Journal Name: International Journal of Cancer and Treatment
Article Type: Analysis
Received date: 05 July, 2021
Accepted date: 21 July, 2021
Published date: 28 July, 2021
Citation: He Z, Hu S, Cai X, He YY, Liu S (2021) Novel Predictive Role of Serum CA19-9 Identified in Colorectal Cancer. Int J Cancer Treat Vol: 4, Issu: 2 (01-10).
Copyright: © 2021 He Z et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
It is unclear that different primary tumor sites have originated and contributed to colorectal cancer (CRC). A total of 1,039 consecutive CRC patient profiles were collected and the predictive role of multiple biomarkers was investigated during the survival period. Correlation and survival analysis was used to explore the predictive value of clinical features (primary tumor sites, tumor subtypes and incidence rates) and multiple biomarkers. The incidence of terminal intestinal tumor site (sigmoid and rectum, 73%) was significantly higher than other primary tumor sites (27%). Patients with tubular adenocarcinoma, primarily originated from terminal intestinal tumor sites, have significantly lower 5-year survival rates, and shorter overall survival time. The serum level of CA19-9 was significantly positive correlated with sigmoid and rectum (Cor =0.88, p=2.2e16), and higher serum CA19-9 (>37 U/mL) was significantly associated with tubular adenocarcinoma. Therefore, CA19-9 could be a promising biomarker for the diagnosis of terminal colorectal cancer, especially in tubular adenocarcinomas originating from sigmoid and rectum, and contribute to improve survival outcomes.
Keywords: Colorectal cancer, tubular adenocarcinoma, CA19-9,Biomarker.
Abbreviations: CA19-9: Carbohydrate antigen 19-9; CRC: Colorectal Cancer; CEA: Carcinoembryonic antigen; CRP: C-reactive protein; ALB: Albumin; TNM: Classification of Malignant Tumors; OS: Overall Survival
Abstract
It is unclear that different primary tumor sites have originated and contributed to colorectal cancer (CRC). A total of 1,039 consecutive CRC patient profiles were collected and the predictive role of multiple biomarkers was investigated during the survival period. Correlation and survival analysis was used to explore the predictive value of clinical features (primary tumor sites, tumor subtypes and incidence rates) and multiple biomarkers. The incidence of terminal intestinal tumor site (sigmoid and rectum, 73%) was significantly higher than other primary tumor sites (27%). Patients with tubular adenocarcinoma, primarily originated from terminal intestinal tumor sites, have significantly lower 5-year survival rates, and shorter overall survival time. The serum level of CA19-9 was significantly positive correlated with sigmoid and rectum (Cor =0.88, p=2.2e16), and higher serum CA19-9 (>37 U/mL) was significantly associated with tubular adenocarcinoma. Therefore, CA19-9 could be a promising biomarker for the diagnosis of terminal colorectal cancer, especially in tubular adenocarcinomas originating from sigmoid and rectum, and contribute to improve survival outcomes.
Keywords: Colorectal cancer, tubular adenocarcinoma, CA19-9,Biomarker.
Abbreviations: CA19-9: Carbohydrate antigen 19-9; CRC: Colorectal Cancer; CEA: Carcinoembryonic antigen; CRP: C-reactive protein; ALB: Albumin; TNM: Classification of Malignant Tumors; OS: Overall Survival
Introduction
Colorectal cancer (CRC) is a global health threat to all populations in global. The incidence rate of CRC has increased rapidly, leading it to be commonly death-caused cancer in the world [1,2]. Particularly in the last ten years, CRC morbidity and mortality have increased to the top ten among many types of cancer in China [3]. CRC is treated as complex diseases: more than 90% of colorectal carcinomas are identified as adenocarcinomas originating from epithelial colorectal mucosa cells. Typically, intestinal subtypes of adenocarcinoma include tubular adenocarcinoma, mucinous adenocarcinoma, adenocarcinoma with necrosis, and mixed adenocarcinoma [4]. Tubular adenocarcinoma, with high cell density and rapid cell proliferation, is the dominant pathological type of CRC [5].
Beyond cell characterizations, these clinical features have been identified as potential indicators for predicting clinical outcomes, including age, gender and tumor stage, etc. The incidence rate and gender preference are varied with a wide difference between colorectal carcinomas, as evidenced by investigations in the western and eastern populations [3,6,7]. The majority (80%) of patients with colon cancer were age over 60-years and the incidence rate of male’s patients was higher than the female’s [6]. In addition, the primary tumor sites of the CRC indicated a different contribution to the overall survival of patients. CRC patients who have left-sided tumor metastasized (distal, splenic flexure to rectosigmoid) have a better survival rate than those whose right-side tumor metastasized (proximal, cecum to transverse). Similarly, patients with metastasized rectal cancer had a better clinical outcome than those with metastasized colon cancer [8]. However, the exact prognostic value of primary tumor sites has not been well established to predict the clinical outcome of the CRC in previous studies.
With the exception of clinical features, serum biomarkers have been widely used to predict clinical outcomes. Multiple biomarkers, including carcinoembryonic antigen (CEA), C-reactive protein (CRP) and carbohydrate antigen 19-9 (CA19-9), have been successfully translated into clinical practice in the assessment and management of CRC patients [9]. Among them, CEA was considered a promising prognosis biomarker for tumor progression and metastases in CRC and integrated into clinically applied patient monitoring [10]. The serum level of CRP was adopted as a potential biomarker for assessing the potential risk of colorectal cancer. However, it is still not clear that there is a positive correlation between the elevated CRP and CRC serum levels [11]. Serum CA19-9 is a well-known tumor biomarker used to screen and detect carcinomas in the digestive tract, and approximately 18% of CRC cases are associated with higher serum CA19-9 levels. Although CA19-9 has been used as a tumor biomarker in colon carcinoma for more than 40 years, particularly as a prognosis indicator of advanced stage and metastatic colorectal cancer, it was still controversial that the specificity and sensitivity of CA19-9 had been applied to CRC clinical screening [12-15].
