Journal Name: International Journal of Cancer and Treatment
Article Type: Case Report
Received date: 10 October, 2018
Accepted date: 23 October, 2018
Published date: 26 October, 2018
Citation: Hanine N, Bouahafa T, Sbai A, Mezouar L, Hassouni K (2018) Malignant Mullerian Endometrial Tumor After Tamoxifen for Breast Cancer. Int J Cancer Tremnt. Vol: 1, Issu: 1 (43-46).
Copyright: © 2018 Hanine N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Tamoxifen is an antiestrogen widely used in the treatment of breast cancer. Its paradoxical antiestrogenic action on the breast and its estrogenic action on the endometrium is well known. Some rare and pejorative histological forms such as mixed Mullerian tumors characterized by their dual epithelial and mesenchymal proliferative contingents are observed following prolonged administration of tamoxifen. Since 1988, there have been some cases of malignant mixed Mullerian tumors after tamoxifen. We report a new case of mixed Mullerian uterine malignancy four years after tamoxifen for breast cancer.
Keywords
Breast cancer, Tamoxifen, Mixed Mullerian tumor, Therapy with aromatase inhibitors.
Abstract
Tamoxifen is an antiestrogen widely used in the treatment of breast cancer. Its paradoxical antiestrogenic action on the breast and its estrogenic action on the endometrium is well known. Some rare and pejorative histological forms such as mixed Mullerian tumors characterized by their dual epithelial and mesenchymal proliferative contingents are observed following prolonged administration of tamoxifen. Since 1988, there have been some cases of malignant mixed Mullerian tumors after tamoxifen. We report a new case of mixed Mullerian uterine malignancy four years after tamoxifen for breast cancer.
Keywords
Breast cancer, Tamoxifen, Mixed Mullerian tumor, Therapy with aromatase inhibitors.
Introduction
Carcinosarcoma or mixed Mullerian tumor (TMM) is a rare and aggressive malignant tumor of the endometrium. Its incidence is estimated between 5-7% of malignant tumors of the endometrium and 1-3% of gynecological tumors (2,3). TMM occurs most often in postmenopausal women, with an average age of 65 years. This pathology developed from the endometrium most often concerns women coming for postmenopausal metrorrhagia [1-3]. The prognosis is often poor, with a five-year survival rate estimated between 15 and 50%.
There are two histological types of TMM tumors [1]. On the one hand, those whose sarcomatous component develops from natural elements of the uterus, they are called TMM homogeneous; On the other hand, those whose sarcomatous component contains elements from other origins (cartilage, skeletal muscle, etc.), these are called heterogeneous TMM and have a poorer prognosis.
Adjuvant tamoxifen therapy in postmenopausal women after breast cancer surgery has been shown to be effective in increasing the recurrence-free survival rate and decreasing the rate of local recurrence and recurrence. contralateral breast. However, prolonged exposure to tamoxifen appears to be responsible for the onset of malignant mixed malignant tumors of the uterus. The very first case was described in 1988 and since then only 65 cases have been reported in the literature [4].
Clinical Case
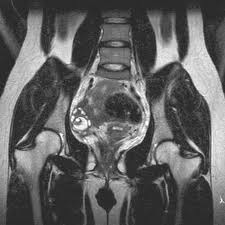
Mrs. T.K, aged 47, fifth gesture third par, still regulated; comes to consult for metrorrhagia. The family history is without particularity. The personal history is marked by breast cancer discovered in 2009, which was treated by mastectomy axillary dissection, followed by chemotherapy, radiotherapy and tamoxifen hormone therapy (20 mg/d) for 4 years (arrested for three years). Clinically the general condition was good the abdomen was supple the uterus was increased in volume. The rest of the exam was normal. The patient underwent a biopsy curettage of the endometrium, the histology of which concluded that there was a malignant mixed malignant pelvic tumor. Pelvic MRI evoked significant heterogeneous endometrial thickening with more than 50% invasion of the myometrium and discontinuation of the junctional zone in some places (Figure 1).
Figure 1: Frontal cut of a T2-weighted pelvic MRI showing heterogeneous thickening of the endometrium.
After oncogynecological consultation, it was decided to adopt the following protocol: surgery followed by radiotherapy; chemotherapy was retained after radiotherapy; but the patient refused chemotherapy.
