Journal Name: Journal of Pediatrics and Infants
Article Type:Analysis Article
Received date:06 June, 2019
Accepted date:08 July, 2019
Published date:13 July, 2019
Citation:Gamagedara TP, Riska MRF, Rajapakse RMG (2018) Effects of Hydroxyapatite - Polymethyl Methacrylate Nanocomposites on Human Red Cell Indices and Serum Proteins. Int J Nano Rech Vol: 2, Issu: 1 (09-14).
Copyright:© 2019 Gamagedara TP. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
HA - PMMA nanocomposites can be used as a potential biomaterial for orthopedic applications. The wear NPs would be prone to bind with proteins to form protein-particle complexes and to interact with visible components in the blood. Therefore, this study was focused to determine the effects of HA - PMMA nanocomposites (10% HA) on human red cell indices and serum proteins by in vitro method. Blood samples and serum samples of healthy adults were mixed with the nanocomposites and incubated. Red cell indices were calculated using hemoglobin level, packed cell volume and red blood cell counts which were determined by cyanomethemoglobin, microhematocrit and counting chamber methods respectively and serum total protein and serum albumin were analyzed by using Biuret method and colorimetric Bromocresol green methods respectively. Descriptive statistical method and t paired test ware used to analyze data. The study shows HAP-PMMA nanocomposites interact with RBCs, hemoglobin (Hb) and serum proteins. There was a significant decrease in the values of MCV, MCH, MCHC, serum total proteins and serum albumin due to the effect of the nanocomposite. In addition, shape and size of red cells were changed. Therefore, there can be an effect on hemocompatibity by releasing particles of nanocomposites to the blood stream. Within the study, importance of in vitro tests for evaluation of the compatibility of nanocomposites with human blood could be demonstrated.
Keywords: Hydroxyapatite (HA), Polymethyl Methacrylate (PMMA), Nanocomposites, Nanoparticles (NPs), Red Blood Cells (RBCs), Serum proteins, Hemocompatibility.
Abstract
HA - PMMA nanocomposites can be used as a potential biomaterial for orthopedic applications. The wear NPs would be prone to bind with proteins to form protein-particle complexes and to interact with visible components in the blood. Therefore, this study was focused to determine the effects of HA - PMMA nanocomposites (10% HA) on human red cell indices and serum proteins by in vitro method. Blood samples and serum samples of healthy adults were mixed with the nanocomposites and incubated. Red cell indices were calculated using hemoglobin level, packed cell volume and red blood cell counts which were determined by cyanomethemoglobin, microhematocrit and counting chamber methods respectively and serum total protein and serum albumin were analyzed by using Biuret method and colorimetric Bromocresol green methods respectively. Descriptive statistical method and t paired test ware used to analyze data. The study shows HAP-PMMA nanocomposites interact with RBCs, hemoglobin (Hb) and serum proteins. There was a significant decrease in the values of MCV, MCH, MCHC, serum total proteins and serum albumin due to the effect of the nanocomposite. In addition, shape and size of red cells were changed. Therefore, there can be an effect on hemocompatibity by releasing particles of nanocomposites to the blood stream. Within the study, importance of in vitro tests for evaluation of the compatibility of nanocomposites with human blood could be demonstrated.
Keywords: Hydroxyapatite (HA), Polymethyl Methacrylate (PMMA), Nanocomposites, Nanoparticles (NPs), Red Blood Cells (RBCs), Serum proteins, Hemocompatibility.