In this study, over 1,000 clinical profiles of hospitalized CRC patients were collected in Wuxi, a local area in Jiangsu province of China. Multiple clinical attributes and biomarkers were associated with these CRC patient profiles. The potential accurate predictor role of these biomarkers were analyzed in colorectal cancer patients.
Methodology
Patient cases information collection
This retrospective cohort study included an evaluation of the patient’s medical records from the hospital database. It included 1,039 consecutive cases with surgical and pathological identification, representative of CRC patients between November 2006 and February 2014 through a surgical resection with curative intent for hospital assessment. This cohort included a total of 1006 cases with clear primary tumor site information and distributed as 566 males and 440 females. Serum levels of CA19-9, CRP, ALB (albumin) and CEA were identified in 334, 602, 602 and 602 cases, respectively.
Ethics approval and consent to participate
The scientific use of the patient profile was approved by the Ethics Committee of the Affiliated Hospital of the University of Jiangnan (Center No. 4321) and acquiesced to the requirement of informed consent (Dr. Shudong Hu worked as committee member and provided). In addition, the study was reviewed, discussed and approved by the Ethics Committee of Kunming Institute of Botany, Chinese Academy of Sciences, and the related Ethics Regulatory Rules followed.
Histopathological grade evaluation
Original histopathological slides were evaluated by gastrointestinal pathologists in the Department of Pathology. The pathologist reviewed the hematoxylin and eosin sections of each colorectal tumor and used well-established criteria to evaluate the pathological malignancy of the tumor. Tumor stage, tumor grade and tumor infiltration of cases were determined by the 7th edition of the TNM (Classification of Malignant Tumors) classification for differentiated colorectal carcinomas of the American Joint Committee on Cancer [16].
Detection of serum biomarkers
Serum biomarker detection (CA19-9, CEA, ALB, and CRP) was performed by standard preoperative protocol (within 2 weeks) prior to surgery. These biomarkers have been identified by the local pathological unit for all patients. Serum CA19-9 was measured with electrochemiluminescence immunoassay using the Roche Cobas E601 (Roche, Switzerland) immunoassay system. The serum level of CA19-9 below or equal to 37 U/mL was identified as a normal reference value (defined as level 1) and greater than 37 U/mL was determined as an abnormal value (defined as level 2). According to the hospital defined normal range, the threshold values for CEA were 0~5 µg/mL, CRP was 0~8 mg/L and ALB was 40~55 g/L.
Lymph node metastases detection
The involvement of lymph node metastases was diagnosed with PET-CT in all patients. The regional number of lymph nodes was identified and calculated on the basis of PET-CT images.
According to TNM standard, the patients with different lymph node metastases were defined as N0 (no nearby lymph nodes), N1(less than 4 nodes) and N2 (equal or more than 4 nodes) patients.
Statistical analysis
Statistical analysis was conducted with R software (version 3.6.1; http://www.Rproject.org) and GraphPad Prism 6 (GraphPad Software, Inc.). The Pearson correlation (r) was employed to measure a linear dependence correlation analysis between two variables of patient’s physiological indicators, and demonstrated as correlation Matrix. In the analysis, CA19-9 level below 37 U/mL, other primary tumor sites, early stage, infiltration tumor and normal indicators were defined as “1”; CA19-9 level over 37 U/mL, terminal tumor sites, advanced stage, non-infiltration tumor and abnormal were defined as “2”. The category study variable was used independent t test or one-way analysis of variance test. The overall survival probabilities were estimated by Kaplan-Meier method and compared using log-rank test. The statistical significance level was set as p value <0.05.
Results
Clinical characterization of cases
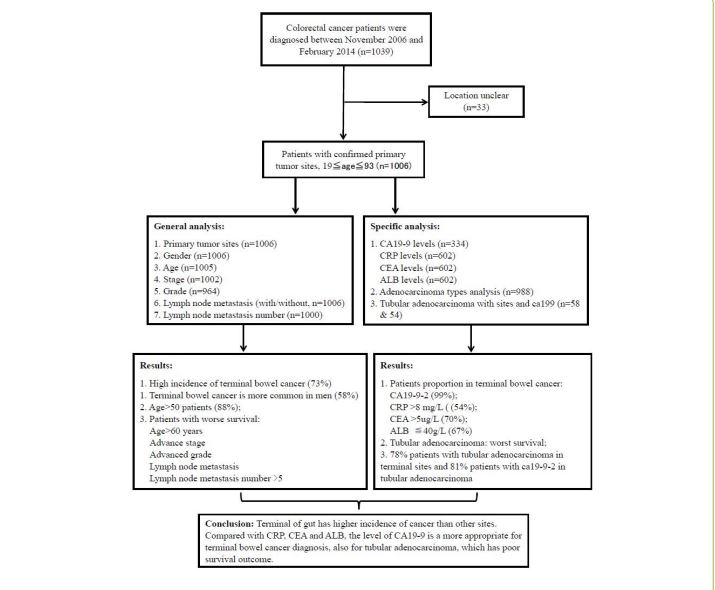
These original cases were selected from diagnosed CRC patients, and these cases with missing the key parameters or clinical markers were excluded for next step general and specific level analysis. The process and characteristic analyses were demonstrated by flow chart (Figure 1). Characterization of these cases was summarized, including the corresponding clinical characteristics, 5-year survival rate and distribution of primary tumors site (Table 1). First of all, the qualified patients were divided into four age groups (Table 1), including less than 50-years-age (124 cases, 12.3%), 50-60 years-age (273 cases, 27.1 %), 61~70 -years-age (326 cases, 32.4 %) and over 70-years-age group (282 cases, 28.0%). The majority of cases (882 cases, 87.7%, p<0.001) were significantly distributed in the group that older than 50-years-age (Figure S1A). The 5-year survival rate of patients over 60-years-age (median 55.5 months, 44%) was significantly lower than that of patients under 60-years-age [median 62 months, 52%, log rank p=0. 0004] (Figure S1B). It is proposed that the patients group that older than 60-year-old have higher risk of developing CRC and lower survival rates. There are no significant differences between gender groups in the five-year survival rate.
