Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:7 May, 2019
Accepted date:26 June, 2019
Published date: 03 July, 2019
Citation:Bendjemil B, Cleymand F, Pichler T, Knupfer M, Fink J (2019) Functionalized Carbon Nanostructures in Cancer Diagnosis and Therapy. Int J Nano Rech Vol: 2, Issu: 2 (01 -20).
Copyright:© 2019 Bendjemil B et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
During the past years, carbon nanotubes (CNTs) have attracted considerable interest since their first discovery. great progress has been made in the field of nanomaterials given their great potential in biomedical applications. Carbon nanotubes (CNTs), due to their unique physicochemical properties, have become a popular tool in cancer diagnosis and therapy. They are considered one of the most promising nanomaterials with the capability of both detecting the cancerous cells and delivering drugs or small therapeutic molecules to these cells because of the unique structure, extremely high specific surface area to-volume ratio enable them to use in an intense real time applications such as detection and treatment of cancerous cells, nervous disorders, tissue repair. and excellent electrical and mechanical properties carbon nanotubes composed of excellent mechanical strength, electrical and thermal conductivities makes them a suitable substance toward developing medical devices., CNTs have been explored in almost every single cancer treatment modality, including drug delivery with small nanomolecules, lymphatic targeted chemotherapy, thermal therapy, photodynamic therapy, and gene therapy and demonstrate a great promise in their use in targeted drug delivery systems, diagnostic techniques and in bio-analytical applications. Majority of the biomedical applications of CNTs must be used after successful functionalization for more potential applications than pristine CNTs. There are several approaches to modify pristine CNTs to potentially active. CNTs poised into the human life and exploited in medical context. Here in, we reviewed the following topics (i) Functionalization of CNTs (ii) CNTs in real time applications such as drug delivery, gene therapy, biosensors and bio imaging; (iii) CNTs 3D printed scaffolds for medicine and (iv) Biocompatability and Biodegradability.
Single-walled carbon nanotubes (SWCNTs) were synthesized using the high-pressure carbon monoxide disproportionation process (HiPCO). The SWCNT diameter, diameter distribution and yield can be varied depending on the process parameters. The obtained HiPCO product present an iron nanoparticle encapsulated heteronanocarbon (coreshell nanoparticles) at low pressure (1 bar) after removing of iron metal catalyst nanoparticle and amorphous carbon by acid immersion and oxidation. The resulting therapeutic molecule in the form of core-shell nanoparticles and single walled carbon nanotubes after functionalization by filling of iron can be use as therapeutic nanomaterials in nanomedicine in diagnosis and treatment of cancer tumor. This paper describes the synthesis method and role of multifunctional nanoparticle in diagnosis and treatment of cancer. Therefore, the aim of this review is to provide basic information on nanoparticles, describe previously developed methods to functionalize nanoparticles and discuss their potential applications in nanobiomedical and mention the therapeutic nanoparticle large scale production and commercialization challenges. In the final part of the review, emphasis is given on the pharmacokinetic aspects of carbon nanotubes including administration routes, absorption mechanisms, distribution and elimination of carbon nanotubes based systems. Lastly, a comprehensive account about the potential biomedical applications has been given followed by insights into the future carbon nanotubes from synthesis to in vivo biomedical applications.
Keywords: Carbon nanotubes; HiPCO process; Optical absorption spectroscopy (OAS); Raman scattering; Magnetic iron core-shell nanoparticles; Purification; Sterilization; Functionalization; Fe@SWCNTs; Therapeutic nanoparticles; Cellular mechanisms; Nanomedicine application; Cancer tumor therapy and diagnosis; Nanomaterials; Nanobiotechnology; Nanotechnology; Cancer diagnosis; Cancer therapy; In vivo nanobiomedical applications.
