Journal Name: International Journal of Cancer and Treatment
Article Type: Research
Received date: 22 March, 2021
Accepted date: 29 April, 2021
Published date: 06 May, 2021
Citation: Fan C, Dai Y, Wang Y, Miao M, Rui C, et al. (2021) Prognostic Function of Platelet-to-Lymphocyte Ratio in Gynecologic Cancers: A Systematic Review and Meta-Analysis. Int J Cancer Treat Vol: 4, Issu: 1 (08-19).
Copyright: © 2021 Fan C et al,. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Although along with the diagnosis and treatment level enhanced, current situation of the gynecologic malignancies improved, the risk of gynecologic tumor remains high. Nowadays, inflammatory markers increasingly employed as a tumor predictive factor. Herein, we focused on the association between the platelet-to-lymphocyte ratio (PLR) and gynecological cancers, including cervical, ovarian, and endometrial cancer.
Methods: In this meta-analysis, we searched systematically on the EMBASE and PubMed databases from June 1, 1989 to May 31, 2019. After excluded some unqualified articles, we calculated the pooled hazard ratio (HR) with 95% confidence interval (CI) mainly to detect the relationship between PLR and prognostic survival, including overall survival (OS) and progression-free survival (PFS). Random-effect model was adopted when I2 >50% after Higgins I² test. Subgroup analysis and funnel plot were used to seek for the possible source of heterogeneity and publication bias, respectively. All statistical tests were two-sided.
Results: After a series of searching and selection, twenty-eight literatures containing 8290 participants totally. Among those recruited trials, 26 studies comprising 8109 patients reported HR for OS and 15 researches enrolled 4283 patients for PFS. Overall, a high value of PLR means a worse OS and PFS in women with gynecologic cancer except those with endometrial cancer for OS (pooled HR =1.35, 95% CI =0.73 to 2.53, P =0.33). Subgroup analyses indicated that the source of heterogeneity may be primarily from the sample size, PLR cut-off, study location, published year, and the cut-off year for the study. Publication bias manifested that bias was not evident.
Conclusion: Elevated pretreatment PLR portends a poor prognosis among patients with gynecological tumor, as well as in women with cervical and ovarian malignancies for both OS and PFS. However, in patients with endometrial cancer, this connection is broken for OS but still available for PFS.
Keywords: Platelet-to-lymphocyte ratio (PLR), Biomarker,Gynecological cancer, Prognosis.
Abstract
Purpose: Although along with the diagnosis and treatment level enhanced, current situation of the gynecologic malignancies improved, the risk of gynecologic tumor remains high. Nowadays, inflammatory markers increasingly employed as a tumor predictive factor. Herein, we focused on the association between the platelet-to-lymphocyte ratio (PLR) and gynecological cancers, including cervical, ovarian, and endometrial cancer.
Methods: In this meta-analysis, we searched systematically on the EMBASE and PubMed databases from June 1, 1989 to May 31, 2019. After excluded some unqualified articles, we calculated the pooled hazard ratio (HR) with 95% confidence interval (CI) mainly to detect the relationship between PLR and prognostic survival, including overall survival (OS) and progression-free survival (PFS). Random-effect model was adopted when I2 >50% after Higgins I² test. Subgroup analysis and funnel plot were used to seek for the possible source of heterogeneity and publication bias, respectively. All statistical tests were two-sided.
Results: After a series of searching and selection, twenty-eight literatures containing 8290 participants totally. Among those recruited trials, 26 studies comprising 8109 patients reported HR for OS and 15 researches enrolled 4283 patients for PFS. Overall, a high value of PLR means a worse OS and PFS in women with gynecologic cancer except those with endometrial cancer for OS (pooled HR =1.35, 95% CI =0.73 to 2.53, P =0.33). Subgroup analyses indicated that the source of heterogeneity may be primarily from the sample size, PLR cut-off, study location, published year, and the cut-off year for the study. Publication bias manifested that bias was not evident.
Conclusion: Elevated pretreatment PLR portends a poor prognosis among patients with gynecological tumor, as well as in women with cervical and ovarian malignancies for both OS and PFS. However, in patients with endometrial cancer, this connection is broken for OS but still available for PFS.
Keywords: Platelet-to-lymphocyte ratio (PLR), Biomarker,Gynecological cancer, Prognosis.
Introduction
Although a great deal of modern therapeutic methods have been expanded for gynecologic cancer, including cervical cancer, ovarian cancer, and endometrial cancer, the estimated deaths are still high last year as before, only after lung, stomach, and liver cancer [1]. Meanwhile, with an estimated over 1,250,000 new gynecological cancer cases in 2018 worldwide [1]. These cancers can result in weight loss, abdominal pain or distension, increased abdominal size, urinary tract symptoms, and subsequentlymental discomfort and economic burden, affecting patient’s life quality severely [2-6]. However, symptoms of ovarian and cervical cancer are not obvious in its early stage lead to the early diagnosis rate remains low, while advanced cancer is hard to manage. In addition, current screening methods for gynecological tumors are neither costly nor actual. For ovarian cancer, bimanual pelvic examination and transvaginal ultrasound are short of enough specificity and sensitivity, and the image examination costs are enormous [2-6]. At the meantime, radioimmunoassay for cancer antigen 125 (CA125) only rises in 50% patients with ovarian cancer [6]. As regards endometrial cancer, clinical examination and ultrasound annually may miss the possible lesions, and the acceptability to women of endometrial biopsy remains in doubt [4]. With respect to cervical cancer, the dominant screening approaches are Papanicolaou (Pap) smear and cervical cytology in the past 60 years, but the same question, limited specificity and sensitivity, appears [7]. Therefore, seeking for the newly satisfied predictive factors with economical and convenient benefits are warranted.
In the past few decades, systemic inflammation has gradually been associated with cancer pathogenesis and thought as a hallmark of cancer [8-11]. Systemic inflammation usually involves in changes in neutrophil, eosinophil, platelet, lymphocyte, and other peripheral blood cell count [10,12]. In tumor ambient environment, the cancer cells could recruit inflammatory cells like platelets and lymphocytes [13]. Meanwhile, some researchers have revealed that platelet deposition selectively enhance lymphocyte adhesion in the case of arterial blood flow, while the majority of tumor tissues are rich in blood vessel [14]. Thus, it is undoubtedly imperative to explore the potential correlations between the inflammatory associated blood bio-markers with gynecological cancer.