Characterization of tumor primary sites
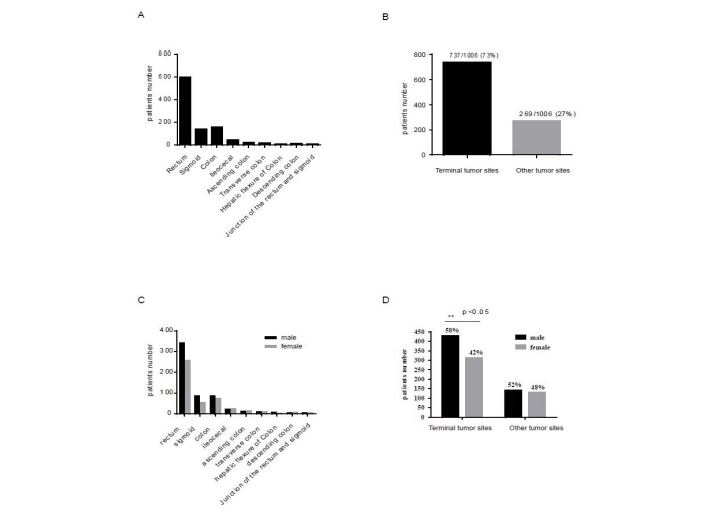
A total of 737 cases (73.3 %) were distributed at the terminal tumor site (median survival time = 57 months, 5-year survival rate = 48.4%), while other cases (n=269, 26.7 %) were allocated at other tumor sites (median survival time = 59 months and 5-year survival rate = 48.3%) (Table 1). Overall, nine primary tumor sites were identified as rectum (59%), sigmoid (14%), colon (15.7%), ileocecal (4.5 %), descending colon (1.1 %), hepatic flexure colon (0.8 %), ascending colon (2.4%), transverse colon (1.7 %), rectum junction and sigmoid (0.6 %). Rectum and sigmoid have been classified as terminal tumor sites, and with higher incidence rate compared to other tumor sites (Figure 2A,B) [17].
Combined gender bias with primary tumor site analysis, male patients (58%) had significantly higher incident rates than female patients (42%), particularly at terminal tumor sites. The distribution ratio for male patients (61 %, 86 cases; 57 %, 341 cases) is higher than for female patients (39 %, 54 cases; 43 %, 256 cases) with sigmoid and rectal cancer, respectively (Figure 2C,D). Furthermore, 74.8% of 50-70-year-old cases were spread to terminal tumor sites, while 25.2% of patients were assigned to other tumor sites. The diagnosis rate of early-stage terminal colorectal cancer (41.1%) was significantly higher than that of other primary tumor sites (31.2%) (Table 2), While the advanced stage of terminal colorectal cancer (58.9%) was significantly lower than that of other primary tumor sites (68.8 %). The OS and five-year survival rates of terminal tumor sites (sigmoid and rectum, 48.4%) were similar to other primary tumor sites (48.3 %).
In this study, the percentage of non-infiltrated tumor cases was significantly higher than that of infiltrated tumor cases. The OS of non-infiltrated tumor cases (mediansurvival time = 54 months, 40%, log rank p=2e-15, figure S4A) was significantly lower than that of tumor-infiltrated cases (median survival time = 67 months, 57%, p<0.01). The majority of non-infiltrated tumor cases were similarly distributed at terminal tumor sites (441/656, 67 %) and other tumor sites (163/229, 71 %), but 98 % of tubular adenocarcinoma cases were identified as non-infiltrated tumors (Figure S4B).
Figure 1:Workflow chart of selecting colorectal cancer patients with the primary tumor sites for category analysis and summary.
Figure 2:Characterization of colorectal cancer cases distribution with primary tumor sites. (A) Distribution of patients at each primary tumor sites (B) Distribution of patients to terminal and other tumor sites (C) Distribution of patients with each primary tumor sites in males and females (D) Distribution of terminal and other tumor sites in males and females.