Introduction
Bone has a complex hierarchical structure. The basic building blocks are the extremely small plate-shaped crystals of carbonate apatite, just hundreds of angstroms long and wide and some 20-30 A thick. They are arranged in parallel layers within the collagenous framework [1]. Engineered nanoparticles show remarkable structural diversity, each structure exhibiting their own individual characteristics, such as tubes, dots, wires, fibers and capsules [2]. Nanomaterials have unique physicochemical properties, such as ultra-small size(1-100nm), large surface area to mass ratio, and high reactivity, which are different from bulk materials of the same composition [3]. Hydroxyapatite (Ca10(PO4)6(OH)2) is the main inorganic component of natural bone, constituting 70% of the mass of the bone matrix. Synthetic hydroxyapatite prepared by sintering processes also exhibits bioactivity, and was commercialized in the late 1980s as bone substitutes in the form of dense and porous body implants and granules [4]. In a comparison study between Nano size HAP filler and micro size HAP filler using a rat calvarial defect model, histological analysis and mechanical evaluation showed a more advanced bone formation and a more rapid increase in stiffness in the defects with the Nano size HAP augmented poly (propylene glycol fumaric acid), suggesting an improved biological response to the NanoHAP particles [4]. Nowadays, numerous synthetic bone graft materials, both single- and multi-phase, are available, which are capable of alleviating some of the practical complications associated with the natural bone grafting materials. A singlephase material (monolithic) does not always provide all the essential features required for bone growth, which leads to continual investigation in search of an ideal bone graft. There is a great need for engineering multi-phase materials (composites) with structure and composition similar to natural bone. Since bone is a typical example of a nanocomposite, designing bone graft in the form of nanocomposite is perceived to be beneficial over monolithic and micro composite materials [4,5].
Polymethyl Methacrylate (PMMA) was the first synthetic polymer to be used in clinical practice in 1937. Since then, numerous polymers were developed and used in a variety of orthopedic and other medical applications. To simulate physiological characteristics (stiffness, bending, compressive, and tensile strengths) for load-bearing circumstances, it may be advantageous to use polymers in the composites [4,6]. Particle reinforcement has been used to improve the properties of bone cement. For example, inclusion of bone particles in PMMA cement somewhat improves the stiffness and improves the fatigue life considerably.
Even though advanced research has been carried out on many different types of nanocomposites, their use in medical devices is very much less than expected as there is not enough reliable experimental and clinical data supporting the long-term performance of nanocomposites with respect to monolithic traditional materials. This problem can be avoided by using clinically well-known constituents in the preparation of nanocomposites. Nano-scale HA has received much attention owing to its superior functional properties over its microscale counterpart. PMMA is another constituent that can be used in the preparation of nanocomposites along with Nano-HA. Since the composition of acrylic bone cements has primarily remained unchanged over the last 50 years, and the fixation of THRs with PMMA bone cements is currently regarded as the gold standard and it is approved by the FDA of USA for specific human clinical applications such as a bone cement [4,7].
There have been some concerns expressed that the small polymeric particles produced by degradation of these type of polymers may stimulate an inflammatory reaction, although this remains an area of debate and investigation [4,8,9]. NPs have toxicity effect on physiological systems of animals and human [10]. There are research studies focus on toxicological effect of HAP NPs used in bone replacement and therapy. NPs can be released in to blood circulation and potential nanoparticles can effect to alter normal physiology by interacting with biomolecules in living cells [11]. Specific areas of concern include carcinogenicity, teratogenicity, developmental toxicity, acute toxicity and interaction with components of the immune system [12].
NPs, when entered into the body, will interact initially with proteins present in blood. Binding to plasma components and forming a nanoparticle-protein corona potentially determine the fate of NPs in the systemic circulation and influence their biological activity. In human plasma, a typical protein corona formed on NPs consists of proteins such as serum albumin, immunoglobulins, fibrinogen, and apolipoproteins, etc. NPs can enter the vascular system intentionally and can interact with blood. In vitro, NPs’ sol did not cause hemolysis but did induce the aggregation of rabbit red blood cells [13]. HAP nanoparticles exhibited Nano size and surface charge effects on the aggregation of RBCs in RBC suspensions [2,14].