Previous studies have manifested platelet-to-lymphocyte ratio (PLR) can be widely used as a prognostic marker for diverse cancers [15-18]. In these literatures, authors demonstrated that a higher level of preoperative PLR was an indicator of poor survival of different cancers, as well as in most survey results of gynecological cancers. Yet, consequence from Prachratana Nuchpramool and Jitti Hanprasertpong illustrated that PLR could not be applied as a prognostic biomarker in early-stage cervical cancer after receiving primary treatment of radical hysterectomy with pelvic lymph node dissection [19]. Because of lacking the firm uniformity in the field, making the mentioned association above clear is crucial.
In this study, we accomplished a systematic review and meta-analysis to explore the conclusive connection between the PLR and the prognosis, including overall survival (OS) and progression-free survival (PFS), of patients with ovarian, cervical, or endometrial malignancies.
Methods
Search strategy
A computerized search of EMBASE and PubMed databases was performed for our research. We searched for MeSH terms and keywords in title and abstract and the main search terms were as follows: gynecology, gynecological, cervical, cervix, ovarian, ovary, endometrial, platelet lymphocyte. We included all publications between June 1, 1989 and May 31, 2019.
Study selection
Articles were eligible for inclusion if they met the following criteria: (1) patients diagnosed with cervical, ovarian or endometrial cancer; (2) provided pre-treatment PLR and cut-off values; (3) studies that reported the hazard ratio (HR) and corresponding 95% confidence interval (CI) for overall survival (OS) and/or progression-free survival (PFS), the HR and 95% CI to be calculated via univariate or multivariate analysis. The exclusion criteria were as follows: (1) review articles, guidelines, letters, case reports and conference proceedings; (2) non-English language publication; (3) title and/or abstract only and no full text provided; (4) only relevant graphic data but not numerical value for HR provided; (5) had no identified gynecologic tumor type.
The literature search and study selection process were conducted by three authors independently. Disagreements between three authors were consulted until a consensus was reached.
Data extraction
Three reviewers independently extracted the detailed information using predetermined forms from the included studies with disagreements discussed until consensus finished. For each study, we extracted characteristics on first author, year of publication, research location, study duration, number of available patients, median or mean age and age range of participants, stage and grade of diverse cancer, histopathologic subtype of tumor, lymph node metastasis whether or not, treatment methods, median or mean followup time. OS, PFS, as well as its HR with associated 95% CI, were also be recorded.
Statistical analysis
Extracted data from the enrolled studies were analyzed using RevMan 5.3 software (Cochrane Collaboration, Copenhagen, Denmark). Survival outcomes, both OS and PFS included, were the primary interests in this meta-analysis. Therefore, the Log (HR) and standard error were calculated according to HRs and their 95% confidence intervals. Heterogeneity was evaluated via Cochran’s Q test and the Higgins I² statistic. While P <0.05 was calculated by Cochran’s Q test, heterogeneity between literatures was manifested. In the Higgins I² test, the value of I² could considered as a criterion to estimate the degree of heterogeneity and the reference standards were as follows: if I² no more than 40%, heterogeneity could be negligible; if I² between 30% and 60%, moderate heterogeneity might be shown; while I² falls into 50-90%, evident heterogeneity may be presented; when I² =75-100%, inevitable heterogeneity exists [20]. Meanwhile, I² >50% in the Higgins I² test were termed as significant heterogeneity, and then a random effects model was chosen; or else, if I² <50%, a fixed effects model was performed. We also conducted subgroup analyses to detect the potential sources of heterogeneity though RevMan 5.3 software. And the items of subgroup analysis contain sample size, PLR cut-off, study location, the cut-off year for the research, published year of the articles, median age, and other subgroup that may impact the heterogeneity between the studies. In addition, funnel plots were employed for testing publication bias. P-values <0.05 was considered as statistically significant, and all adopted tests were two-sided.
Results
Literature search results and characteristics
The literature search results and detailed study selection steps are shown in figure 1. The database searching yielded 556 publications originally. After removing 154 duplicates, and screening residual titles and abstracts of 402 articles, 83 articles remained. Of the remaining studies, 53 literatures were further excluded for various reasons: eight reviews, two with non-English language, thirteen provided conference summary or abstract only, thirty-one did not offered numerical value for hazard ratio, and one with only gynecologic cancer but no primary cancer. Finally, a total of 28 articles and 8290 participants were included in this review and meta-analysis. Among them, one concerning cervical cancer was disposed separately cause of providing HRs and survival outcomes for patients with two different treatments [19,21-34]. As a consequence, fifteen, nine, and four publications regarding to cervical, ovarian, and endometrial cancer were enrolled, respectively [35-47].
Characteristics of all enrolled studies are shown in table 1. The publication of all enrolled studies ranged from 2011 to 2019 and the duration of experiments were from 1988 to 2016. Six studies were from Europe (Spain, Poland, the United Kingdom, and Italy) and the remained twenty-two were from Asia (Korea, China, Japan, Turkey, and Thailand). The number of study population recruited in each research were ranged from 36 to 795 patients. The outcomes of all included cancer were also recorded. PLR cut-off were extracted from the above-mentioned 28 researches, too.