| Tumor location | tumor location number | Gender | Age group (years) | CA19-9 levels | ALB | CRP | CEA | OS(mean,months) | 5-year survival rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | <50 | 50-60 | 61-70 | >70 | 1 | 2 | (≤40g/L)/All (%) | (>8mg/L)/All(%) | (>5ug/L)/All(%) | ||||
| Transverse colon | 17/1006(1.7%) | 8 | 9 | 4 | 7 | 3 | 3 | 2 | 0 | 3 | 3 | 3 | 57 | 7/17 (41%) |
| Ileocecal | 45/1006(4.5%) | 21 | 24 | 4 | 4 | 17 | 20 | 24 | 0 | 16 | 9 | 8 | 53 | 14/45 (31%) |
| Descending colon | 11/1006 (1.1%) | 4 | 7 | 3 | 3 | 1 | 4 | 0 | 4 | 2 | 2 | 3 | 54 | 4/11 (36%) |
| Colon | 63/1006 (6.3%) | 35 | 28 | 9 | 15 | 22 | 17 | 0 | 0 | 34 | 16 | 23 | 72 | 49/63 (78%) |
| Hepatic flexure of Colon | 8/1006 (0.8%) | 6 | 2 | 0 | 1 | 4 | 3 | 6 | 0 | 2 | 1 | 1 | 39 | 1/8 (12%) |
| Ascending colon | 24/1006 (2.4%) | 11 | 13 | 7 | 6 | 6 | 5 | 14 | 0 | 3 | 1 | 6 | 56 | 9/24 (38%) |
| Junction of the rectum and sigmoid | 6/1006 (0.6%) | 3 | 3 | 0 | 1 | 2 | 3 | 0 | 3 | 2 | 1 | 2 | 39 | 1/6 (17%) |
| Left hemicolon | 19/1006 (1.9%) | 9 | 10 | 3 | 7 | 4 | 5 | 0 | 6 | 5 | 4 | 6 | 56 | 8/19 (42%) |
| Right hemicolon | 76/1006 (7.6%) | 42 | 34 | 11 | 21 | 27 | 17 | 27 | 0 | 26 | 16 | 15 | 59 | 37/56 (49%) |
| Sigmoid | 140/1006 (14%) | 86 | 54 | 10 | 36 | 49 | 45 | 1 | 55 | 37 | 15 | 22 | 62 | 70/140 (50%) |
| Rectum | 597/1006 (59%) | 341 | 256 | 73 | 172 | 191 | 160 | 1 | 191 | 122 | 47 | 131 | 59 | 287/597(48%) |
| Terminal tumor site | 737/1006 (73%) | 427 | 310 | 83 | 208 | 240 | 205 | 2 | 246 | 159/439 (36%) | 62/439 (14%) | 153/439 (35%) | 60 | 357/737 (48%) |
| Other tumor sites | 269/1006 (27%) | 139 | 130 | 41 | 65 | 86 | 77 | 73 | 13 | 93/163 (57%) | 53/163 (33%) | 67/163 (41%) | 59 | 130/269 (48%) |
Table 1: Statistics summary of each primary tumor site in colorectal cancer.
| Tumor Characteristics | Stage | |||
|---|---|---|---|---|
| Primary Site location | Early | Advanced | ||
| Rectum | 247 | 41.7% | 346 | 58.3% |
| Sigmoid | 54 | 38.6% | 86 | 61.4% |
| Right hemicolon | 23 | 30.3% | 53 | 69.7% |
| Colon | 26 | 41.3% | 37 | 58.7% |
| Ileocecal | 13 | 28.9% | 32 | 71.1% |
| Ascending colon | 7 | 29.2% | 17 | 70.8% |
| Left hemicolon | 4 | 21.1% | 15 | 78.9% |
| Transverse colon | 4 | 23.5% | 13 | 76.5% |
| Hepatic flexure of Colon | 0 | 0.0% | 8 | 100.0% |
| Descending colon | 6 | 54.5% | 5 | 45.5% |
| Junction of the rectum and sigmoid | 1 | 16.7% | 5 | 83.3% |
| Summary | ||||
| Terminal tumor sites | 301 | 41.1% | 432 | 58.9% |
| Other tumor sites | 84 | 31.2% | 185 | 68.8% |
Table 2: Chacterization of different primary tumor sites in early and advanced stage CRC.
Tumor stage, lymph node metastasis, adenocarcinoma subtypes and survival outcomes
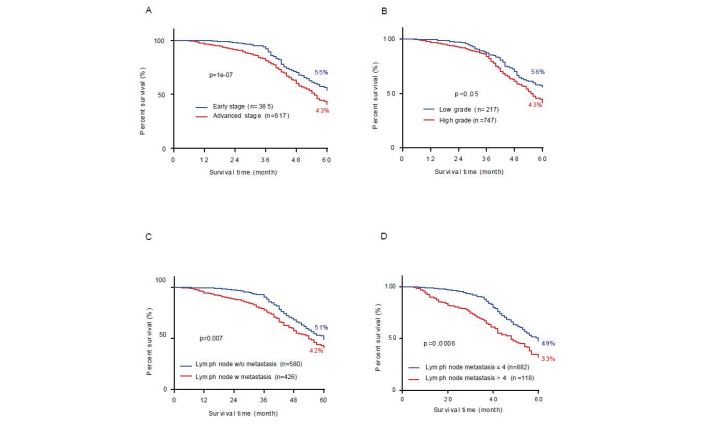
Patients with advanced stage (median survival time = 55 months, 43 %), high differentiation (median survival time = 55 months, 43%), lymph node metastases (median 54 months, 42 %) and severe lymph node metastases (more than 4, median survival time = 44 months, 27 %) have significantly shorter OS and a lower 5-year survival rate than those with early stage (median survival time = 64 months, 55 per cent, log rank p=7e-10, figure 3A), lower differentiation (median survival time = 67 months, 56 %, log rank p=0.024, figure 3B), without lymph node metastases (median survival time = 61 months, 51 %, figure 3C, log rank p=0.007) and mild lymph node metastases (lower 4, median survival time = 60 months, 49 %, log rank p=1e-05, figure 3D).
More interesting, the tumor stage (r=-0.18, p=5.77e-09), lymph node metastases (r=-0.14, p=1.47e-05) and the number of lymph node metastases (r=-0.17, p=3.79e-08) were significantly negative correlated with OS of patients (Figure S2A). The lymph node metastases (r=0.67, p<2.2e-16) and the number of lymph node metastases (r=0.55, p<2.2e-16) showed a significantly positive correlation with the tumor stage. Lymph node metastases (r=0.82, p<2.2e-16) were significantly positive correlated with the number of lymph node metastases (Figure S2A). The 5-year survival rate of patients with varied number of lymph node metastases decreased from 50% (N0) to 34% (N2) (Figure S2B) and the survival rate of patients with lymph node metastases less than 4 (N1) was no significant difference than those without (N0) (Figure S2C, p = 0.22).