Hysterectomy without preservation of the appendages by laparotomy, bilateral pelvic lymphadenectomy, peritoneal and epiploic biopsy, and peritoneal cytology were performed. Histology of the operative specimens concluded that carcinosarcoma (homologous mixed Mullerian tumor, partially infiltrating the myometrium (less than 50%, 1cm/3cm). There was a carcinomatous component of grade 2 and a sarcomatous component of: grade 3. The parameters, cervix, ovaries, fallopian tubes were not infiltrated. There were no vascular emboli or perineurous infiltration. There were no lymph node metastases on 11 lymph nodes removed. The immunohistochemical study showed a mixed Mullerian endometrial tumor, the sarcomatoid component of which is H-caldesmon-and PS 100.
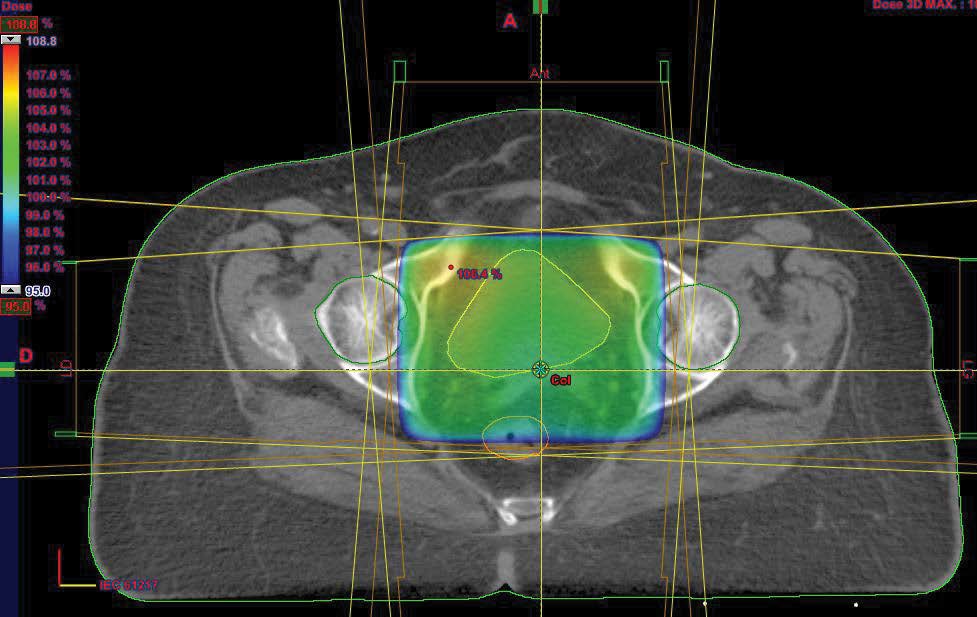
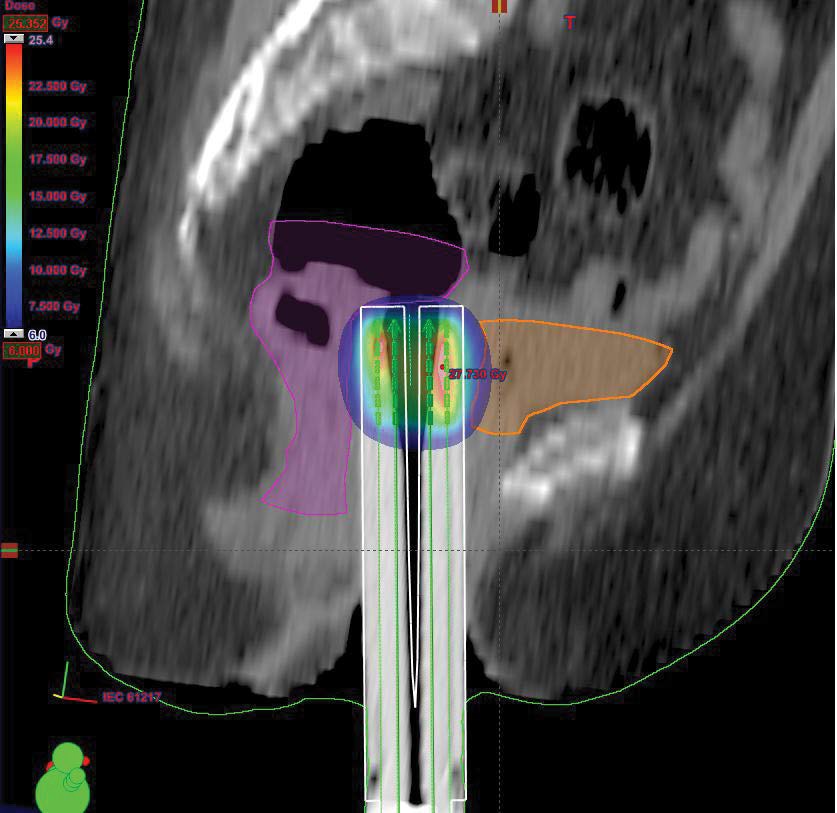
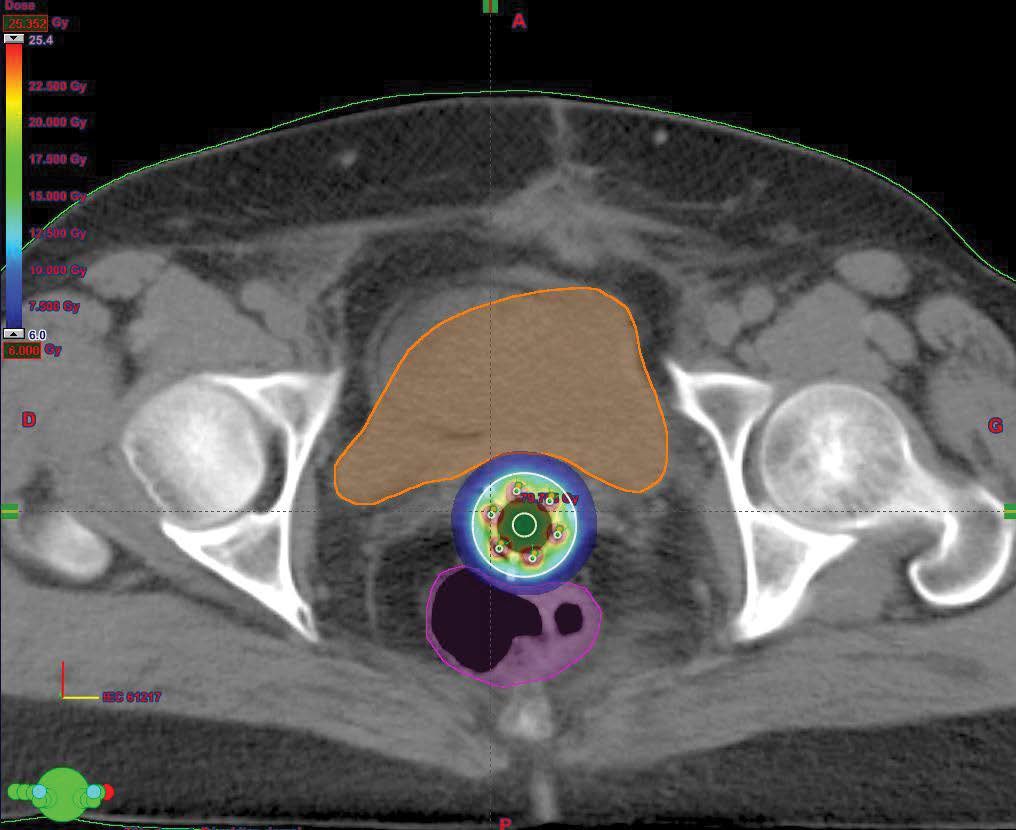
External pelvic radiotherapy was performed at a total dose of 46Gy; 2Gy/Fr in 23 sessions (Figure 2); followed by two 5Gy barrier brachytherapy sessions (Figure 3,4). Three months after the end of the treatment, the clinical examination, the thoraco-abdominopelvic CT scan were normal. During the quarterly controls, the patient is still in good locoregional control and at a distance from the disease.
Figure 2: Axial cut of optimization of dosimetry in external radiotherapy.
Figure 3: 3D dosimetry of sagittal arch applicator brachytherapy Miami.
Figure 4: 3D dosimetry of a Miami applicator barrier brachytherapy in axial section.
Discussion
Tamoxifen is a molecule developed in 1966 in the United Kingdom by Imperial Chemical Industries [5]. At first, this molecule was used in the context of contraception, for its antiestrogenic effect [5]. Paradoxically, it was later discovered a role in the treatment of infertility by anovulation. Finally, for years, tamoxifen has become very important in the adjunctive treatment of breast cancer [6]. It is considered the standard adjuvant treatment for hormone receptor-positive breast cancer in premenopausal women. In fact, it has been shown that treatment with breast cancer with tamoxifen for five years reduces the risk of annual cancer recurrence and mortality by 41% and 34%, respectively [7].
