Journal Name: International Journal of Cancer and Treatment
Article Type:Analysis Article
Received date:01 September, 2021
Accepted date:24 September, 2021
Published date:01 October, 2021
Citation:Johnson MA, Smits MM (2021) Revisiting Szent- Györgyi’s Cancer Hypothesis. Int J Cancer Treat Vol: 4, Issu: 2 (26-21).
Copyright: © 2021 Johnson MA et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Rationale for this communication
•The promine/retine hypothesis on the control of cancer never reached a definite conclusion.
The chemical natures of promine and retine remain unsettled though usually assumed to be glyoxalases and methylglyoxal.
•Many years ago we published some data that indicated the hypothesis may be operating in plants in which glyoxalase I may exist in normal cells in an inhibited state rather than compartmentalized as in early versions of the hypothesis.
•Manju Ray in India has published many papers claiming that methylglyoxal can be used to successfully treat cancer in humans.
We present here previously unpublished data that shows depriving glyoxalase I of GSH allows methylglyoxal to kill mouse lymphoma cells. During treatment of two human cancer cell lines, killing of one line was enhanced by blocking thioredoxin as well as GSH.
It is hoped that what is conveyed here may reignite interest in the near term. Nuclear methodology and statistics can be found in figure 1. The data show a strong interaction between the hypothesis and thiols. It is concluded that the hypothesis has yet to be thoroughly investigated.
Keywords: Methylglyoxal, Glutathione, Buthionine Sulfoximine, Auranofin, Glyoxalase.
Abbreviations: BSO = L-buthionine sulfoximine; NFCR = National Foundation for Cancer Research.
Abstract
Rationale for this communication
•The promine/retine hypothesis on the control of cancer never reached a definite conclusion.
The chemical natures of promine and retine remain unsettled though usually assumed to be glyoxalases and methylglyoxal.
•Many years ago we published some data that indicated the hypothesis may be operating in plants in which glyoxalase I may exist in normal cells in an inhibited state rather than compartmentalized as in early versions of the hypothesis.
•Manju Ray in India has published many papers claiming that methylglyoxal can be used to successfully treat cancer in humans.
We present here previously unpublished data that shows depriving glyoxalase I of GSH allows methylglyoxal to kill mouse lymphoma cells. During treatment of two human cancer cell lines, killing of one line was enhanced by blocking thioredoxin as well as GSH.
It is hoped that what is conveyed here may reignite interest in the near term. Nuclear methodology and statistics can be found in figure 1. The data show a strong interaction between the hypothesis and thiols. It is concluded that the hypothesis has yet to be thoroughly investigated.
Keywords: Methylglyoxal, Glutathione, Buthionine Sulfoximine, Auranofin, Glyoxalase.
Abbreviations: BSO = L-buthionine sulfoximine; NFCR = National Foundation for Cancer Research.
Background: The thesis research of Smits, followed by some additional research in our lab, led us plant biochemists into cancer research but neither of us has had access to research facilities beyond the 1980’s. There was a brief period in the early 1980’s when I(MAJ) had a small research grant from the National Foundation for Cancer Research (NFCR). NFCR had been founded to support research on the cancer hypothesis of Albert Szent-Györgyi. In the mid 1980’s NFCR largely abandoned his ideas in favor of molecular biological approaches. Szent-Györgyi passed away in 1986, but we (along with a few others) remain with possibly valuable insights into the cancer problem. It has now been 40 years since we reported the presence of methylglyoxal in higher plants [1]. Although many publications focus on methylglyoxal as a toxic substance, our interest at the time was to determine whether plants contained biochemicals concordant with the promine/ retine hypothesis of cancer [2-7]. In the hypothesis, promine is associated with unorganized states and retine with organized states. We were conducting research on the biochemistry involved when plants were taken into tissue culture (a new field) and then regenerated via organogenesis or somatic embryogenesis. We wondered if callus might be a state with a high promine/retine ratio.
Szent-Györgyi had proposed that methylglyoxal might be a good candidate for the role of retine whereas the glyoxalase enzyme system, which destroyed methylglyoxal, could serve as promine if that were the case. Other chemicals have been put forward for these roles, but these two appear to be made for each other, promine promoting and retine retarding mitosis.