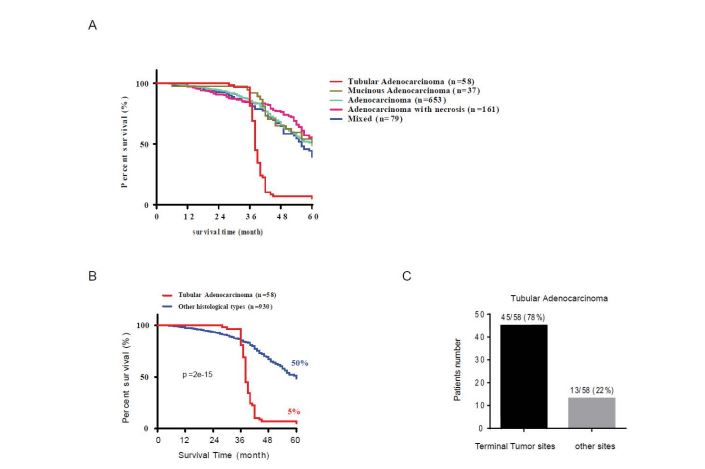
According to the pathohistological characteristics and tumor subtypes classify standard, 988 patients were sorted as tubular adenocarcinoma, mucinous adenocarcinoma, adenocarcinoma, adenocarcinoma with necrosis and mixed type (without tubular adenocarcinoma, more than two other subtypes), respectively (Figure 4A). Among the four subtypes of adenocarcinoma, the survival rate of tubular adenocarcinoma patients (median survival time = 38 months, 5 %) was significantly lower than other histological tumors (median survival time = 60.5 months, 50 %, log rank p = 2e15, figure 4B). In addition, the majority of cases of tubular adenocarcinoma were mainly distributed at the terminal tumor site (78 %) and, in particular, at sigmoid (14/58, 24 %) and rectum (31/58, 53 %) sites (figure 4C, p = 2e-15).
Correlation analysis between serum biomarkers and the CRC primary tumor sites
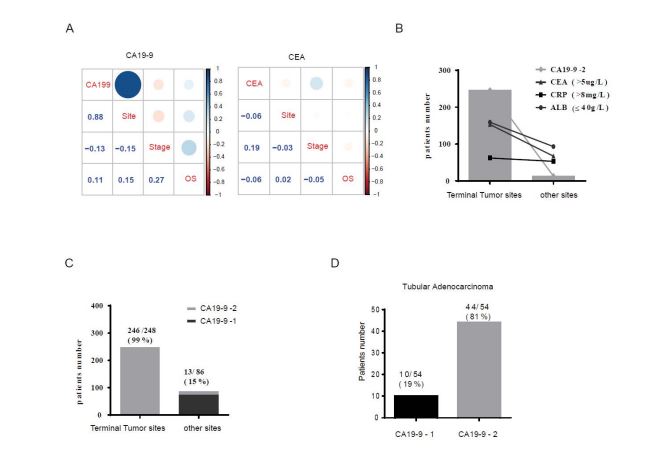
Multiple CRC patient’s serum indicators were tested and summarized (Table 1). Correlation analysis showed that different patterns of these physiological indicators were correlated with primary tumor sites (Figure S3A), tumor stage (Figure S3B) and overall survival time. First of all, the serum level of CA19-9 was positive correlated with CRC tumor sites (r=0.88, p=2.2e-16), overall survival time (r=0.11, p=0.05454) and negative correlated with tumor stage (r=-0.13, p=0.0214) (Figure 5A). The CEA serum level was significantly positive correlated with the CRC tumor stage (r=0.19, p=3.53-e06) and very weak negative correlated with the CRC tumor site (r=-0.06, p=0.16) and overall survival time (r=-0.06, p=0.1546) (Figure 5A). In the meantime, the other two markers CRP (r=-0.21, p=2.65e-7) and ALB (r=-0.19, p=3.54e-6) were significantly negatively correlated with CRC tumor sites (Figure S3C) and showed a weak correlation with CRC tumor stage (CRP, r=0.06, p=0.1544; ALB, r=-0.05, p=0.23) and overall survival time (CRP, r=-0.08, p=0.3882; ALB, r=0.00159, p=0.969) (Figure S3C). Other physiological indicators show little correlation with the CRC stage and tumor sites. Patients with advanced stage tumors with higher abnormal levels of CEA (>5 µg/L, 41%), CRP (>8 mg/L, 33%) and ALB (< 40µg /L, 57%) were mainly distributed at terminal tumor sites (Figure 5B). The remaining seven physiological indicators (Table S1), including leukocyte, blood platelet, neutrophil, lymphocyte, monocyte, did not show a significant difference between normal and tumor patients. In addition, patients (99 %) with higher CA19-9 (CA19-9-2) levels were mainly distributed to terminal tumor sites of colorectal cancer, and only a small portion was allocated to other primary tumor sites (Table 1, Figure 5C). However, there was no significant difference in overall survival time between the two different CA19-9 patient groups. In combination with the tumor stage analysis, lower CA19-9 level patients (73 %) were more likely to be associated with advanced stage than higher CA19-9 level patients (58 %). The serum level of CA19-9 was weakly positive correlated with non-infiltration of the tumor (Figure S4C, r=0.07, p=1.2e-6). In addition, the majority of patients diagnosed with tubular adenocarcinoma (81 %) were associated with higher CA19-9 levels (Figure 5D). With the integration of several markers analyzing primary tumor sites (Figure 5B), CA19-9 may work as a promising biomarker for terminal intestinal cancer and CRC tubular adenocarcinoma.
