Journal Name: International Journal of Cancer and Treatment
Article Type: Research
Received date: 25 August, 2021
Accepted date: 06 September, 2021
Published date: 13 September, 2021
Citation: Corremans M, Verroeye A, Wijngaert LV, Goossens E, Vlaemynck G, et al. (2021) Selective Taste Management: A Selfcare Intervention for Cancer Outpatients suffering Chemotherapy-Induced Dysgeusia. Int J Cancer Treat Vol: 4, Issu: 2 (19-25).
Copyright: © 2021 Corremans M et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Currently, limited evidence-based guidelines exist for the effective management of chemotherapy induced dysgeusia in cancer outpatients. In this pilot study, we used innovative insights from gastrological sciences such as selective taste management to improve the taste of bread for cancer outpatients. We investigated whether it is feasible for cancer outpatients and family caregivers to bake personalized bread themselves at home and whether such bread is considered tasty despite their burdensome taste disorder. Included patients (N=112) are randomly divided in a bread-baking group (N=54) and a control group (N=58). The individual taste thresholds profile of all bread baking patients is assessed using the innovative O-Box. Using an algorithm, these profiles are processed into a recipe for personalized bread. Structured questionnaires and anthropometrics are used to compare the effects of personalized bread after one month follow-up. Only 17% of the bread baking group required some telephone or online assistance in order to correctly apply their personalized recipe. In 60% of the cases, the bread was prepared by the family caregiver. Compliance was high and no side effects were observed. Over 80% of the bread baking patients perceived personalized bread as equally or more tasteful despite their stressful chemotherapy-induced dysgeusia. Compared to the control group loss of bodyweight and Body Mass Index in the bread baking group was not significant (p .968 and p .956 respectively). Baking personalized bread at home appeared to be feasible. Cancer patients with chemotherapyinduced dysgeusia enjoyed the taste of bread again by applying selective taste management. This innovative gastrological self-care intervention is very promising and should be studied more in depth using whole meals in a larger cancer outpatients population.
Cancer; Chemotherapy; Dysgeusia; Food intake; Malnutrition.
Abstract
Currently, limited evidence-based guidelines exist for the effective management of chemotherapy induced dysgeusia in cancer outpatients. In this pilot study, we used innovative insights from gastrological sciences such as selective taste management to improve the taste of bread for cancer outpatients. We investigated whether it is feasible for cancer outpatients and family caregivers to bake personalized bread themselves at home and whether such bread is considered tasty despite their burdensome taste disorder. Included patients (N=112) are randomly divided in a bread-baking group (N=54) and a control group (N=58). The individual taste thresholds profile of all bread baking patients is assessed using the innovative O-Box. Using an algorithm, these profiles are processed into a recipe for personalized bread. Structured questionnaires and anthropometrics are used to compare the effects of personalized bread after one month follow-up. Only 17% of the bread baking group required some telephone or online assistance in order to correctly apply their personalized recipe. In 60% of the cases, the bread was prepared by the family caregiver. Compliance was high and no side effects were observed. Over 80% of the bread baking patients perceived personalized bread as equally or more tasteful despite their stressful chemotherapy-induced dysgeusia. Compared to the control group loss of bodyweight and Body Mass Index in the bread baking group was not significant (p .968 and p .956 respectively). Baking personalized bread at home appeared to be feasible. Cancer patients with chemotherapyinduced dysgeusia enjoyed the taste of bread again by applying selective taste management. This innovative gastrological self-care intervention is very promising and should be studied more in depth using whole meals in a larger cancer outpatients population.
Cancer; Chemotherapy; Dysgeusia; Food intake; Malnutrition.
Introduction
Chemotherapy induced taste alterations (dysgeusia) may contribute to the high prevalence of malnutrition in cancer patients. It is believed that 50-70% of patients with cancer suffer dysgeusia [1]. Taste perception comprises the detection and processing of gustatory, olfactory and trigeminal stimulations. Interactions occurring within and across these three systems might lead to taste and smell alterations [2]. Such alterations are common in the general population, with loss of smell occurring more frequently [3]. Several etiologies have been described including physiological alterations in normal aging, injuries to the oral/pharyngeal anatomy, neural injury, medications, nutritional and immune disorders and coronavirus disease [2,4,5]. Taste alterations always have a substantial impact on patients’ eating behavior and quality of life [1,6].
