Journal Name: International Journal of Food and Bioscience
Article Type: Research
Received date: 13 July, 2018
Accepted date: 27 July, 2018
Published date: 06 August, 2018
Citation: Kesen MA, Aiyegoro OA (2018) Beneficial Characteristics and Evaluation Criteria of Probiotics. Int J Food Biosci Vol: 1, Issu: 1 (19-26).
Copyright: © 2018 Kesen MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Probiotics including Lactobacillus, Bifidobacteria, Enterococcus, Streptococcus, Bacillus, Lactococcus and some yeasts are usually present in the human gastrointestinal tract, fermented milk and other dairy products, human faeces and breast milk. Source of strains and the ability to reach the target site in the gastrointestinal tract are important. Strains must be able to survive on different physiochemical, enzymatic and microbial stresses throughout the gastrointestinal transit before they can exhibit beneficial effects on the host’s health. They can improve the intestinal microbial equilibrium and stimulate butyrate production, which promotes the growth of epithelial cells that lead to an increase in the thickness of the cecal and colonic mucosa for a better absorption of nutrients. They also produce a variety of metabolic end products with antagonistic properties against pathogens. These products include bactericidal proteins and antibiotic-like metabolites termed bacteriocins (such as nisin). The inhibitory spectra of several bacteriocins include food spoilage microorganisms and/or food-borne pathogens. Bacteriocins are considered to be safe natural preservatives or bio-preservatives because it is assumed that they can be degraded by the proteases present in the gastrointestinal tract. The joint working group of Food Aid Organization/World Health Organization made guidelines that can be used for assessing probiotics in food. The minimum requirements include assessment of strain identity and safety, and studies of health benefits in the target host. The continuous search for novel probiotics of importance in medical, industrial and agricultural environments is ongoing around the word. This review summarizes the current state of evaluation criteria for determining the candidacy of novel probiotics.
Keywords
Probiotics, Bacteriocins, Gastrointestinal Tract, In vivo studies, Enzymes, Lactobacilli, World Health Organization, Food Aid Organization.
Abstract
Probiotics including Lactobacillus, Bifidobacteria, Enterococcus, Streptococcus, Bacillus, Lactococcus and some yeasts are usually present in the human gastrointestinal tract, fermented milk and other dairy products, human faeces and breast milk. Source of strains and the ability to reach the target site in the gastrointestinal tract are important. Strains must be able to survive on different physiochemical, enzymatic and microbial stresses throughout the gastrointestinal transit before they can exhibit beneficial effects on the host’s health. They can improve the intestinal microbial equilibrium and stimulate butyrate production, which promotes the growth of epithelial cells that lead to an increase in the thickness of the cecal and colonic mucosa for a better absorption of nutrients. They also produce a variety of metabolic end products with antagonistic properties against pathogens. These products include bactericidal proteins and antibiotic-like metabolites termed bacteriocins (such as nisin). The inhibitory spectra of several bacteriocins include food spoilage microorganisms and/or food-borne pathogens. Bacteriocins are considered to be safe natural preservatives or bio-preservatives because it is assumed that they can be degraded by the proteases present in the gastrointestinal tract. The joint working group of Food Aid Organization/World Health Organization made guidelines that can be used for assessing probiotics in food. The minimum requirements include assessment of strain identity and safety, and studies of health benefits in the target host. The continuous search for novel probiotics of importance in medical, industrial and agricultural environments is ongoing around the word. This review summarizes the current state of evaluation criteria for determining the candidacy of novel probiotics.
Keywords
Probiotics, Bacteriocins, Gastrointestinal Tract, In vivo studies, Enzymes, Lactobacilli, World Health Organization, Food Aid Organization.
Introduction
Probiotics are generally considered to be those live microbes providing beneficial health effects to their hosts after they are ingested or consumed together with food [1]. Metchnikoff was the first person who reported an observed enhanced health among the Bulgarian peasants in 1908, which was a result of continuous consumption of yogurt fortified with live lactic acid bacteria (LAB) [1].
LAB including Lactobacillus, Bifidobacteria, Enterococcus, Streptococcus, Bacillus, Lactococcus and some yeasts have been reported widely as probiotics, so, they dominate in the human gastrointestinal tract (GIT), fermented milk and other dairy products, human faeces and breast milk [2]. Lactobacilli and Bifidobacteria are both Gram-positive, non-pathogenic rods or coccobacilli members of the intestinal tract, and Int J Food Biosci 2018 20 are the most reported genera of probiotic strains; both are catalase negative, facultative anaerobes, grow on sugars and produce lactic acid as an end product [3-5]. Probiotics are identified based on criteria that are believed to be important for ensuring their efficacies. These criteria may include the source of strains and the ability to reach the target site in the GI tract, for which the strain has to be able to survive under different physiochemical, enzymatic and microbial stresses throughout the GI transit. The stomach has an acidic environment where microorganisms can be forced to go through the stress of low pH and high bile salts, after which the strain has to be able to colonize the GI tract because it has to compete with the already present microbial community there for available nutrients. The ability to adhere or attach on the mucus surface covering the gut epithelium is also required to achieve such a competitive advantage [1,6].
Probiotics are believed to offer effects that are beneficial to the host’s health through a direct influence on the microbial communities of the gut, i.e. they improve the intestinal microbial equilibrium and the stimulation of butyrate production, which promotes the growth of epithelial cells that lead to an increase in the thickness of the cecal and colonic mucosa for a better absorption of nutrients [7,8]. They also produce a variety of metabolic end products with antagonistic properties against pathogens. These products include bactericidal proteins and antibiotic-like metabolites termed bacteriocins.