However, data from the literature have demonstrated paradoxical effects and estrogen agonists on the female genital tract [1-3,5] leading to the appearance of endometrial polyps, hyperplasia and endometrial cancers. There is no strong case for linking tamoxifen therapy to uterine carcinosarcoma because the mechanism of the oncogenic effect of tamoxifen is not yet clear [8]. However, it is possible that the lesion begins at the endometrial stroma. Due to probable stroma-epithelial interactions in the hormonal modulation of endometrial cell growth, the mechanisms involved in autocrine and paracrine growth factors, from adjacent stromal cells, are probably associated in the pathogenesis of endometrial cancer [1].
The epithelial and mesenchymal components share a common embryologic origin, which may explain why tamoxifen may result in not only an increased risk of endometrial cancer, but also a risk of malignant transformation of stromal cells.
The first case of mixed Mullerian tumor of the posttamoxifen uterus was described by Hardell [4] in 1988 and since then 65 cases have been reported in the literature [9]. The age of patients is between 40 and 90 years old with a peak at 65 years old and an average age of 66 years. All patients were postmenopausal, received an average of 20 mg tamoxifen daily with a cumulative dose ranging from 7 to 100 g, and duration of treatment ranged from three to 11 years, with an average duration of nine years [9,1].
In our case, the patient developed a uterine carcinosarcoma after four years of treatment with tamoxifen, at a dose of 20 mg/day. Given the benefit of tamoxifen in the adjunctive treatment of breast cancer and the rare incidence of uterine sarcoma, the involvement of tamoxifen in the development of uterine carcinosarcoma has not been clearly established. Cases are rare, which is probably due to the low incidence of uterine sarcomas, which account for 3-7% of cancers of the uterus. MccLuggage et al. [10] observed in their prospective study that tamoxifen exerts a weak estrogenic effect on the vagina and uterus of the postmenopausal woman without pre-existing endometrial pathology. Berlière et al. [11], for their part, considered that endometrial evaluation prior to tamoxifen treatment made it possible to identify patients with endometrial pathology.
Currently, there is an alternative to tamoxifen which is represented using anti-aromatases. Indeed, the normal or neoplastic breast expresses aromatase, which is the key enzyme of estrogen biosynthesis and is found in many tissues and not only in the reproductive organs. This enzyme is also found in pathological tissues, such as endometriosis, uterine fibroids or breast cancer. The recent availability of third-generation anti-aromatases has not only renewed. The value of hormone therapy in women with breast cancer, but in addition, they have been effective in menopausal women with advanced breast cancer and resistant to tamoxifen. As an anti-aromatase, exemestane inhibits the production of estrogens by preventing the proliferation of cancer cells. This treatment has proven effective in reducing the risk of breast cancer recurrence while reducing side effects. An international study on exemestane (Intergroup Exemestane Study) has recently been conducted through the Cancer Research Center at Hammersmith Hospital in London [7].
This study involved a randomized trial in 4724 postmenopausal women with unilateral, invasive and positive or undetermined estrogen receptor breast cancer. The authors of this trial concluded that the switch to exemestane, after two to three years of tamoxifen, improves breast cancer recurrence rate, resulting in a modest reduction in mortality with 222 deaths for patients treated with exemestane against 261 patients who continued with tamoxifen. As a result, there is good reason to include pelvic ultrasound in the pretreatment assessment before prescribing tamoxifen or an anti-aromatase. In the case of uterine ultrasound abnormalities, hysteroscopy with endometrial biopsy is necessary and if necessary supplemented with pelvic MRI. It is also essential to inform the patient of the risk of developing uterine carcinosarcoma.
There is no consensus on the management of carcinosarcoma [12] with respect to debulking surgery, radiotherapy and choice of chemotherapy agents. This consensual vacuum is due to the presence of malignant carcinomatous and sarcomatous elements in the same tumor and the scarcity of it. We propose a complete surgery of resection of the tumor followed by a radiotherapy and a chemotherapy with base of platinum salts. Radiation therapy can reduce the risk of local recurrence but does not have a major impact on survival [13].
Conclusion
Further studies are needed to evaluate a therapeutic strategy integrating surgery, radiotherapy, chemotherapy, as well as molecular therapy, in order to improve the prognosis of these patients, which currently remains dark with a high recurrence rate.
There is no references