Figure 1:Flow diagram of study selection.
| Study | Published year | Duration of study | Country | Number | Age | Analysis | PLR cut-off |
|---|---|---|---|---|---|---|---|
| Lee [22] | 2017 | 2011.03-2014.12 | Korea | 377 | 52(29-79) | PFS | 170.00 |
| He [23] | 2018 | 2007.09-2009.03 | China | 229 | 44(28-79) | OS | 149.27 |
| Nakamura [24] | 2018 | 1997.01-2013.07 | Japan | 98 | 65(32-86) | OS | 212.00 |
| Onal [25] | 2016 | 2006.10-2014.09 | Turkey | 235 | 57(21-86) | OS, PFS | 133.02 |
| Zheng [26] | 2016 | 2005.05-2012.12 | China | 795 | 49.5±10.7 | OS, PFS | 128.30 |
| Huang [27] | 2019 | 2006-2015 | China | 328 | 45(22-86) | OS | 118.00 |
| Holub [28] | 2018 | 2009.06-2016.07 | Spain | 151 | 51(25-92) | OS | 210.00 |
| Wang [29] | 2017 | 2012.01-2014.05 | China | 129 | 51(25-79) | OS | 148.90 |
| Zhang [30] | 2017 | 2005.01-2009.12 | China | 235 | 46(29-78) | OS, PFS | 176.50 |
| Nuchpramool [19] | 2018 | 2001.01-2016.06 | Thailand | 460 | 47 | OS, PFS | 119.00 |
| Jonska-gmyrek [31] | 2018 | 2003.11-2008.11 | Poland | 52 | 53(20-81) | OS | 158.00 |
| Zhu [32] | 2018 | 2012.07-2014.12 | China | 339 | 45(21-76) | OS, PFS | 143.79 |
| Haraga(CCRT) [21] | 2016 | 2007.04-2013.03 | Japan | 131 | 61.5(25-88) | OS, PFS | 172.50 |
| Haraga(RT alone) [21] | 2016 | 2007.04-2013.03 | Japan | 131 | 61.5(25-88) | OS, PFS | 128.00 |
| Chen [33] | 2016 | 2006.01-2009.12 | China | 407 | 44 | OS | 138.35 |
| Ida [34] | 2018 | 2004.04-2015.12 | Japan | 79 | 52.4(25-78) | OS | 260.00 |
| Ovarian cancer | |||||||
| Miao [35] | 2016 | 2005-2010 | China | 344 | 55(45-84) | OS, PFS | 207.00 |
| Raungkaewmanee [36] | 2012 | 2004.01-2010.12 | Thailand | 166 | 53(23-85) | OS, PFS | 200.00 |
| Badora-Rybicka [37] | 2017 | 2007-2013 | Poland | 315 | 54(22-77) | OS, PFS | 129.78(OS) |
| 62.31(PFS) | |||||||
| Liu [38] | 2017 | 2006.06-2012.07 | China | 200 | 53(18-83) | OS | 165.00 |
| Supoken [39] | 2014 | 2003.01-2013.10 | Thailand | 36 | 52 | PFS | 300.00 |
| Asher [40] | 2011 | 1988-1998 | The UK | 235 | 62 (24–90) | OS | 300.00 |
| Li [41] | 2017 | 2000-2010 | China | 654 | 63(28-93) | OS | 273.50 |
| Farolfi [42] | 2018 | 2007.01.01-2015.06.30 | Italy | 375 | 60,63(19-85) | OS, PFS | 169.00 |
| Zhang [43] | 2015 | 2000.01-2012.12 | China | 190 | 50.6±11.1(24-76) | OS, PFS | 203.00 |
| Endometrial cancer | |||||||
| Cummings [44] | 2015 | 2005.01-2007.12 | The UK | 605 | 65(28-95) | OS | 240.00 |
| Aoyama [45] | 2019 | 2007-2013 | Japan | 197 | 59(31-85) | OS, PFS | 206.00 |
| Haruma [46] | 2015 | 2002.01-2012.12 | Japan | 320 | 57.5(23-86) | OS, PFS | 175.72 |
| Li [47] | 2015 | 2007.09-2009.06 | China | 282 | 53(21-76) | OS | 250.00 |
Table 1: Characteristics of recruited studies.
Overall survival and progression-free survival
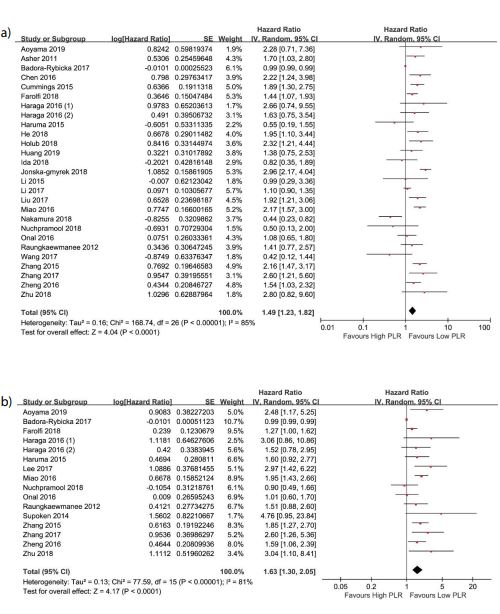
Two forest plots of all articles for OS and PFS are displayed as figure 2a and figure 2b, respectively. Overall, higher PLR represents worse survival in this data, both for OS (HR =1.49, 95%CI =1.23-1.82) and PFS (HR =1.63, 95%CI =1.30-2.05). Among all included studies, twenty-six studies consisting of 8109 participants reported HR for OS. In the meantime, for PFS, fifteen of the eligible twenty-eight literatures comprising 4283 patients. Thereinto, seven articles reported HR from univariate analysis for OS or PFS and the remained twenty-one were from multivariate analysis. The patients had a median age (age range =18 to 95) from 44 to 63 years old in twenty-four studies which reported the median age. The median cut-off for PLR was 169 (range =138.35 to 300) for OS, while it was 172.50 (range =62.31 to 300) for PFS. Relevant follow-up information (duration of median or mean follow-up) were recorded in nineteen researches, ranged from 0.1 to 175.3 months. A random-effects model was performed since the presence of heterogeneity (I²=85%, P<0.0001 and I² =81%, P<0.00001 for OS and PFS, respectively) existed between the applicable literatures.