Abstract
During the past years, carbon nanotubes (CNTs) have attracted considerable interest since their first discovery. great progress has been made in the field of nanomaterials given their great potential in biomedical applications. Carbon nanotubes (CNTs), due to their unique physicochemical properties, have become a popular tool in cancer diagnosis and therapy. They are considered one of the most promising nanomaterials with the capability of both detecting the cancerous cells and delivering drugs or small therapeutic molecules to these cells because of the unique structure, extremely high specific surface area to-volume ratio enable them to use in an intense real time applications such as detection and treatment of cancerous cells, nervous disorders, tissue repair. and excellent electrical and mechanical properties carbon nanotubes composed of excellent mechanical strength, electrical and thermal conductivities makes them a suitable substance toward developing medical devices., CNTs have been explored in almost every single cancer treatment modality, including drug delivery with small nanomolecules, lymphatic targeted chemotherapy, thermal therapy, photodynamic therapy, and gene therapy and demonstrate a great promise in their use in targeted drug delivery systems, diagnostic techniques and in bio-analytical applications. Majority of the biomedical applications of CNTs must be used after successful functionalization for more potential applications than pristine CNTs. There are several approaches to modify pristine CNTs to potentially active. CNTs poised into the human life and exploited in medical context. Here in, we reviewed the following topics (i) Functionalization of CNTs (ii) CNTs in real time applications such as drug delivery, gene therapy, biosensors and bio imaging; (iii) CNTs 3D printed scaffolds for medicine and (iv) Biocompatability and Biodegradability.
Single-walled carbon nanotubes (SWCNTs) were synthesized using the high-pressure carbon monoxide disproportionation process (HiPCO). The SWCNT diameter, diameter distribution and yield can be varied depending on the process parameters. The obtained HiPCO product present an iron nanoparticle encapsulated heteronanocarbon (coreshell nanoparticles) at low pressure (1 bar) after removing of iron metal catalyst nanoparticle and amorphous carbon by acid immersion and oxidation. The resulting therapeutic molecule in the form of core-shell nanoparticles and single walled carbon nanotubes after functionalization by filling of iron can be use as therapeutic nanomaterials in nanomedicine in diagnosis and treatment of cancer tumor. This paper describes the synthesis method and role of multifunctional nanoparticle in diagnosis and treatment of cancer. Therefore, the aim of this review is to provide basic information on nanoparticles, describe previously developed methods to functionalize nanoparticles and discuss their potential applications in nanobiomedical and mention the therapeutic nanoparticle large scale production and commercialization challenges. In the final part of the review, emphasis is given on the pharmacokinetic aspects of carbon nanotubes including administration routes, absorption mechanisms, distribution and elimination of carbon nanotubes based systems. Lastly, a comprehensive account about the potential biomedical applications has been given followed by insights into the future carbon nanotubes from synthesis to in vivo biomedical applications.
Keywords: Carbon nanotubes; HiPCO process; Optical absorption spectroscopy (OAS); Raman scattering; Magnetic iron core-shell nanoparticles; Purification; Sterilization; Functionalization; Fe@SWCNTs; Therapeutic nanoparticles; Cellular mechanisms; Nanomedicine application; Cancer tumor therapy and diagnosis; Nanomaterials; Nanobiotechnology; Nanotechnology; Cancer diagnosis; Cancer therapy; In vivo nanobiomedical applications.
Introduction
The unique quasi one-dimensional nature of single wall carbon nanotubes (SWCNTs) [1-4] has stimulated immense interest in these structures. In addition, SWCNTs can themselves serve as templates for the formation of other nanostructures [5-10] further highlighting their versatility.
Their properties open their use in many applications from nanotechnologies to the bio-molecular science as a result of their unique and exceptional physical, electronic [2-4,11] and mechanical properties [12,13]. Their electronic properties depend sensitively on their diameter and chirality, giving rise to metallic and semi-conducting properties [14-16]. The semi-conducting phases provide a superb opportunity for nanoscale device applications such as single molecule transistors and are considered ideal building blocks for nanomedicine application in diagnosis and therapy of cancer in the body.
The application of these nanoscale devices depends on a detailed knowledge of SWCNTs surface properties and the ability to functionalize them in a controlled manner. However, synthesizing SWCNTs in high yield with accurate diameter and chirality control remains a challenge.