Discussion
In this study, the first-hand clinical data were collected from 1006 patients in the local hospital to discover those risk factors that have contributed to the colorectal carcinogenic, pathological process and to explore potential novel treatment strategies. The contribution of patient age and gender, tumor stage and differentiated grade, lymph node metastases were evaluated and thoroughly analyzed. The majority (88%) of patients identified with colorectal cancer were distributed primarily in the over 50-year-old group, which is twice the incidence rate of patients below the 50-year-old group. In addition, the 5-year survival rate of patients over 60-years-old was significantly decreased in the group. Previously cohort studies have shown that millions of patients have been diagnosed with colorectal cancer and that the median age has decreased from 70 to 50 years [3,18]. It is suggested that more than 50-year old patients are at higher risk of developing as a CRC and would be identified as a screening target during an annual physical examination. Survival analysis of patients diagnosed with advanced stage tumor, higher differentiated grade, with lymph node metastases, and higher lymph node metastases significantly reduces overall survival time and 5-year survival rate (Figure 2A-C). Correlation has been identified between overall survival time and the stage of tumor, lymph node metastases and the number (>5) of lymph node metastases. In addition, the number of lymph node metastases was negatively correlated with patient’s 5-year survival rate. Compared to the 8th edition of the AJCC staging system, these results match the trend previously reported, which had identified the prognosis of lymph node metastases and the stage of tumor in colorectal cancer [19]. In addition, the number of lymph node metastases could be translated as a clinically measurable predictor of tumor progression and 5-year survival outcomes for patients. At the same time, the prognostic value of tumor infiltration in these cases was also evaluated. Non-tumor-infiltration cases showed significantly lower survival rates and shorter overall survival times than tumor-infiltration cases. In addition, the majority of tubular adenocarcinoma cases (53/54) were identified as non-tumor infiltration. It is suggested that tumor-infiltration lymphocytes may be the overall survival prognostic biomarker for CRC. It is consistent with the results of a previously large population meta-analysis study that identified a high level of tumor-infiltration lymphocytes associated with improved patient survival and worked as a prognosis indicator for colorectal cancer [20,21].
Colon adenocarcinoma mainly originated from adenomatous polyps, including three histological types of tubular, tubular and villous adenomas. Tubular adenomas contribute about 85 % of the adenomatous polyps and only 5 % of the adenomatous polyps is transformed as malignancy [22]. CRC originated from different anatomical positions with a variety of molecular genetic alternations and pathogenic mechanisms [1,23,24]. The traditional dichotomy of colon and colorectum was challenged and potentially delayed in early diagnosis and affected the survival of the patient [25]. In this study, 90% of patients were identified as adenocarcinoma or mixed subtype, and only small portion of cases (6 %) were identified as tubular adenocarcinoma with a significantly lower 5-year survival rate and shorter overall survival time (Figure 4C). In order to further explore the heterogeneity of colorectal cancer, this study investigated the distribution of multiple primary tumor sites in the CRC population. The 73% of cases were concentrated at terminal tumor sites. Rectum and sigmoid (terminal tumor sites) were identified as the top two tumor sites with the highest incidence rate. In addition, the incidence rate for male patients was significantly higher than for female patients (Figure 3C,D). Although the early diagnosis rate of terminal colorectal cancer (41%) was significantly higher than other primary sites (31%), overall survival time was not a significant difference between primary tumor sites. In addition, cases of tubular adenocarcinoma have mainly been spread to terminal tumor sites (Figure 4C). It is strongly suggested that rectum and sigmoid would be identified as the primary screening target for colorectal health during annual physical examination and benefit for early diagnosis of tubular adenocarcinoma.
Multiple serum biomarkers, such as CEA, CA19-9 and CRP, have been identified as the standard for CRC patient screening in clinical practice [9,10,26,27]. The serum levels of CA19-9 and CEA had differentiated prognostic value in the CRC. Higher serum CA19-9 (>200 U/ml) was reported as a significant predictor of poor survival of colorectal cancer patients with liver metastases [28]. Serum CEA was the best tumor biomarker for chemotherapy drug response prediction, while CA19-9 was one of the best predictors of advanced colorectal carcinoma [27,29]. In this study, we investigated the internal linkage and potential prediction of these biomarkers for the clinical outcome of the patient. The correlation analysis showed a significant correlation between the serum level of CA19-9 with the patient’s CRC tumor sites and the stage, while the serum level of CEA was significantly positive for the CRC tumor stage (Figure 5A). In addition, terminal tumor cases (99 %) were mainly associated with abnormally higher levels of these markers (CA19-9, CEA, CRP and CEA) (Figure 5B). More interesting, abnormally higher serum CA19-9 was significantly positive correlated with terminal tumors, especially those associated with tubular adenocarcinoma (81%, Figure 5C,D) and non-lymphocyte infiltrating tumors (90%, Figure S4C). It is suggested that the serum level of CA19-9 works as an accurate predictor of terminal colorectal cancer.