Fractures of bone often occur in the world. According to the National Institutes of Health, approximately 1.5 million hip fractures occur worldwide each year, and this number might increase to 2.6 million by 2025 and 4.5 million by 2050 [13]. Therefore, bone grafting is very important for the repair and replacement of bone. Many circumstances call for bone grafting owing to bone defects either from traumatic or from non-traumatic destruction. Nanophase HAP is a class of bio ceramic material that mimic the bone mineral in composition and structure and possess unique capabilities for surface interactions with biological entities than conventional HAP; therefore, it can be used as a scaffolding system in engineering bone tissue. Although preliminary investigations seem to support the impact of nanophase HAP in bone tissue engineering, significant advancement are still necessary to realize their full potential in clinical use. This is exciting time to be involved in nanophase HAP in order to formulate them as a clinically ideal system for bone tissue engineering, with great challenges and also great expectation ahead. For the application of HAP nanoparticles in biological systems as carriers or fluorescent labels, there is a significant chance that the nanoparticles will interact with the complicated components of blood such as blood cells on account of their size [2]. In addition, small polymeric particles may cause adverse effects. Therefore, a study of HAP-PMMA nanocomposite to determine whether they will exhibit specific properties toward such blood components is of major importance before going for a clinical application. The current study will be carried out to determine effects of HAP-PMMA nanocomposite on blood components. In in vitro methods HAP-PMMA nanocomposite will be introduced to blood and blood will be analyzed to determine the effects. Therefore, by carrying out this study, influence on red cell indices, serum total protein and serum albumin by HAPPMMA nanocomposite may be identified. In addition, there are no evidences of previous studies to evaluate the compatibility of HAP-PMMA nanocomposite with human blood using red cell indices and serum proteins. Therefore, this study focuses on determine the effects of hydroxyapatite – polymer (polymethyl methacrylate) nanocomposites on human red cell indices and serum proteins by in vitro method.
Methodology
60 blood samples were collected from healthy students of the Faculty of Allied Health Sciences. Self-administered questionnaire was used to collect information regarding the health status of students. Ethical clearance was obtained from the ethical review committee, Faculty of Allied Health Sciences, University of Peradeniya. Blood samples and serum samples were mixed with 50% (mg/dL) HAP-PMMA nanocomposite which was previously prepared [4] and it was incubated for one hour at room temperature. Red cell indices were calculated using hemoglobin level, packed cell volume and red blood cell counts which were determined by using cyanmethemoglobin, microhematocrit and counting chamber methods respectively and serum total protein and serum albumin were analyzed by using Biuret method and colorimetric Bromocresol green (BCG) methods respectively. Descriptive statistical method and t paired test ware used to analyze the data.
Red cell indices determination
50% (mg/dL) of HAP-PMMA nanocomposite suspension was prepared using concentrated phosphate buffered solution (PBS) (pH 7). Two tubes were taken; One for normal test and one for HAP test. 1 mL of EDTA blood was mixed with 20 µL of 50% suspension in HAP tube and at the same time 1 mL of EDTA blood was mixed with 20 µL of PBS (pH 7) normal tubes. Both tubes were placed for one hour at room temperature (24 o C). After that following tests were done for both normal and HAP tubes to collect the data.
Haemoglobin estimation
Hb value was determined by cyanmethemoglobin method. Test tubes were labeled as test, 20 µL of test sample blood was mixed with 5.0 mL of Drabkin’s reagent and was kept at room temperature for 5 minutes. The absorbance of this mixture was measured by using a spectrophotometer (Tomos Biotools, Shanghai Co. Ltd. Shanghai, China) at 540 nm wavelength. The absorbance of the standard blood was measured by the same procedure. This absorbance values were used to find the haemoglobin concentration.
Red blood cell count
Red blood cell count was measured manually. 20 µL of blood was mixed with 4 mL of RBC diluting fluid and counting chamber was charged with mixed fluid. After that, the red cells of this fluid were counted by using microscope at a magnification of ×40 objectives. This counted red cell number was used to calculate the total RBC.