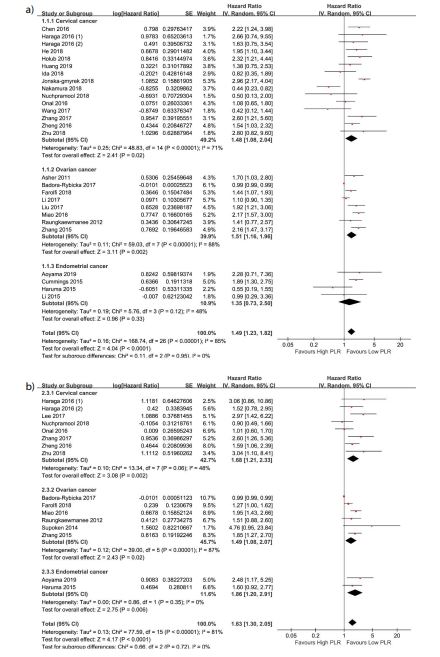
Overall survival and progression-free survival by primary tumor
The results of subgroup analysis by primary tumor are shown in figure 3a and figure 3b. From the figures 3a and 3b, our work manifested that the lower PLR represented the better prognostic except for OS with endometrial cancer. In patients with endometrial cancer, the HR and its 95% CI for OS was 1.35(0.73, 2.50). But for PFS, the corresponding outcome was 1.86(1.20, 2.91) and Higgins I² test declared I² =0%.
Subgroup analysis of overall survival and progression-free survival
In the subgroup analyses, three details should be stated initially. In the subgroup of median age, one research provided two different median age (60 and 63) for diverse group [42]. However, this did not influence the classification of subgroup in this data due to the cut-off value of median age was 50 years old. Another issue worth noting was that the Turkey was identified as a European country in this data on account of their living habits and ethnics were closer to Europe but not Asia. One last thing to note is that the subgroup analysis of endometrial cancer did not be carried out because of a relatively low number of recruited literatures.
Gynecologic cancer: The results of gynecologic cancer subgroup analyses were exhibited in table 2 and several sources of heterogeneity were found. In women with gynecological tumor, stratified analysis discovered several sources of heterogeneity and almost all heterogeneity decreased after subgroup analyses. Concerning OS, we found heterogeneity reduced in the published year (pooled HR =1.49; 95% CI =1.03 to 1.17; P <0.0001) and median age (pooled HR =1.47; 95% CI =1.18 to 1.82; P =0.0005) subgroup in all 26 eligible literatures containing 27 different trials. However, as for PFS, despite the heterogeneity equally shortened in the subgroup of the published year (pooled HR =1.63; 95% CI =1.30 to 2.05; P <0.0001), heterogeneity switched softly in the median age (pooled HR =1.58; 95% CI =1.22 to 2.06; P =0.0006) stratified group in all 16 trials. Among other subgroup analyses, including PLR cut-off, study location, the cut-off year for the study, and sample size subgroup, significant pooled HR and lessened heterogeneity were also observed visibly for PFS but not so remarkable for OS. Regarding study location, we did further classification and found that I² dropped to 0% after Higgins I² test in women who suffered from ovarian cancer and endometrial cancer despite the elevation in the cases with cervical cancer in Asian. With respect to OS, whether it was in Asia or Europe, we failed to explore the exact origin of the heterogeneity even heterogeneity declined to some degree but far from enough.
Cervical cancer: The analytical consequences of cervical cancer were presented in table 3 and table 4. Four subgroups were designed to search the underlying sources of heterogeneity for OS but three for PFS and matching trials were fifteen and eight, respectively. Similar to overall analysis for OS above, the cut-off year for the study (pooled HR =1.48; 95% CI =1.08 to 2.04; P=0.02) and published year subgroup analytical results displayed the significant heterogeneity reduction in one of the layered groups, whereas in the sample size and the PLR cut-off subgroup the decline did not illustrate significant heterogeneity. For PFS, after three subgroup analyses, opposite change occurred between groups of one subgroup: heterogeneity descended in the groups with sample size ≤200 patients, the cut-off year for the study from 2009 to 2012, and published year between 2011 and 2016, but conversely added in the other group.
Ovarian cancer: The corresponding results of ovarian cancer were displayed in table 3 and table 4. Still four and three subgroups were exploited for heterogeneity analyses for OS and PFS. Heterogeneity of the stratified group of published year (pooled HR =1.51; 95% CI =1.16 to 1.96; P<0.002) from 2011 to 2016 illustrated a shrinkage as before, as well as sample size in spite of group altered. In regard to PFS, several favorable survival results were recognized about ovarian cancer and resembled to women with cervical cancer.
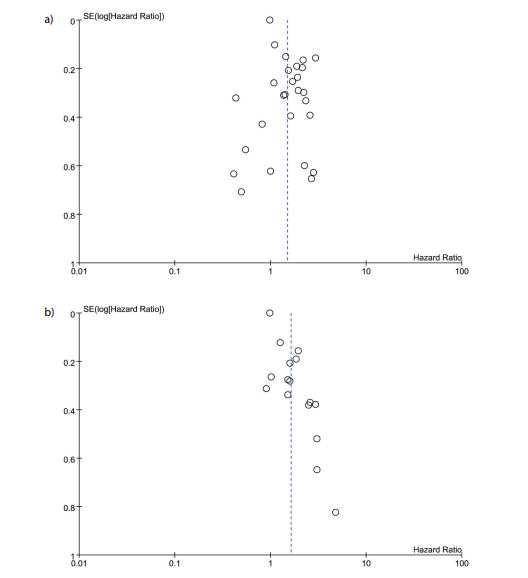
Publication bias analysis
From funnel plot (Figures 4a & 4b), our results suggested that publication bias was low for both OS and PFS.
Figure 2:Forest plots showing hazard ratio for overall survival (A) and progression-free survival (B) in all studies for platelet-to-lymphocyte ratio greater or less than the cut-off. Hazard ratio for each study are represented by the squares, the size of the square represents the weight of the study in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). All statistical tests were two-sided.