Carbohydrate antigen 19-9 (CA19-9) is a modified Lewis blood group antigen associated with specific malignancies, and significantly increases in patients with gastrointestinal cancer [30]. CA19-9 was used as a sensitive biomarker to evaluate colon and rectum adenocarcinomas and advanced stage colorectal cancer with metastases [24,31- 33]. However, it is unclear that the correlation between the local expression level of CA19-9 and the colorectal terminal tumor sites (sigmoid and rectum). Several factors limit the effective assessment of CA19-9 in the clinical assessment for colorectal cancer. First of all, about 5~7 % of the patients population are Lewis-negative, who have fucosyltransferase defect and do not produce CA19-9 in their blood [34]. As a result, their serum level of CA19-9 keeps much lower or undetectable level even cancer recurrence [15,35]. Secondary, the concentration of serum CA19-9 is affected by liver metabolism or by environmental epidemiology [24]. Third, the serum level of CA19-9 also increased frequently in patients with other cancers, such as aspancreatic adenocarcinomas, which interferes the clinical diagnostics accuracy for colorectal cancer [36]. In this study, lacking of the information on Lewis blood identification or more detail on clinical chemotherapy drug treatment in original patient data, it is hard to deeply dig the internal connection of CA19- 9 in colorectal cancer. It causes less samples of patients with higher serum CA19-9 identify the correlation with terminal tumor sites, even which was associated with advanced CRC. Taking these factors into account in the future study, it will be helpful to shed light on the precise application of CA19-9 in the clinical assessment of colorectal cancer.
There were some limitations in this study. First of all, although the study has been retrieved for many years, the lack of patient pathological data caused the exclusion of some cases and destroyed the statistical power of large samples. Second, the lack of somatic mutations and the microsatellite instability status of the primary tumor, which indicated patterns of CRC metastases, prevented further exploration of the internal CRC connection. Third, without the details of clinical chemotherapy and multiple prognosis scores and the CA19-9 serum level of patients before and after chemotherapy, the precise application as a clinical prognosis biomarker has been deterred. Finally, the lack of genetic mutation status of key oncogenes in patients, such as mutations in KRAS (exons 2-4) and BRAF (V600E), which are primarily associated with CRC metastases and widely used in the management of CRC patients [37-40], will impair the predicting effect of CA19-9. Despite these limitations, our results demonstrated novel vision of CA19-9 as a precise biomarker for terminal colorectal cancer, especially for tubular adenocarcinoma. In future studies, larger patient sizes and multiple centers will conduct independent studies to verify the prognostic value of CA19-9 in colorectal tubular adenocarcinoma.
Figure 3:Overall survival analysis the contribution of stage, grade, lymph node metastasis in CRC cases. (A) Overall survival time of all colorectal cancer cases with different stages; (B) different histological grading; (C) lymph node metastasis; and (D) lymph node metastasis with different number.
Figure 4:The survival rate and tumor subtype analysis. (A) Survival rate analysis for different adenocarcinoma types (B) Survival analysis of different histological types of colorectal adenocarcinoma. (C) Distribution of tubular adenocarcinoma in terminal and other primary tumor sites (n=58).
Figure 5: Correlation analysis of CA199 and CEA with primary tumor sites, Stage and survival time in CRC. (A) CA199 and CEA correlation analysis with the tumor site, stage and overall survival time of the CRC. (B) Distribution of CA19-9-2 (n=248), abnormal CEA (n=220), CRP (n=115) and Alb (n=252) in terminal and other primary tumor sites. (C) Distribution of patients with different levels of CA199 at terminal and other primary tumor sites (n=334). (D) Distribution of patients with different levels of CA199 in tubular adenocarcinoma (n=54).
Conclusion
In conclusion, our results provided clinical evidence that the incidence rate and overall survival outcomes were significantly different in terminal tumor sites and other colorectal cancer tumor sites. Colorectal tubular adenocarcinoma shows a significantly lower survival rate at terminal tumor sites (sigmoid and rectum). The serum level of CA19-9 is a promising accurate diagnostic biomarker for terminal colorectal cancer, especially for tubular adenocarcinoma. Integrated CA19-9 level detection with accurate terminal tumor sites, clinical surgery may significantly change clinical outcomes and extend survival time for CRC patients.
Ethics Statement
This retrospective study was approved by the local ethics committee of the hospital affiliated university and waved for requirement of informed consent.
Authors Contributions
Conception and design the overall project study: S.L., and YY. H. Collection and assembly of data: S. H., Z.H. Data analysis and interpretation: Z.H., S.L. Data interpreted and summarized the results: Z.H., X. C., S.L., and YY. H. Manuscript writing: Z.H., S.L., and YY. H. wrote and revised the manuscript. Final approval of manuscript: all authors have read and approved the final version of manuscript.
Funding
This work was supported by Chinese Academy of Science “CAS PioneerHundred Talents Program” (E0241211H1) and startup program (Y8677211K1, Y8690211Z1) from State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, the Chinese Academy of Sciences to Dr. Shubai Liu. The roles of these grants were to support the activities of study design and data collection, analysis and interpretation, manuscript writing and publication cost.
Conflict of Interest
All author(s) announced no potential conflicts of interest.
Supplementary Material
The datasets used and/or analyzed during the current study are available online in supplementary materials.