The mainly characterised active ingredient produced by probiotics are bacteriocins (such as nisin), which are considered to be safe natural preservatives or biopreservatives because it is assumed that they can be degraded by the proteases present in the GIT [9]. The inhibitory spectra of several bacteriocins include food spoilage microorganisms and/or food-borne pathogens [10,11].
The continuous search for novel probiotics of importance in medical, industrial and agricultural environments is ongoing around the word. Potential probiotics must be able to pass basic probiotic attributes test, survive in the gastrointestinal conditions particularly at low pH and high bile concentration, produce antimicrobial compounds and be adhesive to the intestinal mucosa. This review summarizes the current state of evaluation criteria for determining the candidacy of novel probiotics.
Common Views of Probiotics
In contemporary life, the live bacteria with beneficial health effects on humans are generally referred to as probiotics. Probiotic is a word coined from Greek word meaning “for life” [12]. The word probiotic has been given many similar definitions. Lilly and Stillwell described probiotic as the “substances secreted by one microorganism which stimulate the growth of another” [13]. It was also defined by Parker as “organisms which contribute to intestinal microbial balance” [12]. Fuller came with another definition: “A live microbial supplement which beneficially affects the host by improving the intestinal microbial balance” [12]. The Food and Agriculture Organization (FAO) and World Health Organization (WHO) have defined probiotics as live microorganism cultures which offer health benefits to the host when they are consumed in sufficient quantities [14]. The FAO/WHO definition is the most approved and widely accepted definition of probiotics. Such microorganisms may not necessarily be perpetual inhabitants of the GIT, but these microbes should have a beneficial effect on the general health of the host. “In relation to food, probiotics are considered as viable preparations in foods or dietary supplements to improve the health of humans and animals” [15].
Brief Historical Development of Probiotics
Metchnikoff claimed that LAB found in yoghurt inhibited the proliferation of toxigenic bacteria in the gut, resulting in increased longevity of the host [14]. Evidence to back up the claims made by Elie Metchnikoff has been validated over time. For instance, an E. coli strain known as EcN was successfully used to treat constipation and colitis in Germany [16]. Bifidobacteria discovered in breast-fed infants by Tissier in 1906, was reported to have clinical benefits from modulating the microbial flora in infants with intestinal disorders. Another probiotic bacterium, Bacillus coagulans was used in therapy for relieving rheumatoid arthritis [1].
Now, probiotics are obtainable in a wide range of live bacterial supplements which are used to improve health [3,7]. The beneficial effects of probiotics on the host are through growth and activity of the probiotic strain and its ability to stay viable and effective at the targeted site [6]. The joint working group of FAO/WHO made guidelines that can be used to assess probiotics in food. The minimum requirements include: Assessment of a strain identity such as genus and species; In vitro tests for potential probiotic properties such as resistance to bile acid and digestive enzymes; Assessment for safety ensuring no contamination; In vivo studies of healthy effects on the host [6].
Sources of Probiotics
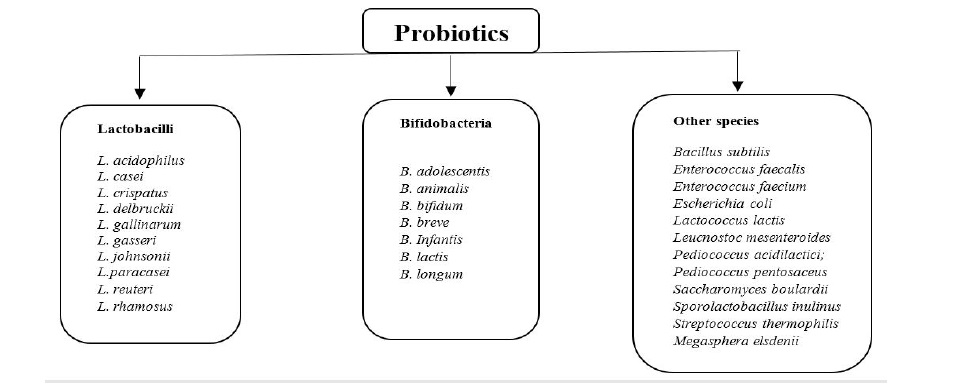
Probiotic bacteria belong to Staphylococcus, Streptococcus, Lactobacillus, Bifidobacterium and Enterococcus have been isolated from various sources, such as human breast milk, plant- and meat-based foods, human and animal faecal materials, and guts of animals [17,18]. The common probiotics are shown in figure 1. However, LAB are microorganisms mostly recognized as probiotics that can be isolated from sources such as fermented food products, for example, yogurt and kefir [3,14,16]. Most fermented milk products have a large composition of the LAB, which make them useful sources of probiotics [5].
Figure 1: Some microorganisms commonly recognised as probiotics [21].
Probiotics have been proven to have beneficial effects on the host by helping in alleviation of lactose intolerance, decrease in fecal enzymes and treating some types of diarrheas e.g. traveler’s diarrhea. The intake or consumption of probiotics is done in many different ways, because many food products containing probiotics are made, including pelleted feed, fermented feed, paste and powder [7]. Other sources of probiotics include cheese, cereal, smoothies, infant and toddler formula and nutritional bars. Probiotics are often sold as drugs, medical foods and dietary supplements, where the probiotics can be dried, and packaged into capsules, tablets or sachets [19,20].
General Methods Used for the Isolation and Characterization of Probiotics
Each selected probiotic strain must be pure, but when used in combination of pure strains, the proportion of each should be known. Probiotics must be non-pathogenic and non-toxic and able to help in the healthy functioning of human body systems, therefore, strains selected as probiotic, should be fully characterized and identified.
