumor necrosis factor – induced intercellular adhesion molecular -1 upregulation in vivo. Microcirculation, 3: 279-286[ Ref ]
Lepley DM, Li B, Birt DF, Pelling JC (1996) The chemopreventive flavonoid apigenin-5-β-D-glucopyranoside induces G2/M arrest in keratinocytes. Carcinogenesis 17: 2367-2375.[ Ref ]
Lepley DM, Pelling JC (1997) Induction of p21/WAF1 and G1 cell cycle arrest by the chemopreventive agent apigenin-5-β-D-glucopyranoside. Mol Carcinog 19: 74-82.[ Ref ]