The causes of taste alterations specific to cancer patients are very diverse and include chemotherapy and other drugs affecting taste and smell, xerostomia, infection, and direct neurotoxicity to taste buds [7,8]. Additional known distressing side effects of chemotherapy are fatigue, nausea, vomiting, and hair loss [6,9,10]. The nature of taste and smell changes varies among cancer patients during chemotherapy [11]. The type of chemotherapy in itself is also a risk factor for the development of taste alterations. Agents such as cyclophosphamide, dacarbazine, doxorubicin, 5-FU, methotrexate, nitrogen mustard, cisplatin, and vincristine have been already associated with taste alterations and heightened sensitivity to one or several flavors [12,13]. Patients treated with gemcitabine plus a platinum agent reported the lowest levels of taste alterations [13]. Steinbach et al. found taxane-based chemotherapies to cause the most severe taste alterations, while Wickham et al. reported cisplatin and doxorubicin to be the agents most likely to cause taste alterations [14,15]. Zabernigg et al. reported a possible effect of cumulative toxicity caused by previous cytostatic treatments. Cranial nerves VII (facial), IX (glossopharyngeal), and X (vagus) all control integral sensory functions in the tongue, and damage to them has been implicated in taste alterations [7,16]. Some chemotherapy agents are secreted in saliva and gain direct contact with taste receptors. As a consequence, patients may experience a metallic or chemical taste when chemotherapy is delivered, which is consistent with drug secretion in saliva [16]. Taste alterations are important factors in the development of decreased food intake and malnutrition in cancer patients [1,17]. Currently, very limited evidence-based practice guidelines exist for the pharmacological or culinary management of dysgeusia and decreased food intake in cancer outpatients [18]. Suggestions from best practices, though useful, do not accurately resolve this stressful situation for the cancer patients involved.
This paper reports on the results of a pilot study in which an innovative approach of dysgeusia was tested on the basis of personalized and own baked bread in cancer outpatients. Since bread is important in our Western food culture, and because taste control of full meals is particularly complex, it was decided to test this innovative gastrological approach exclusively with bread in the first instance. The primary aim was to determine whether this bread was perceived as tasteful by cancer outpatients despite their chemotherapy induced dysgeusia. The secondary aim was to determine whether this intervention is feasible for outpatients and their family caregivers.
Materials and Methods
Study design
This descriptive study with an intervention and control group is a first phase pilot study of a complex intervention and was conducted similar in two outpatient chemotherapy units, one in a large university hospital and another in a regional hospital, both located in Belgium.
Patients
Adult cancer outpatients were considered eligible if they reported taste disturbances after receiving at least once intravenous chemotherapy, and if they were willing to give written informed consent. The type of cancer or chemotherapy was not an exclusion or inclusion criterion. However, patients suffering head-neck cancer, mucositis grade 2, chewing- and swallowing problems and patients receiving a combination of radiotherapy and chemotherapy were excluded in order to avoid bias in food intake. Data regarding patient characteristics were obtained with a structured identification form and from patients’ medical records.
Sample size
This trial is a pilot study primarily intended to test the feasibility of an innovative and patient centered intervention in the home setting and to determine whether personalized bread is actually perceived as tasty despite the chemotherapy-induced taste problems. For statistical analysis to be meaningful the minimum pilot trial sample size was set at 30 participants in each group.
Chemotherapy induced taste disturbances
The Chemotherapy-induced Taste Alteration Scale (CiTAS) enables valid, reliable measurement of specific symptoms of chemotherapy-induced taste alterations. CiTAS is a 5-point Likert-type scale with 18 items and 4 subscales, that was first developed by Kano and Kanda [19].
• 1st Subscale (2nd–6th items) Decline in Basic Taste: The condition of sensing the bitter, sweet, salty, sour, and umami taste by individuals is assessed.
• 2nd Subscale (13th–18th items) Discomfort: The relationship between taste alterations and nausea-vomiting, experiencing alterations in the sense of smell, having difficulty eating hot/oily/meat, and reduced appetite is assessed.