A Gram stain reaction is a standard laboratory procedure used to detect the presence and morphology of bacteria in a sample. The outcome of the result is generally either interpreted as Gram-positive or Gram-negative. It gives relatively quick results as to which types of bacteria present in a sample. The Gram stain involves preparing a pure bacterial isolate smear on a glass slide and allowing it to dry. The slide is then stained with special dye, decolourised and then counter stained. The procedure is based on microorganisms’ ability to retain color of the stains/dyes used during the Gram staining reaction. Gram-negative bacteria are decolorized by the use of alcohol, losing the purple color of the primary stain and taking on the pink colour of the counter stain. Gram-positive bacteria are not decolorized by the alcohol and will retain the purple colour of the primary stain/dye. The Gram stain is a very important initial step in the characterization of probiotic bacteria [22].
Furthermore, it is also an important step in the screening of potential infectious/pathogenic bacteria, so that such undesired isolates will be immediately dropped off as a candidate or potential probiotic. The mostly common morphologies of probiotic bacteria are the following:
- a) Cocci and spherical bacteria may be in a pair (diplococci), a group of four (tetracocci), a grape-like cluster (Staphylococci), a chain (Streptococci) or a cubical arrangement of eight or more (sarcinae), for example, Megasphaera elsdenii, Streptococcus thermophilis, Lactococcus lactis.
- b) Bacilli and rod-shaped bacteria occur singly, but can sometime be found in a pair (diplo-bacilli) or a chain (streptobacilli) such as Lactobacillus rhamnosus, Leucnostoc mesenteroides, Sporolactobacillus inulinus.
- c) Some probiotic bacteria may have other shapes such as coccobacilli with elongated spherical or ovoid form, filamentous bacilli occurring in long chains or threads, and fusiform bacilli with tapered ends, for example, Streptomyces, Bifidobacterium breves, B. bifidum.
Scanning electron microscope (SEM) is useful to observe and identify the shape and surface structure of the bacterium under study from a liquid culture. Nation described detailed procedures for using SEM [23].
Catalase production test is another important preliminary screening step, the enzyme catalyzes the breakdown of hydrogen peroxide into oxygen and water. Catalase test is usually performed on a single isolated colony that is picked and streaked on a glass slide, and one drop of 15% hydrogen peroxide added on to the smear. The effervescence of oxygen indicates the positive response of the bacteria to catalase test [24]. The catalase test is one of the diagnostic tests for the recognition of catalase positive bacteria due to its simplicity. In performing catalase test, if no bubble was observed, this is an indication that the isolated bacterium is catalase negative and cannot mediate the decomposition of H2O2 to O2 [25,26]. In general, 3% (v/v) of H2O2 is used for the aerobic culture while 15% (v/v) of H2O2 is used for the detection of catalase in anaerobes. The culture used for the test should not be more than 24 hours old. The main uses of catalase test are to differentiate between the morphologically/structurally similar Enterococcus or Streptococcus (catalase negative) and Staphylococcus (catalase positive) and between aerobic and obligate anaerobic bacteria.
Sugar fermentation test is performed using different sugar substrates, such as arabinose, sucrose, maltose, lactose, sorbitol, and glucose [28]. About 0.1 g of each sugar substrate will be added to 100 mL of the medium (0.1%, w/v). Five ml of sugar-containing medium will be transferred into different test tubes. For gas detection, Durham tube will be inserted into the test tube containing different sugars with indicators (for colour changes during sugar fermentation). All the tubes will be sterilized for 15 min at 121 °C. The tubes will then be inoculated with a single colony of the bacterium under study. The positive reaction will be indicated by the appearance of bubbles and/or the changes in the colour of the medium.
Biolog is an automated system that was first introduced in 1989. Pyar and Peh described detailed procedures for testing carbohydrate utilization spectra of a number of anaerobic bacteria [28]. The isolated bacterium is cultured on MRS agar plates at 37 °C for 48-72 hrs. A single cell colony from MRS agar is now sub-cultured in Brain Heart Infusion (BHI) medium for 36-48 hrs. The cultured bacterium is then suspended in anaerobic Biolog fluid. The turbidity of the suspension is monitored and measured using the Biolog turbidity meter until reaching 65% of transmittance. The suspension (100 μL) is then pipetted into each of the 99 wells of the Biolog Micro PlateTM. The plate is then incubated for 24 hrs at 37 °C in an anaerobic jar containing only CO2 gas using oxygen-free atmosphere kit. The plate after incubation is then inserted into the Biolog automatic analysis system and the identification process is carried out using the Biolog software [28].
Criteria for the Selection of Probiotics
A potential probiotic candidate should have more than a few desirable properties, which should be evaluated during the development of novel strains and probiotic products. Nonetheless, no individual candidate ought to pass all probiotic quality attributes.
The original source of a probiotic strain is one of the important factors to be considered since microbial species that are already present in the intestinal flora may have a better chance to survive in their native environment and tolerating harsh gastrointestinal conditions [27]. The ability of microorganisms to colonize the GIT is often considered as one of the main selection criteria for potential probiotics. Below are some of the criteria which a novel probiotic candidate should be able to exhibits.
The list is not exhaustive, but a very promising probiotic strain should be able to pass or inherently exhibit most of these criteria:
Acid tolerance
In order to survive passage through the gastrointestinal tract, resistance to low pH is important. Acid tolerance is determined according to methods described by Hyrominus et al. [29]. Cells of Lactobacillus strains are usually grown on deMan Rogosa Sharpe (MRS) broth at 37 °C overnight, and sub-cultured in fresh MRS (3%, v/v) at pH 2-3. Survival rate is then calculated as the percentage of the CFU after incubation at 37 °C for 60 and 180 min respectively compared to the CFU at time of 0 min. If there would be 50% of the CFU after 180 min, then the isolate is adjudged to be acid tolerant and it is assumed that it will tolerate and survive the acidic condition of the GIT.