Briefly summarizing our previous findings: Consistent with the promine/retine hypothesis Smits found that Douglas –fir needles contained methylglyoxal but needle callus did not whereas the needle callus contained both glyoxalases I and II but needles did not with the exception that needles appeared to have glyoxalase I in an inhibited state [1]. The latter was a deviation from the original hypothesis which suggested compartmentalization of promine. A postdoctoral investigator in the same lab later found evidence that the natural inhibitor was a flavonoid [4]. Also, another student working with wild carrot cells in suspension culture found that somatic embryogenesis therein was enhanced by the addition of buthionine sulfoximine [BSO, a specific inhibitor of glutathione (GSH) biosynthesis] whereas GSH addition blocked somatic embryogenesis [5]. This was important for at least two reasons: (a) GSH is an essential coenzyme for glyoxalase I and (b) in the hypothesis the reducing state (maintained by GSH) provides the milieu for the unorganized primitive cancer cells while organized cellular structures require methylglyoxal to maintain the opposite oxidizing conditions, probably by reacting readily with mitotic sulfhydryl groups. The organized state must be temporarily dismantled whenever there is a need for mitosis. In cancer (or callus? or suspension cells?), which is characterized by runaway cell division, glyoxalases must continuously destroy any methylglyoxal that may form.
Both methylglyoxal and glyoxalase had been metabolic orphans since the beginnings of biochemistry. The glyoxalases were found to be an enzyme pair prior to the emergence of the promine/retine idea and have been the subject of much more research in recent times(3). They are widely distributed and glyoxalase I is among the most active enzymes known. We have proceeded on the basis that Szent-Györgyi’s observations on the existence of promine and retine have never been rigorously disproven and that sulfhydryl groups play an essential role in mitosis and its regulation [3,6].
We occasionally discussed what had been observed in our lab until the new millennium when we read that Dr. Manju Ray in India had begun clinical studies in which she was successfully using methylglyoxal to treat human cancer patients [7]. These studies have been controversial from the start but appear to be ongoing. Most of the criticism is directed at experimental design. We were no longer in a position to conduct any laboratory or clinical studies ourselves but we were spurred to conduct some further investigations if we could find research partners. By 2012 we had enough insight and additional data to secure U.S. patent # 8163796.
Methods and Results
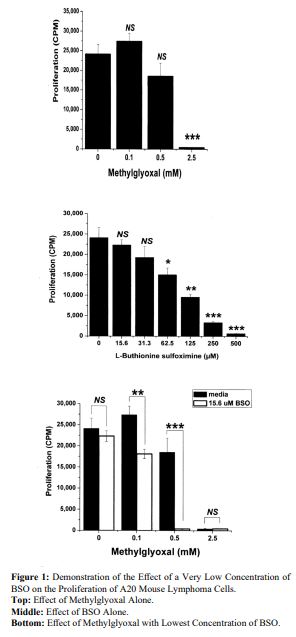
The patent showed the effectiveness of methylglyoxal in blocking the growth of mouse lymphoma cells. The patent examiner saw the additional data in figure 1 where the effect of BSO can be seen. Methodology and Statistics are embedded in the figure.
In each case 2 x 103 A20 cells were plated in 96 well plates in usual growth media without and with indicated concentrations of methylglyoxal (top), BSO (middle) or with indicated concentrations of methylglyoxal without and with 15.6 µM BSO (bottom). Experimental wells were pulsed with 0.5 µCi 3H-thymidine per well from 88 h to the experimental endpoint at 96 h to quantify proliferation/viability. Data points represent the mean of triplicate wells +/- 1 SD. Comparisons were made between the media control (0 mM) and methylglyoxal (top) or BSO (middle) experimental wells using Student’s two sample t-test (equal variance). NS = not statistically significant; * = p value <0.05; ** = p value <0.005; *** = p value <0.0005. Applying the same statistics, comparisons were made between wells containing or absent 15.6 µM BSO at each methylglyoxal concentration (bottom).