Figure 3:Forest plots showing hazard ratio for overall survival (A) and progression-free survival (B) by primary tumor for platelet-to-lymphocyte ratio greater or less than the cut-off. Hazard ratio for each study are represented by the squares, the size of the square represents the weight of the study in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). All statistical tests were two-sided.
| Subgroups | No. of studies | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I² | P value | ||||
| Overall survival | |||||
| Cervical Cancer | |||||
| Sample size | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| ≤200 patients | 6 | 1.47 (0.47, 2.94) | 0.27 | 85% | <0.00001 |
| >200 patients | 9 | 1.52 (1.23, 2.05) | 0.006 | 41% | 0.09 |
| PLR cut-off | 15 | 1.70 (1.46, 1.99) | <0.00001 | 71% | <0.00001 |
| <200 | 12 | 1.89 (1.60, 2.25) | <0.00001 | 57% | 0.007 |
| ≥200 | 3 | 0.94 (0.63, 1.40) | 0.77 | 85% | 0.001 |
| The cut-off year for the stud | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| 1998~2010 | 4 | 2.60 (2.05, 3.29) | <0.00001 | 0% | 0.59 |
| 2011~2016 | 11 | 1.18 (0.82, 1.69) | 0.37 | 59% | 0.006 |
| Published year | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| 2011~2016 | 5 | 1.56 (1.20, 2.03) | 0.0010 | 3% | 0.39 |
| 2017~2019 | 10 | 1.35 (0.83, 2.20) | 0.22 | 80% | <0.00001 |
| Ovarian Cancer | |||||
| Sample size | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| ≤200 patients | 3 | 1.91 (1.47, 2.49) | <0.00001 | 0% | 0.50 |
| >200 patients | 5 | 1.36 (1.03, 1.80) | 0.03 | 88% | <0.00001 |
| PLR cut-off | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| <200 | 3 | 1.33 (0.90, 1.97) | 0.15 | 86% | 0.0009 |
| ≥200 | 5 | 1.64 (1.17, 2.32) | 0.005 | 77% | 0.002 |
| The cut-off year for the study | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| 1998~2010 | 4 | 1.53 (1.04, 2.25) | 0.03 | 77% | 0.005 |
| 2011~2016 | 4 | 1.51 (1.00,2.28) | 0.05 | 90% | <0.00001 |
| Published year | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| 2011~2016 | 4 | 1.97 (1.60, 2.43) | <0.00001 | 0% | 0.56 |
| 2017~2019 | 4 | 1.22 (0.97, 1.54) | 0.09 | 80% | 0.002 |
| Progression-free survival | |||||
| Cervical Cancer | |||||
| Sample size | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| ≤200 patients | 3 | 2.17 (1.35, 3.47) | 0.001 | 4% | 0.35 |
| >200 patients | 5 | 1.49 (0.99, 2.24) | 0.06 | 55% | 0.06 |
| The cut-off year for the study | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| 2009~2012 | 2 | 1.85 (1.19, 2.87) | 0.006 | 25% | 0.25 |
| 2013~2016 | 6 | 1.63 (1.03, 2.56) | 0.04 | 55% | 0.05 |
| Published year | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| 2011~2016 | 4 | 1.43 (1.05, 1.95) | 0.02 | 12% | 0.33 |
| 2017~2019 | 4 | 2.04 (1.08, 3.88) | 0.03 | 65% | 0.03 |
| Ovarian Cancer | |||||
| Sample size | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| ≤200 patients | 3 | 1.80 (1.33, 2.43) | 0.0002 | 0% | 0.40 |
| >200 patients | 3 | 1.31 (0.90, 1.91) | 0.15 | 91% | <0.0001 |
| The cut-off year for the study | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| 2009~2012 | 3 | 1.84 (1.48, 2.29) | <0.00001 | 0% | 0.73 |
| 2013~2016 | 3 | 1.15 (0.85, 1.56) | 0.35 | 74% | 0.02 |
| Published year | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| 2011~2016 | 4 | 1.87 (1.51, 2.32) | <0.00001 | 0% | 0.58 |
| 2017~2019 | 2 | 1.09 (0.86, 1.38) | 0.49 | 76% | 0.04 |
| P values <0.05 are in bold. | |||||
Table 2: Subgroup analyses of main outcome for gynecologic cancer.
| Subgroups | No. of studies | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I² | P value | ||||
| Overall survival | |||||
| Cervical Cancer | |||||
| Sample size | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| ≤200 patients | 6 | 1.47 (0.47, 2.94) | 0.27 | 85% | <0.00001 |
| >200 patients | 9 | 1.52 (1.23, 2.05) | 0.006 | 41% | 0.09 |
| PLR cut-off | 15 | 1.70 (1.46, 1.99) | <0.00001 | 71% | <0.00001 |
| <200 | 12 | 1.89 (1.60, 2.25) | <0.00001 | 57% | 0.007 |
| ≥200 | 3 | 0.94 (0.63, 1.40) | 0.77 | 85% | 0.001 |
| The cut-off year for the stud | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| 1998~2010 | 4 | 2.60 (2.05, 3.29) | <0.00001 | 0% | 0.59 |
| 2011~2016 | 11 | 1.18 (0.82, 1.69) | 0.37 | 59% | 0.006 |
| Published year | 15 | 1.48 (1.08, 2.04) | 0.02 | 71% | <0.00001 |
| 2011~2016 | 5 | 1.56 (1.20, 2.03) | 0.0010 | 3% | 0.39 |
| 2017~2019 | 10 | 1.35 (0.83, 2.20) | 0.22 | 80% | <0.00001 |
| Ovarian Cancer | |||||
| Sample size | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| ≤200 patients | 3 | 1.91 (1.47, 2.49) | <0.00001 | 0% | 0.50 |
| >200 patients | 5 | 1.36 (1.03, 1.80) | 0.03 | 88% | <0.00001 |
| PLR cut-off | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| <200 | 3 | 1.33 (0.90, 1.97) | 0.15 | 86% | 0.0009 |
| ≥200 | 5 | 1.64 (1.17, 2.32) | 0.005 | 77% | 0.002 |
| The cut-off year for the study | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| 1998~2010 | 4 | 1.53 (1.04, 2.25) | 0.03 | 77% | 0.005 |
| 2011~2016 | 4 | 1.51 (1.00,2.28) | 0.05 | 90% | <0.00001 |
| Published year | 8 | 1.51 (1.16, 1.96) | 0.002 | 88% | <0.00001 |
| 2011~2016 | 4 | 1.97 (1.60, 2.43) | <0.00001 | 0% | 0.56 |
| 2017~2019 | 4 | 1.22 (0.97, 1.54) | 0.09 | 80% | 0.002 |
| Progression-free survival | |||||
| Cervical Cancer | |||||