Bile salt tolerance
In order to survive passage through the gastrointestinal tract, resistance to bile and pancreatic enzyme are important. Bile tolerance test is usually conducted using a modified method of Gilliland et al [30]. Normally, late-log phase cultures of Lactobacillus strains are inoculated (3%, v/v) into MRS broth containing 0.3, 0.5 and 1.0% (w/v) oxgall respectively. Survival rate is calculated as the percentage of the CFU after incubation at 37 °C for 240 min compared to that at time of 0 min.
Tolerance to simulated human gastrointestinal tract
Tolerance is measured in vitro by determining the viability of isolate under the simulated human gastrointestinal tract conditions. Gilliland et al [30] described procedures using simulated gastric and pancreatic juices prepared by suspending pepsin (3 mg/mL) and pancreatin USP (1 mg/ mL in sterile 0.5% (w/v) of sodium chloride solution), at pH values of 3.0 and 8.0, respectively. About 0.2 ml of washed cell suspensions of the bacteria in phosphate buffered saline (PBS, pH 7.0) is then inoculated into 1.0 ml of simulated gastric or pancreatic juice and 0.3 ml NaCl (0.5%, w/v) and incubated at 37 °C. Cultures are incubated for 180 min for gastric transit tolerance assay, and for 240 min for small intestinal transit tolerance assay, then, total viable counts are evaluated as CFU.
Antibiotic resistance
Uncontrolled use of antibiotics in human and veterinary medicines, has led to increasing antibiotic resistance in microorganisms [31]. Therefore, checking if a probiotic strain can act as a donor of conjugative antibiotic resistance genes is a wise restraining measure to curtail further spread of antibiotic resistance [32]. Currently, the spread of antimicrobial resistance microorganisms is a serious public health problem, “therefore, it is of utmost importance that potential probiotic strains be tested for antibiotic resistance genes” [33], for the purpose of clearing it off possibility of donating mobile antibiotic resistance genes to the pathogenic microorganisms in the gut. The absence of transferrable genes providing resistance to clinically relevant antibiotics is also desirable [34]. Additionally, it should be made sure that virulence genes is absent from the genome of probiotics, so the safety of selected probiotic strains should therefore be evaluated for potential virulence factors that might cause infection [35]. “Because genes involved in these activities could be present but not expressed, negative phenotypic results should be confirmed by proving the absence of key genes with PCR techniques and/or DNA hybridization” [36]. Nevertheless, antimicrobial resistance in a potential probiotic strain is normally not considered to be a risk factor unless such resistance is transferred to pathogens causing untreatable infections [37].
Antimicrobial activity
To have a positive impact on the colonic flora, it is desirable for probiotic strains to have antagonistic effects towards enteric pathogens. This can happen via antimicrobial substance (Bacteriocins) production or competitive exclusion [31]. The most popular assay for the determination of antimicrobial activity of probiotic candidate is the agar well diffusion assay. The isolated culture may be incubated for 48 hrs in MRS broth at 37 OC. The cell free supernatant is obtained by centrifugation (10,000 x g, 10 min) followed by sterile filtration. Thereafter, 24 hrs broth culture of target strains are inoculated on solid Muller-Hinton agar medium by spread plate method. Wells are then bored on each of the agar plates. These wells are then filled with 100 μL of previously prepared cell free supernatant solution. Target strain inoculated plate with an un-inoculated MRS broth will serve as controls. The plates are incubated at 37 OC for 24 hrs and the inhibitions zones are then visualized and measured, with bigger diameters as an indication of higher antimicrobial activities.
Hemolytic activity and blood biochemistry parameters
Hemolysin production is usually analysed on Columbia agar plates containing 5% (v/v) sheep blood [37]. The presence of β- or α-haemolysin is indicated by the formation of clear or greenish zones around the colonies. Haemolysin is a very common virulence factor among pathogens that frequently cause anaemia and oedema in the host, and hence, haemolytic strains are not recommended for use as probiotic. Therefore, it would be preferable to select only the non-haemolytic strains as probiotic candidates.
The probiotics effect on blood biochemistry parameters such as total serum protein, cholesterol, glucose, globulin and albumin after administration of probiotic strains should be normal; a high concentration of albumin and globulin in blood is an indication of infection and dehydration [38]. Probiotic effect on haematology i.e. neutrophils and basophils after ingestion should also be maintained at normal level, therefore, probiotics should not stimulate production of more neutrophils and basophils which is an indication of infection [39,40].
Antibodies stimulation activity
Probiotics in the intestinal tract could act as immune adjuvant to the immune system, which may result in stimulating antibody production [41]. To verify the antibody stimulation potential of a candidate probiotic, an aliquot (2 mL) of blood samples is usually collected into serum bottles and centrifuged at 10,000 x g, 15 min, and the resulted fractionated sera are then aspirated using a Pasteur pipette into sterile 5 mL centrifuge tubes, which should be performed within 12 hours of preparation. Appropriate enzyme-linked immunosorbent assay (ELISA) kits are used for measuring the degree of antigen-antibody reaction.
Antioxidant activity
A good probiotic should have abilities to demonstrate viable antioxidant potential, and probiotic may exhibit antioxidant activities in different major ways [73], which may include reinforcing the inherent cellular antioxidant defence by secreting enzymes like superoxide dismutase (SOD), releasing and promoting the production of the major non-enzymatic antioxidant (such as the exopolysaccharides, EPSs) and free-radical scavenger glutathione (GSH), exhibiting metal chelating activity. Research data suggest that probiotics may have a potential therapeutic role in reducing reactive oxygen species (ROS) and combating characterized gastrointestinal disorders.