• 3rd Subscale (10th–12th items) Phantogeusia and Parageusia: The condition of individuals based on their experiences of phantogeusia and parageusia are assessed.
• 4th Subscale (1st, 7th–9th items) General taste alterations: The condition of individuals regarding their experiences of ageusia, cacogeusia, and hypogeusia is assessed.
For the assessment of the scale, scores received from each subscale are evaluated rather than the total score received from the entire scale [19]. The subscale scores are obtained by dividing the number of the items into the sum of scores of those items. The maximum score is 5 points, whereas the minimum score is 1 point that can be received from subscales.
The increase in the score shows that the intensity of taste alterations and discomfort are also increased. The CiTAS may also help evaluate the effectiveness of interventions to reduce the symptoms of taste alterations.
Individual food hedonics profile
To assess food hedonics in all individual participants the ‘O-box’ was introduced (Figure 1). The O-box, in which the ‘O’ stands for ‘Oncology’, is developed by the Center for Gastrology, a non for profit organization founded in February 2011 and located in Leuven (Belgium) www.centerforgastrology.com/en/intro.
The O-box contains 21 small bottles each containing natural food products (Table 1), some prepared in a paste others in a liquid form, all in a well-defined and reproducible concentration. These food products can be used in a multitude of concentrations and combinations. It also contains a larger bottle with a neutral yogurt dressing. Before starting the assessment any food allergies are questioned. Possible allergens present in the O-box products are marked in table 1. A full assessment using the O-box can be completed at patient’s bedside by trained chefs gastro-engineering, nurses, dieticians or other healthcare workers. To avoid inter rater variations, all food hedonics assessments in this study were executed by one and the same trained member of staff, a chef gastro-engineering. If the patients’ taste perception changes after the initial O-box assessment, with a negative influence on food intake and the gastrological intervention, a new assessment should be performed. The O-box assessment comprises three steps:
• Step 1: the food hedonics of 13 different food products (table 1) are examined. Each of these products is stimulating the trigeminal system in particular. With a stirrer, the researcher offers a little amount of each of the 13 products to the patient. The patient than indicates whether or not he/she likes it (yes or no).
• Step 2: the food products, approved by the patient in step 1, are now combined with the five basic tastes and in increasing concentrations: sweet, sour, bitter, salt and umami. The patient again indicates the preferred combinations and concentrations.
• Step 3: finally, the preferred combinations in step 2 are now combined with a standard dose (two drops) of three steering products.
It is very important and necessary that in between each food product used in all three steps of the O-box assessment the patient rinses his/her mouth with the provided neutral yogurt dressing. Also, in every assessment the three steps and the food products concerned should be used in the same order. Once all data are completed in a for this purpose designed electronic system, a visual dashboard shows the results of the individual O-box assessment. By using an algorithm it is possible for chefs with a proficiency in gastroengineering, to compose hyper personalized recipes. An O-Box assessment also includes a survey of imposed diets, likes and dislikes of food or food components and also in which stores the patient usually purchases food. This additional information is important to optimally personalize recipes as well as to advise patients on the purchase of prescribed ingredients in the stores they already know. This approach guarantees optimal patient-centered care.
Figure 1: The O-box.
Intervention
The gastrological intervention in this study involves baking personalized bread at home. The recipe for the personalized bread is made by applying an algorithm to the results of each individual O-box assessment. This algorithm determines which food ingredients, and to what amount, should be added to the dough. These natural and balanced additives ensure that the individual gustatory, olfactory and trigeminal systems are selectively and sufficiently stimulated so that food, in this case bread, tastes good despite the dysgeusia present.
Treatment allocation was based on patients’ preferences: all included patients and their primary family caregiver, in most of the cases their partner, were asked if they were willing and able to bake bread at home, at least for the duration of this trial (1 month). If yes, they received a singlebread oven, type Domo B3970 to use at home. If not, they were assigned to the control group, and had to eat bread from their local shop, as usual. Patients in the intervention group received a personalized recipe based on the results of the O-box assessment. All recipes were delivered online within 24 hours after the O-box assessment. In case of any question or doubt, patients or their family caregiver were able to contact a helpline either by telephone or by email during the 1 month follow up period. Patients in the intervention group who definitely stopped the baking of personalized bread, for whatever reason, were relocated to the control group.