Packed cell volume determination
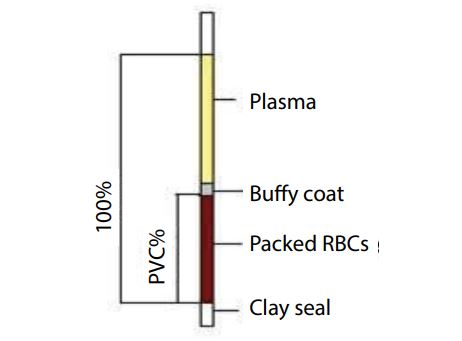
Microhaematocrit method was used to measure packed cell volume. 3/4 of length capillary tubes were filled with blood and the capillary tubes were centrifuged by micro centrifugation (HERMLE Labortechnik. GmbH, Siemenstrass 25, Wehingen, Germany) at 12000 rpm for 5 minutes. Following this procedure, the length of red cell column was measured by using a hematocrit meter. This red cell column length was used to calculate the packed cell volume (Figure 1).
Red cell indices were calculated using Hb value, RBC count and PCV measurement. All laboratory tests were conducted according to the WHO standard operating procedure.
Red cell indices calculation formula
One femtoliter is 10-15 L
One picogram is 10-12g
Serum proteins determination
Blood was collected to plain tube. It was allowed for 30 minutes to complete clotting. Then blood sample was centrifuge for 3000 rpm for 10 minutes to separate serum. Two tubes were taken. One for normal test and one for HAP test. 0.4 mL of was mixed with 40 µL of 50% suspension in HAP tube and at the same time 0.4 mL of serum was mixed with 40 µL of PBS (pH 7) normal tubes. Both tubes were placed for one hour at room temperature (24°C). After that following serum protein tests were done for both normal and HAP tubes to collect the data.
4.7 Serum total protein determination
Serum total protein value was determined by Biuret method. 60 µL of serum was mixed with 5.0mL of Total protein reagent and it was kept at room temperature (25°C) for 10 minutes. After that, absorbance of this mixture was measured by using a spectrophotometer (Tomos Biotools, Shanghai Co. Ltd. Shanghai, China at 546 nm wavelength. This absorbance value was used to find the serum total protein concentration.
(T -test was used to compare the proportions and 95% confidence intervals (95% CI) between normal and abnormal.
Figure 1: Picture of blood separation in PCV measurement
| Normal | With nanocomposites | P value | |||
|---|---|---|---|---|---|
| Mean | Std. Deviation | Mean | Std. Deviation | ||
| MCV (fl) | 88.94 | 6.30 | 88.14 | 8.91 | 0.877 |
| MCH (pg) | 28.81 | 2.45 | 26.16 | 3.59 | 0.000 |
| MCHC (%) | 32.23 | 1.84 | 29.45 | 2.22 | 0.000 |
| Serum total protein (g/dL) | 5.87 | 0.97 | 4.03 | 0.91 | 0.000 |
| Serum albumin(g/dL) | 3.53 | 0.30 | 3.20 | 0.38 | 0.000 |
Table 1: Statistical data of normal samples and samples with nanocomposites in red cell indices and serum proteins values.
Serum albumin determination
Serum total protein value was determined by colorimetric Bromo cresol green BCG method. Serum albumin value was determined by 30 µL of serum was mixed with 3.0 mL of albumin reagent and was kept at room temperature (25 OC) for 5 minutes. After that, absorbance of this mixture was measured using a spectrophotometer (Tomos Biotools, Shanghai Co. Ltd. Shanghai, China at 547 nm wavelength. This absorbance value was used to find the serum albumin concentration.
All laboratory tests were conducted according to the WHO standard operating procedures.
Statistical analysis
Analysis of red cell indices values and serum proteins were done using SPSS (version 16) statistical software. Descriptive statistical method was used to identify deviations from normal values. The paired t test was used to determine the significant difference between normal samples and HAP-PMMA nanocomposite introduced samples in red cell indices and serum proteins. T-test was used to compare the proportion of abnormal conditions in Hb values and red cell indices values (p < 0.05 is considered to be statistically significant, 95% CI was used).