To evaluate the total antioxidative activity (TAA) of probiotic strains, the linolenic acid (LA) test may be used as described by Kullisaar et al. [42]. Using 45 μL of the samples (lysate or whole bacterial cells). The absorbance at 534 nm will be measured using a spectrophotometer, and the percentage of TAA of the samples is expressed as [1 − (As/Ac)] × 100], where As is the absorbance in the presence of the sample and Ac is the absorbance of the control without sample. Reduced and oxidized glutathione and the glutathione redox status may be evaluated using cell-free extracts and the GSH/GSSG Ratio Assay kits. The glutathione content will then be quantified on the basis of a standard curve generated with known amounts of glutathione. The reduced glutathione (GSH) calculated as the difference between the total GSH and the oxidized glutathione (GSSG). The glutathione redox ratio is expressed as GSH/GSSG.
Extracellular Enzyme Production
Extracellular enzyme production assay of selected probiotic strains is usually determined quantitatively, as described by Nandi et al [18]. They described the enzyme assay methods as the following. Selected strains will be cultured in selected broth media that are used for the production of the enzymes. Starch broth, peptone-gelatin broth, carboxymethyl-cellulose broth and lipase production broth media are used for amylase, protease, cellulase and lipase assays, respectively. Cultures will be incubated with shaking (100-120 rpm at 30 ± 1 °C for 72 hrs). After incubation, the contents are centrifuged (10,000 x g, 10 min, 4 °C) and the supernatant collected is used for determining the activities of amylase, protease, cellulase and lipase, and the protein content of the supernatant can be estimated according to Bradford assay [43]. A probiotic strain should not have β-glucuronidase activity, which may have negative effects on the colon because it has been associated with incidence of colon cancer (most cancer cells show higher β-glucuronidase activity) [44]. In contrast, strains that are able to produce β-galactosidase are desired because it is a beneficial enzyme supporting the reduction of lactose intolerance as well as milk acidification [44]. Strains which produce α-glucosidase and β-glucosidase that may contribute to polysaccharide digestion are also desired [45]. Extracellular enzyme producing bacteria in the gut have stimulating effects on the digestive processes of the host. Many bacterial enzymes can be very helpful for digesting carbohydrates, proteins and some special substrates such as cellulose, which can be digested only by a few animals. Therefore, prospecting and application of probiotics with enzyme producing ability is gaining more attention.
Adherence to the intestinal mucosa wall
Adhesion will enable probiotcs attaching on the intestinal mucosa wall, which will prevent colonization of pathogens in the gut [46,47], show antagonistic activity against pathogens, modulate the immune system and increase the primary defense of the body [48]. Assays for testing auto-aggregation ability, cell surface hydrophobicity and co-aggregation may be used as an initial identification of promising adherent bacteria [49].
Mutagenicity (Ames assay)
It is always a good check to determine the antimutagenicity and mutagen-binding ability of the potential probiotic bacterial strain [50-53]. Probiotic strain with antimutagenic potential will play an important role in preventing mutagen formation in the intestinal tract, and such could be used for treating obstructing colon cancer.
Cytotoxicity tests
Many probiotics are marketed as foodstuffs or drugs for human consumption. Therefore consideration of the safety of probiotics is of utmost importance. One of the most important characteristics to establish a probiotic strain is that it must be non-pathogenic and, furthermore, should possess generally accepted as safe (GRAS) status [74]. Probiotics are expected to be selective in their cytotoxicity; should be friendly to the host cells and deadly to the pathogen and cancer cells. Little is known about the mechanisms by which probiotics induce cytotoxicity on cancer cells and anti-inflammatory responses, despite many studies.
Stable and viable for reasonable periods under storage and field conditions
Bacterial viability and maintenance of desirable characteristics during manufacture and storage of products are a necessity for probiotic strains. Shelf life [32] studies should be carried out periodically to assess the viability of the probiotic strains over a period of time.
Capability of exerting a beneficial effect on host
Consumption of food containing LAB may provide a range of health benefits including immune system modulation, stronger resistance to malignancy and infectious diseases. Also, a good probiotic bacterium should have an efficient cholesterol reduction ability. According to Kumer and coworkers [54], probiotics have many healthy biological properties, and one of them is anti-cholesterol assimilation.