Outcome measures
Primary outcome measure of the intervention is the tastefulness of the personalized bread as it is reported once a week, during one month after the start of the intervention. This measure only applies to all patients of the intervention group. All patients in the control group were not exposed to an intervention and eat bread from their local bakery as usual. Body weight, body mass index and CiTAS-scores are compared with the baseline measurement after one month in both groups.
Table 1: Overview of the food products and allergens present in the O-Box.
Ethical Approval
This study was approved by the Antwerp University Bioethical Committee (Decision No. B300201731261). All participants signed informed consent.
Statistical Analysis
The data obtained were analyzed by SPSS 20 (SPSS Inc., Chicago IL, USA) software package. Descriptive statistics reported as means and standard deviations for continuous variables and as numbers and proportions for dichotomous variables. To compare means and differences between groups paired T-tests were used. Differences were judged to be statistically significant when the P value was ≤ 0.05.
Results
A total of 112 patients participated, of which 54 baked personalized bread at home and 58 participated in the control group. Both groups are well matched as there were no significant differences in the distribution of gender (p .386), age (p .601), type of cancer (p .940) or treatment (p .945). Also anthropometric values as well as the impact of chemotherapy induced taste alterations are equally distributed (Table 2).
At baseline, allergies to a variety of products was reported by 20,3% of all patients in the intervention group (N = 54). These notifications also included 6 different food-related allergies. However, none of these allergies contraindicated the use of the O-box or the composition of the personalized recipes. During the intervention no relocation of patients to the control group was needed. All patients performed the intervention without any adverse effects during the entire follow-up period. No patient developed mucositis or chewing and swallowing problems to such an extent that the intervention was compromised. Only a few patients (N = 9) required telephone or online assistance to correctly apply the prescribed recipe. The request for help always turned out to be about the correctness and quantities of ingredients. Bread baking failed once in 1 patient. The cause turned out to be carelessness in measuring prescribed liquid ingredients. A one-off telephone or online intervention always proved to be sufficient to solve the problem that occurred. No problems were reported in connection with purchasing the necessary ingredients in local shops. In 60% of the cases, the personalized bread was prepared by the family caregivers and in 28% by the cancer outpatients themselves. If the caregiver was called in to bake the bread, it is usually because the patient is too sick, too tired, or because the smells during the baking process are not well tolerated. This latter information is lacking in 12% of the cases. For all these reasons we argue that this is a feasible intervention for cancer outpatients and their family caregivers.
Comparison of the outcome variables one month after initiating the intervention demonstrates no significant differences in the intervention group (Table 3). Meaning no loss of body weight (p .968) compared to baseline measurement and by consequence also the BMI remained stable (p .956). However, over the same follow-up period patients in the control group lost significantly body weight (p .021) and their BMI dropped significantly (p .025). Scores for the chemotherapy induced taste disturbances showed the same trend. Follow-up scores of all the CiTAS-subscales in the intervention group were not significantly different compared to the baseline scores, whereas these scores in the control group worsened significantly. Except for the subscale ‘discomfort’. The latter might be explained by the standard use of anti-emetics.
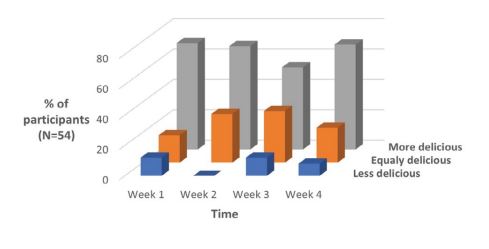
The taste of home baked personalized bread was perceived as equally or more tasteful by the majority of patients in the intervention group (every week >80% of the patients) despite the current dysgeusia (Figure 2). The percentage of patients who could not appreciate the taste of the super personalized bread ranged between 0% and 13% during the 1-month follow up period. Only one patient needed a second O-box assessment in the course of this experiment because of major changes in his taste perception. Newly tailored recipes were immediately offered on-line to him and applied successfully. Finally, we also conducted in-depth interviews of all patients from the intervention group to further understand the impact of this innovative gastrological approach. These qualitative data will be presented in a separate publication.