Kouzminova N, Lu T, Lin AY (2010) Molecular basis of colorectal cancer.N Engl J Med 362: 1245-1246.[ Ref ]
Ogino S, Chan AT, Fuchs CS, Giovannucci E (2011) Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60: 397-411.[ Ref ]
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66: 115-132.[ Ref ]
Carcinoma of the colon and rectum (2010) WHO Classi cation of Tumours of the Digestive System 2010: 134-146.[ Ref ]
Whiteford MH, Whiteford HM, Yee LF, Ogunbiyi OA, Dehdashti F, et al. (2000) Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum 43: 759-767.[ Ref ]
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, et al. (2015) Colorectal cancer. Nat Rev Dis Primers 1: 15065.[ Ref ]
Wong MCS, Huang J, Lok V, Wang J, Fung F, et al. (2020) Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol 19: 955- 966.e61.[ Ref ]
Boisen MK, Johansen JS, Dehlendorff C, Larsen JS, Osterlind K, et al. (2013) Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol 24: 2554-2559.[ Ref ]
Das V, Kalita J, Pal M (2017) Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed Pharmacother 87: 8-19.[ Ref ]
Campos-da-Paz M, Dorea JG, Galdino AS, Lacava ZGM, de Fatima Menezes Almeida Santos M (2018) Carcinoembryonic Antigen (CEA) and Hepatic Metastasis in Colorectal Cancer: Update on Biomarker for Clinical and Biotechnological Approaches. Recent Pat Biotechnol 12: 269-279.[ Ref ]
Guo YZ, Pan L, Du CJ, Ren DQ, Xie XM (2013) Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev 14: 243-248.[ Ref ]
Koprowski H, Herlyn M, Steplewski Z, Sears HF (1981) Specific antigen in serum of patients with colon carcinoma. Science 212: 53-55.[ Ref ]
Wang WS, Lin Jk Fau - Chiou T-J, Chiou Tj Fau - Liu J-H, Liu Jh Fau - Fan FS, Fan Fs Fau - Yen C-C, et al. (2002) CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology 49: 160-164.[ Ref ]
Huo YR, Huang Y, Liauw W, Zhao J, Morris DL (2016) Prognostic Value of Carcinoembryonic Antigen (CEA), AFP, CA19-9 and CA125 for Patients with Colorectal Cancer with Peritoneal Carcinomatosis Treated by Cytoreductive Surgery and Intraperitoneal Chemotherapy. Anticancer Res 36: 1041-1049.[ Ref ]
Thomas WM, Robertson JF, Price MR, Hardcastle JD (1991) Failure of CA19-9 to detect asymptomatic colorectal carcinoma. Br J Cancer 63: 975-976.[ Ref ]
.Ferretti S, Patriarca S, Carbone A, Zanetti R (2010) [TNM classification of malignant tumours, VII edition 2009. Changes and practical effects on cancer epidemiology]. Epidemiol Prev 34: 125-128[ Ref ]
Vandertoll DJ, Beahrs OH (1965) Carcinoma of Rectum and Low Sigmoid; Evaluation of Anterior Resection of 1,766 Favorable Lesions. Arch Surg 90: 793-798[ Ref ]
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet 383: 1490- 1502.[ Ref ]
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, et al. (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67: 93-99.[ Ref ]
Mei Z, Liu Y, Liu C, Cui A, Liang Z, et al. (2014) Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis Br J Cancer 110: 1595-1605.[ Ref ]
.Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, et al. (2020) The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci Rep 10: 3360.[ Ref ]
Amersi F, Agustin M, Ko CY (2005) Colorectal cancer: epidemiology, risk factors, and health services. Clin Colon Rectal Surg 18: 133-140.[ Ref ]
Ahlquist DA (2010) Molecular detection of colorectal neoplasia. Gastroenterology 138: 2127-2139.[ Ref ]
Fearon ER (2011) Molecular genetics of colorectal cancer. Annu Rev Pathol 6: 479-507.[ Ref ]
Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, et al. (2012) Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 61: 847-854.[ Ref ]
Filella X, Molina R, Grau JJ, Pique JM, Garcia-Valdecasas JC, et al. (1992) Prognostic value of CA 19.9 levels in colorectal cancer. Ann Surg 216: 55-59.[ Ref ]
Kouri M, Pyrhonen S, Kuusela P (1992) Elevated CA19-9 as the most significant prognostic factor in advanced colorectal carcinoma. J Surg Oncol 49: 78-85.[ Ref ]
Mitsuyama Y, Shiba H, Haruki K, Fujiwara Y, Furukawa K, et al. (2012) Carcinoembryonic antigen and carbohydrate antigen 19-9 are prognostic predictors of colorectal cancer with unresectable liver metastasis. Oncol Lett 3: 767-771.[ Ref ]
Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, et al. (1995) The prognostic value of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG immunohistochemistry in advanced colorectal cancer. Ann Oncol 6: 581-587.[ Ref ]
Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, et al. (1979) Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 5: 957-971.[ Ref ]
Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, et al. (2002) CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology 49: 160-164.[ Ref ]
Hidaka E, Maeda C, Nakahara K, Wakamura K, Ishiyama Y, et al. (2019) High Serum CA19-9 Concentration Predicts Poor Prognosis in Elderly Patients with Stage IV Colorectal Cancer. Gastrointest Tumors 5: 117-124.[ Ref ]
Wu T, Mo Y, Wu C (2020) Prognostic values of CEA, CA19-9, and CA72-4 in patients with stages I-III colorectal cancer. Int J Clin Exp Pathol 13: 1608-1614.[ Ref ]
Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, et al. (1987) Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 47: 5501-5503.[ Ref ]
Torok N, Gores GJ (2001) Cholangiocarcinoma. Semin Gastrointest Dis 12: 125-132.[ Ref ]
Tian F, Appert HE, Myles J, Howard JM (1992) Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann Surg 215: 350-355.[ Ref ]
Gattenlohner S, Etschmann B, Kunzmann V, Thalheimer A, Hack M, et al. (2009) Concordance of KRAS/BRAF Mutation Status in Metastatic Colorectal Cancer before and after Anti-EGFR Therapy. J Oncol 2009: 831626.[ Ref ]
Tol J, Nagtegaal ID, Punt CJ (2009) BRAF mutation in metastatic colorectal cancer. N Engl J Med 361: 98-99.[ Ref ]
Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. (2015) Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33: 692-700.[ Ref ]
Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, et al. (2017) Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol 28: 562-568.[ Ref ]