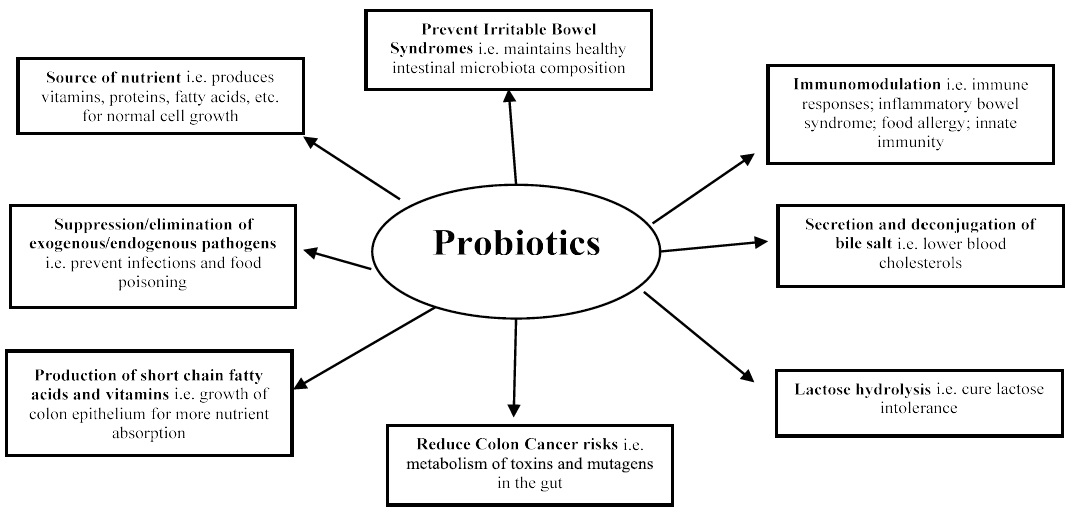
Recently, numerous studies have considerable evidences that probiotics influence several aspects of the acquired and innate immune responses by inducing phagocytes and IgA secretion, modifying T-cell response, enhancing Th1 response and attenuating Th2 response [57]. Probiotics have showed abundant health benefits beyond providing basic nutritional advantages [58], and the evidences supporting effects of the beneficial claims of probiotics have increased (Figure 2). These include the improvement of intestinal health, enhancement of the immune response, decrease in blood cholesterol, and cancer prevention. These healthenhancing properties are attributed to different strains and impacted by various mechanisms [59]. Several studies have reported positive effects due to the administration of probiotics, especially on the public health of humans by reducing antibiotic consumption used in food producing animals. This will ultimately lower the presence of drug and multi-drug-resistant organisms in the environments [60].
Figure 2: Health benefits of probiotic (modified from [56]).
Perspectives of the Development of More Probiotics
Probiotics are a promising future as alternative health therapy. Research studies have shown that beneficial effects of probiotics on humans are potentially enormous [61]. More studies should be done on probiotic strains to check their efficacy on targeted human diseases and conditions, with further investigations on the mechanisms of action behind their conferred beneficial effects [62]. Better knowledge of probiotic mechanisms should be obtained by further studying their mode of action [61,62]. Therefore, individual evaluation of potential probiotic safety should be taken into account. Hence, the presence of genes associated with virulence and resistance factors in the potential probiotic strains must be investigated. It is in our opinion that more studies at screening the candidacy of strains will provide further understanding of the abilities of the chosen candidate strains to colonize and be able to outcompete the pathogens in the gastrointestinal tract with no aptitude to initiate infections and/or cause any harm to humans when consumed.
Recommendations and Conclusion
Probiotics are incorporated into both dairy and non-dairy based foods to enhance their nutrition and health values, such foods may include yoghurt, cheese, infant formulas, breakfast cereals, sausages, chocolate, and ice cream. LAB are used in food fermentation, which offers a very valuable economic importance. For example strains of L. acidophilus well adapted to the intestine are able to ferment milk to produce mildly acidified yoghurts accepted as healthy foods comsummed worldwide [63-67].
The principle behind the selection of good probiotic includes those that are safe for consumption and survival in the gastrointestinal tract. The probiotic strain must be able to overcome the extremely low pH and the emulsifying effect of bile salts, and reach the site of action in a feasible physiological state [68]. Good probiotic should also be accepted by the immune system and it should be pathogen-free, allergenfree, mutagen-free [69], and should be compatible with the host GI environment [70]. Additionally, good probiotic should have good ability to adhere in the intestinal epithelial cells through hydrophobicity, auto-aggregation and coaggregation. Moreover, probiotic microorganisms should have no connection with diarrheagenic bacteria, no ability to transfer antibiotic resistance genes [71], and no expression of virulence genes [72]. For practical and commercial applications, probiotics must be easily culturable on a largescale and must resist technological manipulation such as heating and low oxygen in packages [20].
Acknowledgement
Acknowledgements to the supports from the University of Waterloo and the Agricultural Research Council, South Africa.
Soccol CR, Vandenberghe LPS, Spier MR, Medeiros ABP, Yamaguishi CT, et al. (2010) The Potential of Probiotics: A Review. Food Technol Biotechnol 48: 413-434. [ Ref ]
Getahun L, Tesfaye A, Muleta D (2017) Investigation of the Potential Benefits and Risks of Probiotics and Prebiotics and their Synergy in Fermented Foods. Singapore Journal of Chemical Biology 6: 1-16. [ Ref ]
Azizpour K, Bahrambeygi S, Mahmoodpour S, Azizpour A, Mahmoodpour S (2009) History and Basic of Probiotics. Research Journal of Biological Sciences 4: 409-426. [ Ref ]
Gaudana SB, Dhanani AS, Bagchi T (2010) Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Brit. J. Nutrition 103: 1620-1628. [ Ref ]
Fontana L, Bermudez-Brito M, Plaza-Diaz J, Muñoz-Quezada S, Gil A (2013) Sources, isolation, characterization and evaluation of probiotics. Brit. J. Nutrition 109: S35-S50. [ Ref ]
Vandenplas Y, Huys G, Daube G (2015) Probiotics: an update. J Pediatr (Rio J) 91: 6-21. [ Ref ]
Ohashi Y, Ushida K (2009) Health-beneficial effects of probiotics: Its mode of action. Animal Science Journal 80: 361-371. [ Ref ]
Rigobelo EC, Ávila FA (2012) Protective effect of probiotics strains in ruminants. Probiotics in Animals in, Chapter 2. [ Ref ]
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol 71: 1-20. [ Ref ]
Schillinger U, Geisen R, Holzapfel WH (1996) The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends of Food Science Technology 7: 158-164. [ Ref ]