| Characteristics | Total N 112 | Intervention N 54 | Control N 58 | p |
|---|---|---|---|---|
| N(%) | N(%) | N(%) | ||
| GenderMaleFemale | 35 (31)77 (69) | 19 (54)35 (45) | 16 (46)42 (55) | .386 |
| DiagnosisGastro intestinal cancerUrologic cancerGynaecologic° cancerLung cancerLymphomaOther | 33 (29,5)3 (2,7)54 (48,2)5 (4,5)12 (10,7)5 (4,5) | 16 (48,4)1 (33,3)25 (46,3)3 (60,0)7 (58,3)2 (40,0) | 17 (51,6)2 (66,7)29 (53,7)2 (40,0)5 (41,7)3 (60,0) | .940 |
| Treatment protocolDoxorubicine, Bleomycine, Vinblastine, DacarbazineCarboplatinumCyclofosfamide, Doxorubicine, Vincristine, Prednisone Combi*DecitabineFluorouracil ElvorineDocetaxelEpirubicine CyclofosfamideIrinotecan, Leucovorin, Fluorouracil Oxaliplatin Irinotecan, Levofolinezuur, FluorouracilOxaliplatin, FluorouracilGemcitabine CisplatinumGemcitabine Paclitaxel Mono**PaclitaxelPaclitaxel CarboplatinumTaxotere Cyclofosfamide | 5 (4,5)3 (2,7)4 (3,6)10 (9,0)2 (1,8)2 (1,8)3 (2,7)7 ( 6,2)8 (7,1)2 (1,8) 9 (8,0)5 (4,5)3 (2,7)4 (3,6)33 (29,5)4 (3,6)7 (6,2) | 2 (3,7)2 (3,7)2 (3,7)5 (9,2)1 (1,9)1 (1,9)2 (3,7)3 (5,6)5 (9,2)1 (1,9) 5 (9,2)4 (7,4)0 (0,0)2 (3,7)14 (25,9)1 (1,9)3 (5,6) | 3 (5,2)1 (1,7)2 (3,4)5 (8,6)1 (1,7)1 (1,7)1 (1,7)4 (6,9)3 (5,2)1 (1,7) 4 (6,9)1 (1,7)3 (5,2)2 (3,4)19 (32,7)3 (5,2)4 (6,9) | .945 |
| Mean(SD) | Mean(SD) | Mean(SD) | p | |
| Age in years | 60(13,3) | 59,3 (11,6) | 60,6 (14,7) | .601 |
| Body Weight (Kg) | 72 (16,0) | 71,6 (13,9) | 72,4 (17,9) | .795 |
| Height (meter) | 1,68 (0,09) | 1,69 (9,4) | 1.67 (8,5) | .230 |
| Body Mass Index | 25,2 (5,1) | 24,7 (3,9) | 25,7 (6,1) | .339 |
| CiTAS-scoresDecline in basic tastePhantogeusia and ParageusiaDiscomfortGeneral taste alterations | 1,85 (0,80)2,16 (0,93)1,91 (0,86)2,52 (0,52) | 1,99 (0,91)2,26 (0,88)2,02 (0,84)2,64 (0,53) | 1,71 (0,66)2,07 (0,97)1,81 (0,87)2,41 (0,49) | .074.280.205.278 |
| °includes breast cancer; *Combi: other cytostatica in combinationtherapy **Mono: other cytostaticum as monotherapy. | ||||
Table 2: Patient characteristics
Discussion
In this pilot study we aimed to demonstrate in particular the effect of an innovative gastrological approach on the taste perception of bread as well as the feasibility of this approach for cancer outpatients. First point of consideration was the assessment of the individual patients’ hedonics profile by using the O-box. A variety of approaches to the assessment of taste and smell alterations have evolved in the literature including self-reporting tools that continue to generate a description of the development, duration, and recovery of distorted chemosensory perception in cancer patients. Several groups validated quantitative assessments of gustatory and/or olfactory function in a clinical context using impregnated “taste strips” or “sniffing strips” [20- 22]. The taste from a list of five descriptors, i.e., sweet, sour, salty, bitter, and umami, can be assessed serially using these strips objectively. The duration of these tests range between 8 to 10 minutes and can be completed at patient’s bedside. These tests are all designed to determine the presence of taste alterations and its severity. However, they do not solve the patient’s problem and consequently the negative impact of dysgeusia on food intake persists. The O-box however is not only meant to assess patient’s taste or smell thresholds in it. It assesses them as influenced by the chemotherapy, and in contrast to other methods, these measured results can subsequently be applied in an algorithm that leads to personalized recipes.