Overall Conclusion: A20 lymphoma cell proliferation was not inhibited by 15.6 µM BSO alone nor by methylglyoxal alone at 0.1 or 0.5 mM. However, addition of 15.6 µM BSO with methylglyoxal caused significant inhibition at 0.1 mM and profound inhibition at 0.5 mM.
Figure 1:Demonstration of the Effect of a Very Low Concentration of BSO on the Proliferation of A20 Mouse Lymphoma Cells. Top: Effect of Methylglyoxal Alone. Middle: Effect of BSO Alone. Bottom: Effect of Methylglyoxal with Lowest Concentration of BSO.
Discussion
Some years later we learned that treatment results were improved in one of two cultured human cancer cell lines if the involvements of both GSH and thioredoxin were minimized. A world-wide multiauthored paper came to the same conclusion with a different interpretation not involving methylglyoxal [8]. For a while we employed both BSO and auranofin (to block the thioredoxin) until we found that auranofin stopped both GSH and thioredoxin from interfering with methylglyoxal inhibition of mitosis. With the availability of glyoxalase I inhibitors now we think it may be possible to conduct anticancer efficacy experiments without adding exogenous methylglyoxal.
To reiterate, the essence of the promine/retine hypothesis continues to show some promise for the development of a treatment for cancers of all types. Manju Ray has always added ascorbic acid and has improved results by the addition of creatine to the protocol for her own reasons [7]. We have found it beneficial to focus on the roles of thiols. We recognize that it may prove hazardous to tamper with the concentrations of these thiols in whole body systems given their critical roles in many areas of metabolism; however, to date we have seen large apoptotic or other lethal responses of cancer cells to very small amounts of agents such as BSO and auranofin, so we are hopeful that crucial noncancer related metabolism may remain little affected in the required time frame. Even better is the possibility that that such tampering will prove unnecessary.
Although it may be unlikely that we will contribute much more in our lifetimes, hopefully other biochemists may see fit to pursue this hypothesis to a well- supported conclusion. Too many lives are at stake to continue ignoring this possibility even if it were to result in an application of Occam’s razor which seems to be falling out of favor.
Conclusion
The data shown here in the single figure indicate that promine and retine may exist as glyoxalases and methylglyoxal respectively and their functioning as such is dependent on concomitant thiol concentrations. The employment of glyoxalase I inhibitors now available, perhaps in combination with sulfhydryl control, may allow better testing of the original hypothesis than previously possible.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: All data generated for this report are included in figure 1. The statement about the utility of blocking both GSH and thioredoxin came from observations during an undergraduate student research project.
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: Both authors contributed equally to this research. MAJ did the writing but both read and approved the manuscript.
Funding
Self funded.
Acknowledgements
Our thanks to Dr. Raymond Johnson and Dr. Russell Feirer for their assistance over the past decade or so.
Smits MM, Johnson MA (1981) Methylglyoxal: Enzyme Distributions Relative to Its Presence in Douglas-Fir Needles and Absence in DouglasFir Needle Callus. Arch Biochem Biophys 208: 431-439.[ Ref ]
Kalapos MP (1994) Methylglyoxal Toxicity in Mammals. Toxicol Lett 73: 3-24.[ Ref ]
Kalapos MP (1999) On the Promine/Retine Theory of Cell Division: Now and Then. Biochim Biophys Acta 1426: 1-16.[ Ref ]
Brandt RB, Laux J, Thomson C, Johnson MA, Gross M (1984) Inhibition of Glyoxalase I In Vitro by Flavones. Int J Quantum Chem Quantum Biol Symp 11: 195-200.[ Ref ]
Earnshaw BA, Johnson MA (1985) The Effect of Glutathione on Development in Wild Carrot Suspension Cultures. Biochem Biophys Res Commun 133: 988-993.[ Ref ]
Chiu J, Dawes IW (2012) Redox Control of Cell Proliferation. Trends in Cell Biol 22: 592-601.[ Ref ]
Talukdar D, Ray S, Ray M, Das S (2008) A Brief Critical Overview On the Biological Effects of Methylglyoxal and Further Evaluation of Methylglyoxal-based Anticancer Formulation in Treating Cancer Patients. Drug Metab And Drug Interact 23: 175-210.[ Ref ]
Harris IS (2015) Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 27: 211-222.[ Ref ]