Results and Discussion
A total of 60 undergraduate students of Faculty of Allied Health Sciences participated in this study. 30 individual for red cell indices and 30 individuals for serum protein. Five students were excluded considering the exclusion criteria. Three of these students had red cell indices were not within the range of normal level. The age of the Participant’s ranged between 20 to 28 years and the mean age was 23.76 (± 1.78) years. Table 1 shows the statistical data of normal samples and samples with nanocomposites in red cell indices and serum proteins values. There were differences in MCH and MCHC after introduction of the nanocomposite. There was effect on red blood cells, hematocrit (PCV) and hemoglobin levels after introduction of the nanocomposite. However, there was no difference in MCV mean value after introduction of the nanocomposite. There were differences in serum total protein and serum albumin after introduction of the nanocomposite and there was an effect on serum protein after introduction of the nanocomposite.
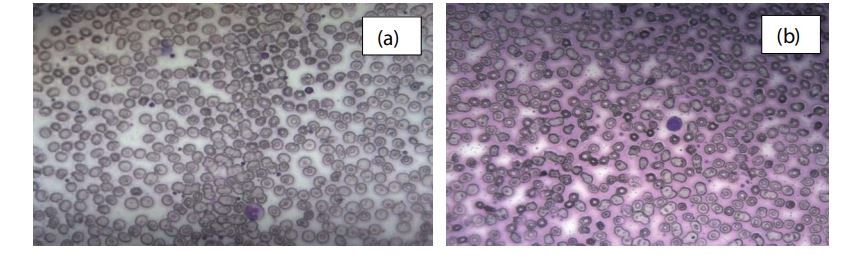
Figure 2 shows the morphology of red cells after onehour incubation with the nanocomposite. Images (a), (b) and (c) shows the obvious RBC irregular membrane without RBC aggregation. Therefore, lifetime of RBC is decreased causing breakdown of RBC. Shape and size of red cells was changed due to the effect of the nanocomposite. In this image, RBC showed Burr cell appearance and irregularity in middle part.
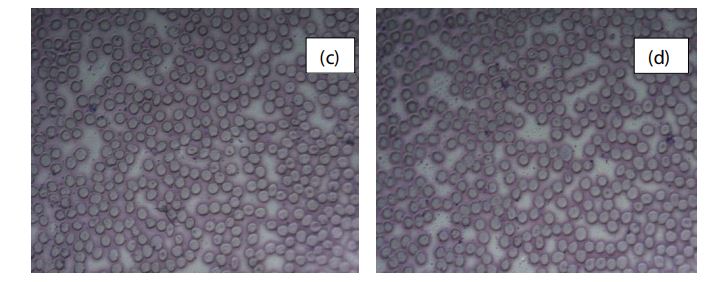
Figure 3 shows the RBC morphological characteristics without the nanocomposite. Images (c) and (d) shows the regular cell membrane with clear cell margin.
In the research findings of RBCs with HAP particles, hemolysis was not observed. However, obvious RBC aggregations were observed as rounded and compressed clumps, and it has been reported in various previous studies. However, in vitro hemolysis assay has been investigated with Nano HAPs and results showed no obvious hemolysis phenomenon was observed after exposure of nanohydroxyapatite.
22% samples’ MCH value was decreased. 3.7% samples’ MCH value was increased than the normal range and the rest of the samples MCV value was within the normal range due to the nanocomposite. 55% of samples’ MCHC value was decreased than the normal range and the rest of samples’ MCHC value was within the normal range. There was no increased MCHC value due the effect of the nanocomposite indicating average volume of RBC was not changed by the nanocomposite.
40% of individuals’ Hb concentrations were lower than the cut off value due to the presence of the nanocomposite.