Gustation and olfactory functions have been demonstrated to be most disturbed by chemotherapy [1,12,13]. Therefore, food products used in the O-box are mainly targeting the less stressed trigeminal function in cancer patients during and after chemotherapy. An individual food hedonics profile can be assessed bedside, however due to the extensive possible combinations and the often large individual differences in taste preferences, the duration of a full O-box assessment ranges between 35 to 45 minutes. In terms of a bedside procedure this assessment takes a long time. This might have led to bias because of a decreased attention among patients or the investigator. However, it is worth the investment because it leads to tailored recipes, in this case of bread that actually helped patients to improve their self-care and to overcome their decreased daily food intake. Also the environment in which the O-box is used is important. Until now the O-box was only used in laboratory conditions. In this study the O-box was used for the first time in the context of busy daycare oncology clinics. As mentioned, the assessment procedure requires patients’ and assessors’ focus for a considerably amount of time. In some cases the assessment was interrupted by other diagnostic or therapeutic procedures. Also odors from the hospital environment might have had a negative effect on the bedside assessment of hedonics. Therefore, it is recommendable to perform the O-box assessment in a low-stimulus room, away from influential odors and other possible disturbances.
Treatment allocation may be a weak point in the design of this study. If patients are allowed to choose whether to perform the intervention, this may bias the results if only the most motivated patients make this choice. Also the motivation of the informal caregivers is an important aspect. They were often more motivated than the cancer patients themselves. In many cases they baked the bread instead of the patient. Blinding the allocation and also the role of informal caregivers in this innovative gastrological intervention are points for improvement in the upscaling of this innovative approach. In general, patients as well as family caregivers considered this self-care intervention as a very helpful tool in coping with the burden of their cancer treatment. As such, the home baking of personalized bread empowers this particular vulnerable group of patients and their family caregivers in a meaningful way. Finally, we demonstrated some effects on anthropometrics. Compared to the control group, body weight in the intervention group remains stable during the intervention and the one month follow up period. This is clinically relevant. Especially in cancer patients as their nutritional condition influences the therapeutical possibilities. The effects of this innovative gastrological approach on cancer cachexia and quality of life will be much more meaningful if it comprises whole meals in a much larger population. This pilot study proved the tools and gastrological approach, like the O-box and the production of personalized recipes, are ready to scale up.
| Variables | Differences before/after | |||
|---|---|---|---|---|
| Intervention group N = 54 | Control group N = 58 | |||
| Mean (SD) | p | Mean (SD) | p | |
| Loss of Body Weight (Kg) | 0,008 (1,46) | .968 | 2,983 (9,12) | .021 |
| Loss of Body Mass Index | 0,004 (0,52) | .956 | 1,051 (3,29) | .025 |
| CiTAS-scoresDecline in basic tastePhantogeusia and ParageusiaDiscomfortGeneral taste alterations | ||||
| 0,05 (0,96) | .719 | -0.31 (0,99) | .042 | |
| 0,07 (1,08) | .685 | -0.38 (1,18) | .034 | |
| 0,01 (0,67) | .903 | -0,19 (0,78) | .103 | |
| -0.10 (0,66) | .349 | 0,32 (0,64) | .001 | |
Table 3: Outcome variables compared to baseline measurements after 1 month follow-up.
Figure 2: Taste perception of personalized bread during chemotherapy as compared to taste of usual bread by the same patients before chemotherapy.
Conclusion
In this pilot study we demonstrate promising and clinical relevant results when applying selective taste management to overcome chemotherapy induced dysgeusia. Home baked personalized bread was perceived as tasteful by over 80% of the participating cancer patients. Compared to baseline measurements, and in contrast with a control group, the mean bodyweight of bread baking patients remained stable after one month follow up. Given the absence of any adverse effects, the minimal need for support and the high degree of adherence we argue that this is a feasible intervention for cancer outpatients and their family caregivers. Therefore, selective taste management should be studied more in depth using whole meals in a larger cancer population.