Chauhan S (2012) Screening of potential probiotic lactic acid bacteria from fresh water fish intestine. MSc Thesis. National Institute of Technology, Rourkela. [ Ref ]
Amenu D (2014) Probiotic Properties of Lactic Acid Bacteria from Human Milk. Journal of Medical Microbiology and Diagnosis S3: 5. [ Ref ]
Heller KJ (2004) Inclusion of Probiotics in Beverages. In: Wilson T, Temple NJ (edn) Beverages in Nutrition and Health. Nutrition and Health. Humana Press, Totowa, NJ. pp 247-257. [ Ref ]
Kober MM, Bowe WP (2015) The effect of probiotics on immune regulation, acne, and photo aging. International Journal of Women’s Dermatology 1: 85-89. [ Ref ]
Holzapfel WH, Haberer P, Geisen R, Björkroth J, Schillinger U (2001) Taxonomy and important features of probiotic microorganisms in food and nutrition. American Journal of Clinical Nutrition 73: 365S-373S. [ Ref ]
Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M (2013) Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med 3: a010074. [ Ref ]
Lara-Villoslada F, Olivares M, Sierra S, Rodriguez JM, Boza J (2007) Beneficial effects of probiotic bacteria isolated from breast milk. British Journal of Nutrition 98: S96-S100. [ Ref ]
Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ring E, Ray AK (2016) Probiotic Potential of Autochthonous Isolated from the Gastrointestinal Tract of Four Freshwater Teleosts. Probiotics & Antimicro Prot 9: 12-21. [ Ref ]
Yirga H (2015) The Use of Probiotics in Animal Nutrition. J Prob health 3: 2. [ Ref ]
Food and Agriculture Organization (FAO) and World Health Organization (WHO) (2001) Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, Cordoba, Argentina, pp: 1-4. [ Ref ]
Iqbal MZ, Qadir MI, Hussain T, Janbaz KH, Khan YB, et al. (2014) Probiotics and their beneficial effects against various diseases. Pakistan Journal Pharmaceutical Sciences 27: 405-415. [ Ref ]
Black J (2012) Microbiology: Principle and Exploration 8th Edn. John Willey and Sons. pp: 68. [ Ref ]
Nation JL (1983) A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technology 58: 347-351. [ Ref ]
Nelson G, George S (1995) Comparison of media for selection and enumeration of mouse fecal flora populations. J Microbiol Methods 22: 293-300. [ Ref ]
Robinson R (1990) Dairy Microbiology Volume (1). The microbiology of milk. 2nd edn Elsevier science publishing Co. INC. USA. 1990. [ Ref ]
Schillinger U (1999) Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. International Journal of Food Microbiology 47: 79- 87. [ Ref ]
Vasiljevic T, Shah NP (2008) Probiotics-from Metchnikoff to bioactive. International Dairy Journal 18: 714-728. [ Ref ]
Pyar H, Peh KK (2014) Characterization and Identification of Lactobacillus acidophilus using Biolog rapid identification system. Int J Pharm Pharm Sc 6: 189-193. [ Ref ]
Hyronimus B, Le C, Marrec A, Sassi H, Deschamps A (2000) Acid and bile tolerance of spore-forming lactic acid bacteria. Inter J Food Microbiol 61: 193-197. [ Ref ]
Gilliland SE, Staley TE, Bush LJ (1984) Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J Dairy Sci 67: 3045- 3051. [ Ref ]
Austin DJ, Kristinsson KG, Anderson RM (1999) The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci (96): 1152-1156. [ Ref ]
Saarela M, Mogensen G, Fonden R, Mättö J, Mattila-Sand-holm T (2000) Probiotic bacteria: safety, functional and technological properties. Journal of Biotechnology 84: 197-215. [ Ref ]
Dlamini ZC, Langa RLS, Aiyegoro OA, Okoh AI (2018) Safety Evaluation and Colonisation Abilities of Four Lactic Acid Bacteria as Future Probiotics. Probiotics & Antimicro Prot https://doi.org/10.1007/ s12602-018-9430-y. [ Ref ]
Wassenaar TM, Ussery D, Nielsen LN, Ingmer H (2015) Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol 5: 44-61. [ Ref ]
Muresan A, Sârbu I, Pelinescu D, Ionescu R, Csutak O, et al. (2017) In vitro selection of some lactic acid bacteria strains with probiotic potential. Rom Biotechnol Lett 23: 1-14. [ Ref ]
Wassenaar TM, Klein G (2008) Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J Food Prot 71: 1734-1741. [ Ref ]
Wei YX, Zhang ZY, Liu C, Malakar PK, Guo XK (2012) Safety assessment of Bifodobacterium longum JDM301 based on complete genome sequences. World J Gastroenterol 18: 479-488. [ Ref ]
Busanello M, Pozza MSS, Pozza PC, Nunes RV, Chambo APS, et al. (2015) Probiotics: viable and inactivated cells on the performance, microflora and blood parameters of piglets. Revista Brasileira de Saúde e Producão Animal 16: 387-396. [ Ref ]
Nurliyani EH, Marsetyawan HS (2011) Leukocytes count and lymphocytes proliferation of dinitrochlorobenzene sensitized rat supplemented with fermented goat milk. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 5: 357-361. [ Ref ]
Al-Saiady MY (2010) Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of Newborn calves J Anim Vet Adv 9: 604-609. [ Ref ]
Naqid IA, Owen JP, Maddison BC, Gardner DS, Foster N, et al. (2015) Prebiotic and probiotic agents enhance antibody-based immune responses to Salmonella Typhimurium infection in pigs. Animal Feed Science and Technology 201: 57-65. [ Ref ]
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, et al. (2002) Two anti-oxidative lactobacilli strains as promising probiotics. Intl Food Microbiol 72: 215-224. [ Ref ]
Olson BJSC, Markwell J (2007) Assays for Determination of Protein Concentration. Current Protocols in Protein Science 48: 3.4.1-3.4.29. [ Ref ]