Therefore, 40% of sample had normocytic normochromic condition. However, microcytic hypochromic conditions and macrocytic normochromic condition were not observed due to the effect of HAP-PMMA nanocomposite. There was significant decrease in MCH and MCHC after introduction of the nanocomposite and there was no significant difference in MCV after introduction of the nanocomposite (p<0.05).
There was no evidence of a previous study to check the effect of the HAP-PMMA nanocomposites on red cell indices. However, hemolysis study was done to determine the effect of HAP nanomaterials. The hemolysis was obtained in our study may be due to the effect of the nanocomposite. Because previous studies used only nanomaterial (1-100 nm) to check the interaction. In my study, nanocomposite was used to check the interaction with red cells. Nanocomposite consists of 10% HAP NPs and 90% PMMA (~200 nm) [4]. Therefore, hemolysis can be due to the interactions with PMMA. PMMA can attach to the red cell membrane. Protein corona formed due to HAP and this protein corona can attach to the membrane of red cells. Large nanocomposite corona can attach to the red cell membrane. It can change the irregular margin of the cell membrane.
22% samples’ serum total value was decreased than the normal range and the rest of the samples’ serum total value was within the normal range due to the nanocomposite. 20% of samples albumin value was decreased than the normal range and rest of the samples’ albumin value was within the normal range. There was no increased serum total protein and albumin value due the effect of the nanocomposite. There was a significant decrease in the serum proteins after introduction of the nanocomposite (p<0.05).
In a study of investigating adverse effects of Nanohydroxyapatite and TiO2 Nanoparticles (NPs) with proteins has been shown that a typical protein corona formed on NPs consists of proteins such as serum albumin, immunoglobulins, fibrinogen, and apolipoproteins in the human plasma [13]. If nanoparticle enters the blood, it is immediately enclosed by thin layer of biomolecules called as a protein corona. Formation of protein corona may cause to the lower albumin level. Previous study showed protein corona formed due to NPs. According to my study, there was obvious significant decrease in total protein and albumin between normal samples and samples with nanocomposites. Therefore, there can be an interaction of proteins with particles of nanocomposites. Whole blood behavior and plasma protein adsorption on Nano-structured polymer material under flow conditions using microfluid setup were investigated and showed surface nano-topography of polymer films influences primarily plasma protein adsorption, which in the control of platelet adhesion and thrombus formation [3]. In a study, protein adsorption on Polystyrene (PS)- and Polymethyl Methacrylate (PMMA)- based microspheres and their modified forms were treated with blood components to evaluate their biocompatibility and showed activated PMMA surfaces show a great capacity for protein adsorption and are phagocytosed as much as PS microspheres. The same trend was observed on the surfaces modified with albumin. According to the results of my study, protein interaction is similar as in the previous study. Therefore, PMMA surfaces may have great capacity for protein adsorption [15,16].
Therefore, this study shows that there can be an effect on hemocompatibity by releasing particles of nanocomposites in the bone graft, etc. to the blood stream. However, sample size can be increased to improve the reliability of the results. In this study, HAP-PMMA nanocomposite was used and interaction with blood components may be due to HAP NPs, PMMA or HAP-PMMA combined effect. Therefore, it is important to investigate the effect of HAP NPs and PMMA individually to check which is responsible mainly for the interactions. The finding can be used in the optimization of the composition of the nanocomposite with better hemocompatibity considering the individual hemocompatibility as well. In my study, nanocomposite concentration used was 50% (mg/dL) which is a compatible concentration of inorganic salts of blood plasma. Therefore, it is important to see the effect of different concentrations of nanocomposite on hemocompatibility. However, within the current study, the importance of in vitro tests for evaluation of the compatibility of nanocomposites with human blood could be demonstrated. In addition, biocompatibility has to be evaluated by in vitro studies using osteoblast cell line before use in a biomedical application.
Figure 2: Light microscopic images ( x 40) of RBCs (a, b) after 1 h incubation with the nanocomposite.