Various important methods already exist for the primary SWCNTs growth like laser ablation [17-19], arc discharge [20,21] and catalytic decomposition of hydrocarbons (CCVD) [22-24]. SWCNTs with high purity of about 90 wt. % have been formed in the gas phase using Fe(CO)5 with high pressure of CO through the (HiPCO) process [25-28]. The synthesis of single-walled carbon nanotubes by CVDHiPCO appears to be a promising method in comparison with arc-discharge and laser ablation. The low cost, high purity, relatively simple apparatus and multiple adjustable parameters are the main advantages for large-scale production.
The catalytic chemical vapor deposition characterized by the decomposition of a hydrocarbon [24,29] on a MgO catalyst is similar to (HiPCO) supported on an iron, but it is unsuitable for large scale production, since the catalyst material is only available in limited amounts.
In contrast to this, the HiPCO process designed by Nikolaev et al. [25] represents a continuous-flow synthesis method. It is possible modeling of the HiPco process for carbon Nanotube production at the reactor-scale analysis [30].
In the HiPCO process, a Fe (CO)5 is injected into a stream of CO gas at high temperature (1170 to 1450 K) and pressure (1 to 40 bars). The iron forms metal clusters that act as catalytic sites to promote the Boudouard reaction figure 1(a,b):
COc+ COh → C (solid) + CO2 (gas) (1)
Fe(CO)5 → Fe + 5CO (2)
2CO + Fe → CNTs + CO2 (3)
where COc and COh is cold and hot carbon monoxide in the turbulent flow regime, respectively.
When the metal clusters achieve a size near that of C60, SWCNTs nucleate and grown into the catalyst nonoparticles. The SWCNTs will continue to grow together with the metal clusters, which are growing with addition of residual free iron atoms. The average diameter of SWCNTs using HiPCO method is a function of CO pressure and is on average less than 1 nm, which is pure and smaller than SWCNTs synthesized by the laser vaporization process.
In the present paper we report the synthesis of singlewalled nanotubes in a self-constructed HiPCO apparatus and discuss the structural composition and catalyst morphology, as well as the optical properties of deposited SWCNTs in relation to the synthesis parameters.
Multifunctional nanoparticles
Multifunctional nanoparticle systems can integrate imaging, targeting and treatment modalities both on the surface and in the core to targeting tumor cells [31]. Multifunctional nanoparticles also use for simultaneous delivery of multiple treatment agents, to apply effective combinatorial therapeutic regimens against cancer [32]. Different factors contribute to multifunctional nanoparticle application resiliency, such as tumor location in the body, the inability of the treatment to reach the tumor cells, and the risk of damaging healthy cells figure 1 [33]. Nanoparticles provide an opportunity to change the pharmacokinetic outline of drugs, reduce toxicity and enhance the therapeutic markers. This causes the development of “multifunctional” nanoparticles. For this reason, more capabilities like targeting and image contrast improvement are attached to the nanoparticles. On the other hand, additional functionality means additional synthetic steps and costs, more in vivo complex behavior and effects, and also greater regulatory impediments [34].
Cancer therapy and diagnosis
Some physicochemical features of available potentially potent therapeutic agents (both biopharmaceutical and small molecule drug related) such as large size, highly charged, too unstable metabolism and high insolubility, necessitate assistance of delivery vehicles to reach cancer target cells [35-37]table 1.
As compared with conventional infusion method, the infusion of antineoplastic drugs with nanomaterials as carriers led to an increased payload of drugs to the tumor. In addition, by the Drug-loaded nanoparticles, surface molecules can be overexpressed in tumor cells and therefore, by adding a targeting component, nanoparticles increased their efficacy of tumor-specific drug delivery beyond EPRmediated tumor homing, for example the polymeric particles such as BIND-014, with a PSMA-targeting moiety on the surface and a docetaxel payload, has been tested in humans clinical trials in early phase for metastatic cancers [38-46] table 2.