Pugnaloni S, Vignini A, Borroni F, Sabbatinelli J, Alia S, et al. (2020) Modifications of taste sensitivity in cancer patients: a method for the evaluations of dysgeusia. Support Care Cancer 28: 1173-1181.[ Ref ]
Verhagen JV, Engelen L (2006) The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev 30: 613-650.[ Ref ]
Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, et al. (2021) The Prevalence of Olfactory Dysfunction in the General Population: A Systematic Review and Meta-analysis. Am J Rhinol Allergy 35: 195-205. [ Ref ]
Mercante G, Ferreli F, De Virgillio A, Gaino F, Di Bari M, et al. (2020) Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngology-Head and Neck surgery 146: 723-728.[ Ref ]
Lechien JR, Chiesa-Estomba CM, De Siati DR (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277: 2251-2261.[ Ref ]
Wagland R, Richardson A, Ewings S, Armes J, Lennan E, et al. (2016) Prevalence of cancer chemotherapy-related problems, their relation to health-related quality of life and associated supportive care: a crosssectional survey. Support Care Cancer 24: 4901-4911.[ Ref ]
Zabernigg A, Gamper EM, Giesinger JM, Rumpold G, Kemmler G, et al. (2010) Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist 15: 913-920.[ Ref ]
Murtaza B, Hichami A, Khan AS, Ghiringhelli F, Khan NA (2017) Alteration in Taste Perception in Cancer: Causes and Strategies of Treatment. Front Physiol 8: 134. [ Ref ]
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, et al. (2010) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 18: 1039-1360.[ Ref ]
Sarenmalm EK, Browall M, Gaston-Johansson F (2014) Symptom Burden Clusters: A Challenge for Targeted Symptom Management. A Longitudinal Study Examining Symptom Burden Clusters in Breast Cancer. Journal of Pain and Symptom Management 47: 731-741.[ Ref ]
Boltong A, Keast R (2012) The influence of chemotherapy on taste perception and food hedonics: a systematic review. Cancer Treat Rev 38: 152-163.[ Ref ]
Camp-Sorrell D (2005) Chemotherapy toxicities and management. In: Yarbo, C., Frogge, M., Goodman, M. (eds) Cancer Nursing. Jones and Bartlett Publishers, Sudbury[ Ref ]
Bernhardson BM, Tishelman C, Rutqvist LE (2008) Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer 16: 275-283. [ Ref ]
Steinbach S, Hundt W, Zahnert T, Berktold S, Böhner C, et al. (2010) Gustatory and olfactory function in breast cancer patients. Support Care Cancer 8: 707-713.[ Ref ]
Wickham RS, Rehwaldt M, Kefer C, Shott S, Abbas K, et al. (1999) Taste changes experienced by patients receiving chemotherapy. OncolNurs Forum 26: 697-706.[ Ref ]
Epstein JB, Barasch A (2010) Taste disorders in cancer patients: pathogenesis and approach to assessment and management. Oral Oncol 46: 77-81.[ Ref ]
Hutton JL, Baracos VE, Wismer WV (2007) Chemosensory Dysfunction Is a Primary Factor in the Evolution of Declining Nutritional Status and Quality of Life in Patients With Advanced Cancer. J Pain Symptom Manage 33: 156-165.[ Ref ]
.Sevryugin O, Kasvis P, Vigano ML, Vigano A (2021) Taste and smell disturbances in cancer patients: a scoping review of available treatments. Support Care Cancer 29: 49-66.[ Ref ]
Kano T, Kanda K (2013) Development and validation of a chemotherapyinduced taste alteration scale. Oncol Nurs Forum 40: E79-E85. [ Ref ]
Mueller C, Kallert S, Renner B, Kobal G (2003) Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 41: 2-6.[ Ref ]
Smutzer G, Desai H, Coldwell SE, Griffith JW (2013) Validation of edible taste strips for assessing PROP taste perception. Chem Senses 38: 529-539.[ Ref ]
Walliczek U, Negoias S, Hähner A, Hummel T (2016) Assessment of Chemosensory Function Using “Sniffin’ Sticks”, Taste Strips, Taste Sprays, and Retronasal Olfactory Tests. Curr Pharm Des 22: 2245-2252.[ Ref ]