| Sample size | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| ≤200 patients | 3 | 2.17 (1.35, 3.47) | 0.001 | 4% | 0.35 |
| >200 patients | 5 | 1.49 (0.99, 2.24) | 0.06 | 55% | 0.06 |
| The cut-off year for the study | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| 2009~2012 | 2 | 1.85 (1.19, 2.87) | 0.006 | 25% | 0.25 |
| 2013~2016 | 6 | 1.63 (1.03, 2.56) | 0.04 | 55% | 0.05 |
| Published year | 8 | 1.68 (1.21, 2.33) | 0.002 | 48% | 0.06 |
| 2011~2016 | 4 | 1.43 (1.05, 1.95) | 0.02 | 12% | 0.33 |
| 2017~2019 | 4 | 2.04 (1.08, 3.88) | 0.03 | 65% | 0.03 |
| Ovarian Cancer | |||||
| Sample size | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| ≤200 patients | 3 | 1.80 (1.33, 2.43) | 0.0002 | 0% | 0.40 |
| >200 patients | 3 | 1.31 (0.90, 1.91) | 0.15 | 91% | <0.0001 |
| The cut-off year for the study | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| 2009~2012 | 3 | 1.84 (1.48, 2.29) | <0.00001 | 0% | 0.73 |
| 2013~2016 | 3 | 1.15 (0.85, 1.56) | 0.35 | 74% | 0.02 |
| Published year | 6 | 1.49 (1.08, 2.07) | 0.02 | 87% | <0.00001 |
| 2011~2016 | 4 | 1.87 (1.51, 2.32) | <0.00001 | 0% | 0.58 |
| 2017~2019 | 2 | 1.09 (0.86, 1.38) | 0.49 | 76% | 0.04 |
| P values <0.05 are in bold. | |||||
Table 3: Subgroup analyses of main outcome for cervical and ovarian cancer.
| Subgroups | No. of studies | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I² | P value | ||||
| Overall survival | |||||
| Asia | 20 | 1.45 (1.16, 1.80) | 0.001 | 65% | <0.0001 |
| Cervical cancer | 12 | 1.35 (0.96, 1.90) | 0.08 | 65% | 0.001 |
| Ovarian cancer | 5 | 1.68 (1.19, 2.38) | 0.003 | 78% | 0.001 |
| Endometrial cancer | 3 | 1.04 (0.45, 2.39) | 0.93 | 37% | 0.20 |
| Europe | 7 | 1.59 (1.04, 2.45) | 0.03 | 92% | <0.00001 |
| Cervical cancer | 3 | 1.98 (1.03, 3.78) | 0.04 | 82% | 0.004 |
| Ovarian cancer | 3 | 1.27 (0.90, 1.80) | 0.18 | 81% | 0.005 |
| Endometrial cancer | 1 | ||||
| Progression-free survival | |||||
| Asia | 13 | 1.82 (1.55, 2.14) | <0.00001 | 7% | 0.38 |
| Cervical cancer | 7 | 1.84 (1.31, 2.59) | 0.0005 | 39% | 0.13 |
| Ovarian cancer | 4 | 1.87 (1.51, 2.32) | <0.00001 | 0% | 0.58 |
| Endometrial cancer | 2 | 1.86 (1.20, 2.91) | 0.006 | 0 | 0.35 |
| P values <0.05 are in bold. | |||||
Table 3: Subgroup analyses of main outcome for gynecologic cancer.
Discussion
Accumulating evidence implies that inflammation acts an absolutely required part in the formation of neoplasm possibly via all kinds of transcription factors, chemokines, as well as cytokines [10,11]. In many published knowledges, the roles of inflammatory factors like neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and C-reactive protein (CRP) have always been delineated in patients who suffered from cancer. Given that, we turned our attention on the association between another inflammatory immune factors, platelet-to-lymphocyte ratio (PLR), and gynecological cancer [48-50].
According to the present review and meta-analysis, we evaluated the prognostic effects of PLR for ovarian, cervical, and endometrial cancers and ultimately found that an elevated PLR was linked to both shorter OS and poorer PFS in the ovarian cancer and cervical cancer, in keeping with many known outcomes with several other sorts of neoplasm such as early stage classical Hodgkin lymphoma, hepatopancreatico-biliary malignancy, early stage non– small-cell lung cancer, gastric cancer, breast cancer, and esophageal cancer [15-18,51,52]. But contrary result appeared in endometrial cancer, pretreatment low or high value of PLR had no impact on OS in endometrial tumor, while higher value of PLR denoted inferior PFS.
After subgroup analyses, we detect some meaningful outcomes likewise. Nearly in all published year subgroups, decreased heterogeneity in stratified group (2011~2016) was observed and P value for all pooled HR in the subgroup for diverse analyses were less than 0.05. Hence, we have adequate reasons to believe that heterogeneity between literatures may come from those which are published later than 2016. Comparably, in the majority of the cut-off year for the study subgroups, our consequences revealed that the relatively later cut-off year for the study, the higher heterogeneity happened to the studies we included these observations. Combined to the published year subgroup analysis, we speculated that the latest researches could exist certain inconsistence since the later the trial stopped, the more recent the study published. And this may be resulted from the rapid medical development particularly in cancer evolution and along with the new controversy appeared, as well as the incompatible evaluation criterion. For this account, inclusion criteria for patients may various and enrolled women with tumor and treatment methods differ followed would have an inherent impact on the outcomes [53]. Additionally, sample size no more than 200 could be taken as another source of the heterogeneity both in cervical and ovarian cancer. Moreover, study location may constitute another sources of heterogeneity for PFS. Heterogeneity was relatively low in Asian patients, especially for endometrial cancer. But this may not make sense because of the few literatures included in our report. Regretfully, for OS, maybe due to the large amount of the studies, we do not ensure sufficient resources of heterogeneity in women with gynecologic tumor.