Figure 3: Light microscopic images ( x 40) of RBCs (c, d) after 1 h incubation without nanocomposite.
Conclusion
Within the current study, the importance of in vitro tests for evaluation of the compatibility of nanocomposites with human blood could be demonstrated. This study shows that there can be an effect on hemocompatibity by releasing particles of nanocomposites to the blood stream.
According to the study HAP-PMMA interact with blood cells, hemoglobin and other blood proteins. Therefore, there is hemolysis of red cells and significant decrease in MCH, MCHC and blood proteins. 40% of the samples show normochromic normocytic anemic state due to the effect of the nanocomposite. However, there is no significant difference in the MCV values due the effect of the nanocomposite. Moreover, it is important to investigate the effect of HAP NPs and PMMA individually to optimize the composition of the nanocomposite with better hemocompatibity.
Weiner S, Traub W (2007) Hawley’s Condensed Chemical Dictionary. Hoboken, NJ, John Wiley & Sons, USA 6: 879-885.[ Ref ]
Han Y, Wang X, Dai H, Li S (2012) Nanosize and surface charge effects of hydroxyapatite nanoparticles on red blood cell suspensions. ACS Appl Mater Interfaces 4: 4616-4622.[ Ref ]
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, et al. (2008) Nanoparticles in Medicine. Therapeutic Applications and Developments 83: 761-769.[ Ref ]
Gamagedara T, Rajapakse R (2019) Facile Bottom-up Approach to Synthesise Hydroxyapatite - Polymethyl Methacrylate Nanocomposites for Possible Applications in Bone Grafting. Int J Nanotechnol Med Eng 4: 1-8.[ Ref ]
Murugan R, Ramakrishna S (2005) Development of nanocomposites for bone grafting. Compos Sci Technol 65: 2385-2406.[ Ref ]
Weir NA, Buchanan FJ, Orr JF (2004) Degradation of poly-L-lactide. Part 1: in vitro and in vivo physiological temperature degradation. Proc Inst Mech Eng H 218: 307-319.[ Ref ]
Deb GKS (2008) Orhopaedic Bone Cements. Orhopaedic Bone Cements. Deb S [Eds] Woodhead Publishing Limited, Cambridge, England; pp: 167-180.[ Ref ]
Athanasiou CM, Niederauer KA, Agrawal GG (1996) Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ poly- glycolic acid copolymers. Biomaterials 17: 93-102.[ Ref ]
Brydone S, Meek D, Maclaine S (2010) Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng Part H J Eng Med 224: 1329-1343.[ Ref ]
Negahdary M, Ranjbar A, Asadi A (2012) The toxicity effect of cerium oxide nanoparticles on ALT. AST and ALP enzymes in male Rat 3: 4386- 4392.[ Ref ]
Adeyemi OS, Adewumi I (2014) Biochemical Evaluation of Silver Nanoparticles in Wistar Rats. International Scholarly Research Notices.[ Ref ]
Ilinskaya AN, Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part II: safety concerns. Nanomedicine (Lond) 8: 969-981.[ Ref ]
Wang J, Wang L, Fan Y (2016) Adverse Biological Effect of TiO2 and Hydroxyapatite Nanoparticles Used in Bone Repair and Replacement. Int J Mol Sci 17: 1-14.[ Ref ]
Gamagedara T, Ziana H (2019) Effects of hydroxyapatite nanoparticles on liver enzymes and blood components. J Clin Investig Stud 1: 1-5.[ Ref ]
Norman M, Williams P, Illum L (1993) Influence of block copolymers on the adsorption of plasma proteins to microspheres. Biomaterials 14: 193-202.[ Ref ]
Tsougeni K, Tserepi A, Constantoudis V, Gogolides E, Petrou PS, et al. (2010) Plasma Nanotextured PMMA Surfaces for Protein Arrays: Increased Protein Binding and Enhanced Detection Sensitivity. Langmuir 26: 13883-13891.[ Ref ]