Monteagudo-Mera A, Rodríguez-Aparicio L, Rúa J, Martínez-Blanco H, Navasa N, et al. (2012) In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. Journal of Functional Foods 4: 531-541. [ Ref ]
Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki E, Kotzekidou P (2003) Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Science 65: 859-867. [ Ref ]
Gómez NC, Ramiro JM, Quecan BXV, de Melo Franco BDG (2016) Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella typhimurium, and Escherichia coli 0157:H7 biofilms formation. Front Microbiol 7: 863. [ Ref ]
Janković T, Frece J, Abraham M, Gobin I (2012) Aggregation ability of probiotic Lactobacillus plantarum strains. Int J of Sanit Eng Res 5: 19-24. [ Ref ]
Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, et al. (2001) In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 73: 389-392. [ Ref ]
Vlková E, Rada V, Smehilova V, Killer M (2008) Auto-aggregation and co-aggregation ability in Bifidobacteria and Clostridia. Folia Microbiol 53: 263-269. [ Ref ]
Shuker DE (2002) The enemy at the gates? DNA adducts as biomarkers of exposure to exogenous and endogenous genotoxic agents. Toxicol Lett 134: 51-56. [ Ref ]
Burns AJ, Rowland IR (2004) Anti-genotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat Res 551: 233-243. [ Ref ]
Pool-Zobel BL, Neudeker C, Domizlaff I, Ji S, Schillinger U, et al. (1996). Lactobacillus and Bifidobacterium mediated anti-genotoxicity in the colon of rats. Nutr Cancer 26: 365-380. [ Ref ]
Vorobieva LI, Khodjaev EY, Cherdinceva TA (1996) The study of induced anti-mutagenesis of propionic acid bacteria. J Microbiol Methods 24: 249-258. [ Ref ]
Kumer M, Nagpal R, Kuman R, Hemalatha R, Verma V, et al. (2012) Cholesterol-lowering probiotics as potential bio-therapeutics for metabolic diseases. Experimental Diabetes Research 5: 902-917. [ Ref ]
Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, et al. (2002) Lactobacillus bacteraemia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Di 35: 1155-1160. [ Ref ]
Parvez S, Malik KA, Kang SH, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology 100: 1171-1185. [ Ref ]
Isalauri E (2001) Probiotics: effect on immunity. Am J Clin Nutr 73: 444s-450s. [ Ref ]
Olnood CG, Beski SSM, Iji PA, Choct M (2015) Delivery routes for probiotics: Effects on broiler performance, intestinal morphology and gut microflora. Animal Nutrition 1: 192-202. [ Ref ]
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, et al. (2013) Health benefits of probiotics: a review. International Scholarly Research Notices Nutrition Article ID 481651. [ Ref ]
Fijan S (2014) Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. International Journal of Environmental Research and Public Health 11: 4745-4767. [ Ref ]
Chaucheyras-Durand F, Durand H (2010) Probiotics in animal nutrition and health. Benef Microb 1: 3-9. [ Ref ]
Musa HH, We SL, Zhu CH, Seri HI, Zhu GQ (2009) The potential benefits of probiotics in animal production and health. J Anim Vet Adv 8: 313- 321. [ Ref ]
Collins M, Samelis J, Metaxopoulos J, Wallbanks S (1993) Taxonomic studies on some Leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Bact 75: 595-603. [ Ref ]
Dave R, Shah N (1997) Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. International Dairy Journal 7: 31-41. [ Ref ]
64 Roy D, Sirois S, Vincent D (2001) Molecular discrimination of lactobacilli used as starter and probiotic cultures by amplified ribosomal DNA restriction analysis. Current microbiology 42: 282-289. [ Ref ]
Talwalkar A, Kailasapathy K (2004) A review of oxygen toxicity in probiotic yoghurts: Influence on the survival of probiotic bacteria and protective techniques. Comprehensive Reviews in Food Science and Food Safety 3: 117-124. [ Ref ]
Vinderola C, Reinheimer J (2000) Enumeration of Lactobacillus casei in the presence of Lactobacillus acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. International Dairy Journal 10: 271-275. [ Ref ]
Agaliya PJ, Jeevaratnam K (2012) Screening of Lactobacillus plantarum isolated from fermented idli batter for probiotic properties. African Journal of Biotechnology 11: 12856-12864. [ Ref ]
Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, et al. (2012) Probiotics, their health benefits and applications for developing healthier foods: a review. Federation of European Microbiological Societies 334: 1-5. [ Ref ]
Dhama K, Verma V, Sawant PM, Tiwari R, Vaid RK, et al. (2011) Applications of probiotics in poultry: enhancing immunity and beneficial effects on Production performances and health-a review. J Immunol Immunopathol 13: 1-19. [ Ref ]
Sharma P, Tomar SK, Goswami P, Sangwan V, Singh R (2014) Antibiotic resistance among commercially available probiotics. Food Research International 57: 176-195. [ Ref ]
Bennedsen M, Stuer-Lauridsen B, Danielsen M, Johansen E (2011) Screening for antimicrobial resistance genes and virulence factors via genome sequencing. Appl Environ Microbiol 77: 2785-2787. [ Ref ]
Spyropoulos BG, Misiakos EP, Fotiadis C, Stoidi CN (2011) Antioxidant Properties of Probiotics and Their Protective Effects in the Pathogenesis of Radiation-Induced Enteritis and Colitis. Dig Dis Sci 56: 285-294. [ Ref ]
Anadón A, Martínez-Larrañaga MR, Ares I, Martínez MA (2016) Probiotics: Safety and Toxicity considerations. In nutraceuticals: Efficacy, safety and toxicity, pp: 777-798. [ Ref ]