To our knowledge, this study firstly summarizes the potential prognostic for PLR on overall gynecologic cancer patients, as well as cervical cancer, ovarian cancer, and endometrial cancer. Further, we detected the underlying sources of heterogeneity, and possible causes displayed subsequently. In addition, we recruited the ample researches in the present report. Despite the heavy workload, we furnished the convincing proof, which could offer a reference for clinical management with gynecologic cancer patients.
Some limitations still inevitably exist in this study. First, almost all of the contained observations in this data were retrospective but not prospective cohort study, which may lead to bias in data processing. At the same time, because significant results were easier to be accepted by journal now, the role of PLR may be overestimated virtually. Besides, available data extracted from the above-mentioned articles were gathered instead of specific personal information also further exaggerated the potential bias. Second, PLR was susceptible to influence via other diseases not only gynecological cancer. For example, several authors had reported chronic hepatitis B virus infection, Helicobacter pylori infection, acute kidney injury all had an impact on the value of PLR [53-56]. But many selected studies did not eliminate such corresponding conditions in our work and subsequently imbalance between groups came. Therefore, PLR cannot be used as an independent judgment prognostic indicator, but can be applied as an auxiliary indicator. Third, the other inconformity between the literatures were that not all researches adopted the multivariate model. Nearly a quarter researches used the univariate model to explore the correlation between the PLR and gynecologic cancer. Meanwhile, not all results from the recruited studies were statistically significant. Moreover, different therapeutic methods for gynecologic cancer could have influence on the observed index, but this is hard to manage.
Figure 4: Funnel plot of hazard ratio for overall survival (A) and progression-free survival (B) for high platelet-to-lymphocyte ratio (horizontal axis) and the standard error (SE) for the hazard ratio (vertical axis). Each study is represented by one circle. The vertical line represents the pooled effect estimate.
Conclusion
In conclusion, our currently results manifest that a higher value of pretreatment PLR indicates a worse prognosis among patients with gynecologic malignancies, as well as in patients with cervical, ovarian, and endometrial cancer for both OS and PFS except in those with endometrial cancer for OS. This provides us a therapeutic thought of that we could improve the prognosis of gynecological cancers by reducing the value of PLR, that is to say, attenuating the platelet count within certain range correspondingly should be considered. Though PLR do not set up as an independent prognostic indicator, it can help clinicians judge the prognosis of gynecologic cancer. But for all that, more perspective investigations need to be performed and the appropriate cut-off for PLR remains to be determined in the near future.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 82071602 and 82073392), Six Talent Project in Jiangsu Province (WSY-119, WSW-120), Nanjing Medical Science and Technique Development Foundation (QRX17072), and Science and Technology Fund of Nanjing Medical University (NMUB2018070).
Acknowledgement
None.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.[ Ref ]
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA (2014) Ovarian cancer. The Lancet 384: 1376-1388.[ Ref ]
Goff BA, Mandel LS, Melancon CH, Muntz HG (2004) Frequency of Symptoms of Ovarian Cancer in Women Presenting to Primary Care Clinics. JAMA 291: 2705-2712.[ Ref ]
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. The Lancet 387: 1094-1108.[ Ref ]
Cohen PA, Jhingran A, Oaknin A, Denny L (2019) Cervical cancer. The Lancet 393: 169-182.[ Ref ]
Jelovac D, Armstrong DK (2011) Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 61: 183-203.[ Ref ]
Goodman A (2015) HPV testing as a screen for cervical cancer. BMJ 350: h2372-h2372.[ Ref ]
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.[ Ref ]
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, et al. (2013) Inflammationinduced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13: 759-771.[ Ref ]
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436-444.[ Ref ]
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. The Lancet Oncology 15: e493-e503.[ Ref ]
Jenne CN, Kubes P (2015) Platelets in inflammation and infection. Platelets 26: 286-292.[ Ref ]
Chen X, Wang Q, Liu L, Sun T, Zhou W, et al. (2018) Double-sided effect of tumor microenvironment on platelets targeting nanoparticles. Biomaterials 183: 258-267.[ Ref ]
Spectre G, Zhu L, Ersoy M, Hjemdahl P, Savion N, et al. (2012) Platelets selectively enhance lymphocyte adhesion on subendothelial matrix under arterial flow conditions. Thromb Haemost 108: 328-337.[ Ref ]
Cannon NA, Meyer J, Iyengar P, Ahn C, Westover KD, et al. (2015) Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-smallcell lung cancer. J Thorac Oncol 10: 280-285.[ Ref ]
Chen XD, Mao CC, Wu RS, Zhang WT, Lin J, et al. (2017) Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS One 12: e0175074.[ Ref ]
Liu C, Huang Z, Wang Q, Sun B, Ding L, et al. (2016) Usefulness of neutrophilto-lymphocyte ratio and platelet-to-lymphocyte ratio in hormonereceptor-negative breast cancer. Onco Targets Ther 9: 4653-4660.[ Ref ]
McLaren PJ, Bronson NW, Hart KD, Vaccaro GM, Gatter KM, et al. (2017) Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios can Predict Treatment Response to Neoadjuvant Therapy in Esophageal Cancer. J Gastrointest Surg 21: 607-613.[ Ref ]
Nuchpramool P, Hanprasertpong J (2018) Preoperative NeutrophilLymphocyte Ratio and Platelet-Lymphocyte Ratio Are Not Clinically Useful in Predicting Prognosis in Early Stage Cervical Cancer. Surg Res Pract 2018: 9162921.[ Ref ]
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2018) Diagnostic Performance of Magnetic Resonance Imaging for the Detection of Bone Metastasis in Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 73: 81-91.[ Ref ]
Haraga J, Nakamura K, Omichi C, Nishida T, Haruma T, et al. (2016) Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol 5: 567-574.[ Ref ]
Lee JW, Jeon S, Mun ST, Lee SM (2017) Prognostic Value of Fluorine-18 Fluorodeoxyglucose Uptake of Bone Marrow on Positron Emission Tomography/Computed Tomography for Prediction of Disease Progression in Cervical Cancer. Int J Gynecol Cancer 27: 776-783.[ Ref ]
He X, Li JP, Liu XH, Zhang JP, Zeng QY, et al. (2018) Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer 9: 1877-1884.[ Ref ]
Nakamura K, Nakayama K, Tatsumi N, Minamoto T, Ishibashi T, et al. (2018) Prognostic significance of pre-treatment neutrophil-tolymphocyte and platelet-to-lymphocyte ratios in non-surgically treated uterine cervical carcinoma. Mol Clin Oncol 9: 138-144.[ Ref ]
Onal C, Guler OC, Yildirim BA (2016) Prognostic Use of Pretreatment Hematologic Parameters in Patients Receiving Definitive Chemoradiotherapy for Cervical Cancer. Int J Gynecol Cancer 26: 1169-1175.[ Ref ]
Zheng RR, Huang M, Jin C, Wang HC, Yu JT, et al. (2016) Cervical cancer systemic inflammation score: a novel predictor of prognosis. Oncotarget 7: 15230-15242.[ Ref ]
Huang H, Liu Q, Zhu L, Zhang Y, Lu X, et al. (2019) Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep 9: 3284.[ Ref ]
Holub K, Biete A (2019) Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin Transl Oncol 21: 836-844.[ Ref ]
Wang L, Jia J, Lin L, Guo J, Ye X, et al. (2017) Predictive value of hematological markers of systemic inflammation for managing cervical cancer. Oncotarget 8: 44824-44832.[ Ref ]
Zhang W, Liu K, Ye B, Liang W, Ren Y (2018) Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer. Cancer Med 7: 105-113.[ Ref ]
Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, Kowalska M, Fuksiewicz M, et al. (2018) Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Cancer Manag Res 10: 6029-6038.[ Ref ]
Zhu M, Feng M, He F, Han B, Ma K, et al. (2018) Pretreatment neutrophillymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clin Chim Acta 483: 296-302.[ Ref ]
Chen L, Zhang F, Sheng XG, Zhang SQ, Chen YT, et al. (2016) Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: Lymphocyte. Medicine (Baltimore) 95: e4381.[ Ref ]
Ida N, Nakamura K, Saijo M, Kusumoto T, Masuyama H (2018) Prognostic nutritional index as a predictor of survival in patients with recurrent cervical cancer. Mol Clin Oncol 8: 257-263.[ Ref ]
Miao Y, Yan Q, Li S, Li B, Feng Y (2016) Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark 17: 33-40.[ Ref ]
Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T (2012) Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 23: 265-273.[ Ref ]
Badora-Rybicka A, Nowara E, Starzyczny-Slota D (2016) Neutrophil-tolymphocyte ratio and platelet-to-lymphocyte ratio before chemotherapy as potential prognostic factors in patients with newly diagnosed epithelial ovarian cancer. ESMO Open 1: e000039.[ Ref ]
Liu Y, Chen S, Zheng C, Ding M, Zhang L, et al. (2017) The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer 17: 285.[ Ref ]
Supoken A, Kleebkaow P, Chumworathayi B, Luanratanakorn S, Kietpeerakool C (2014) Elevated preoperative platelet to lymphocyte ratio associated with decreased survival of women with ovarian clear cell carcinoma. Asian Pac J Cancer Prev 15: 10831-10836.[ Ref ]
Asher V, Lee J, Innamaa A, Bali A (2011) Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 13: 499-503.[ Ref ]
Li Z, Hong N, Robertson M, Wang C, Jiang G (2017) Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep 7: 43001.[ Ref ]
Farolfi A, Petrone M, Scarpi E, Galla V, Greco F, et al. (2018) Inflammatory Indexes as Prognostic and Predictive Factors in Ovarian Cancer Treated with Chemotherapy Alone or Together with Bevacizumab. A Multicenter, Retrospective Analysis by the MITO Group (MITO 24). Target Oncol 13: 469-479.[ Ref ]
Zhang WW, Liu KJ, Hu GL, Liang WJ (2015) Preoperative platelet/ lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 36: 8831-8837.[ Ref ]
. Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, et al. (2015) Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer 113: 311-320.[ Ref ]
Aoyama T, Takano M, Miyamoto M, Yoshikawa T, Kato K, et al. (2019) Pretreatment Neutrophil-to-Lymphocyte Ratio Was a Predictor of Lymph Node Metastasis in Endometrial Cancer Patients. Oncology 96: 259-267.[ Ref ]
Haruma T, Nakamura K, Nishida T, Ogawa C, Kusumoto T, et al. (2015) Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer Res 35: 337-343.[ Ref ]
Li J, Lin J, Luo Y, Kuang M, Liu Y (2015) Multivariate Analysis of Prognostic Biomarkers in Surgically Treated Endometrial Cancer. PLoS One 10: e0130640.[ Ref ]
Grenader T, Waddell T, Peckitt C, Oates J, Starling N, et al. (2016) Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 27: 687-692.[ Ref ]
Choi YH, Lee JW, Lee SH, Choi JH, Kang J, et al. (2019) A High Monocyteto-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Advanced Gallbladder Cancer Receiving Chemotherapy. Cancer Epidemiol Biomarkers Prev 28: 1045-1051.[ Ref ]
Koch A, Fohlin H, Sorenson S (2009) Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 4: 326-332.[ Ref ]
Reddy JP, Hernandez M, Gunther JR, Dabaja BS, Martin GV, et al. (2018) Pre-treatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio are prognostic of progression in early stage classical Hodgkin lymphoma. Br J Haematol 180: 545-549.[ Ref ]
Spolverato G, Maqsood H, Kim Y, Margonis GA, Luo T, et al. (2015) Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol 111: 868-874.[ Ref ]
von Loga K, Gerlinger M (2017) Cancer (r)evolution. Nat Ecol Evol 1: 1051-1052.[ Ref ]
Zhao Z, Liu J, Wang J, Xie T, Zhang Q, et al. (2017) Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int Immunopharmacol 51: 1-8.[ Ref ]
Farah R, Hamza H, Khamisy-Farah R (2018) A link between platelet to lymphocyte ratio and Helicobacter pylori infection. J Clin Lab Anal 32.[ Ref ]
Zheng C-F, Liu W-Y, Zeng F-F, Zheng M-H, Shi H-Y, et al. (2017) Prognostic value of platelet-to-lymphocyte ratios among critically ill patients with acute kidney injury. Critical Care 21: 238.[ Ref ]