Carbon nanomaterials, including nanotubes, nanohorns, fullerenols, graphene, and NDs, have been used in delivering a wide spectrum of therapeutic compounds with regards to their versatile surface properties [47-52].
RNA interferences [RNAi] also can enhance drug delivery by silencing cancer-causing genes, if can overcome enzymatic degradation in the body table 3 [53-62]. Cyclodextrin-based polymers [CDPs] is a good example that are designed to protect and deliver siRNA tometastatic solid malignancies cancer cells [63-65]. Lipid based carriers with tumor-targeting and tumor-penetrating moieties have been designed to improve delivery and enhance the circulatory half-life of siRNA [66], for example siRNA nanocomplexes that display a cyclic nanopeptide on its surface is able to penetrate cells after being proteolytically processed by endogenous proteases.
These approaches ensured that the payload was only released once inside the cancer cell and ultimately cause that the particle was more toxic to tumors than to healthy tissue.
One of the most important approaches to cancer diagnosis and therapy is thermal therapy. Several classes of nanoparticles are used for cancer thermal therapy such as magnetic, metallic and liposomes [67].
Photothermal therapy by using the heat to remove the cancer cells, has gained interest for the inducible and noninvasive nature of this triggered therapy and it can be applied to a huge number of solid tumors. Interesting features of gold nanoshell encapsulating a silica core clinically include biocompatibility of the particles as well as the tunability of the surface plasmon resonance with regards to the ratio of the dielectric core radius to shell thickness, make this class of nanoparticles to be effective in near infrared laserinduced cancer therapy in wide range of cancers such as lung, prostate, and gliomas [68-70]. This especial nano shell’s design provides highly efficient and localized conversion of light to heat. During the administration and photothermal ablation, the cancerous tissue vasculature was destroyed, whereas healthy tissue stays intact [71,72]. The technology of nano shells has been commercialized by Nano spectra Biosciences as Auro Lase, with clinical trials about the head and neck cancer and primary and metastatic lung tumors.
In addition to photothermal therapy, magnetic and radiobased strategies are being used for improving drug delivery and efficacy [53,73]. The basic concept of Radiotherapy is based on greater absorption of X-rays in cancerous tissue rather than the normal tissue.
One of the best examples of Radio-induced nanomedicine is hafnium oxide nanoparticles that enhanced energy deposit in nanoparticle containing subcellular cell structures and finally lead to enhanced energy release and subsequent localized cellular destruction from nanoparticle clusters. This method was tested clinically in patients with soft tissue sarcoma of advanced squamous cell carcinoma of the oral cavity [73].
Figure 1: Nanoparticle application in cancer diagnosis and therapy. [35].
| Formulation | Drugs | Indication | Type | Ref. |
|---|---|---|---|---|
| Core-shell nanoparticles | Paclitaxel and Bcl-2-targeted | siRNA Breast cancer | Polymeric nanoparticle | [36] |
| Nanoparticle aptamer types bioconjugates | Doxorubicin and docetaxel | Prostate cancer and various cancer types | Polymeric nanoparticle | [37] |
Table 1 : Liposomes, Polymeric nanoparticles and dendrimers for combination cancer therapy.
| Drug delivery | ||||
|---|---|---|---|---|
| Agent delivered key | Material[s] | Translational status | Details of study | Ref. |
| Cisplatin | Carbon | Preclinical | Drug-loaded carbon nanohorns enhanced efficacy in human lung cancer mouse xenograft tumors in vivo compared to unmodified cisplatin | [40] |
| Paclitaxel | Carbon | Preclinical | Drug-loaded single-walled carbon nanotubes enhanced circulation half-life, tumor killing, and overall survival in 4T1 murine breast cancer model compared to unmodified paclitaxel | [41] |
| Doxorubicin | Carbon | Preclinical | Drug-loaded NDs enhanced blood circulation, tumor killing, and animal survival in chemoresistant mouse breast and liver tumors compared to unmodified doxorubicin | [42] |
| Epirubicin | Carbon | Preclinical | EGFR-targeted, drug-loaded ND-lipid hybrid particle enhanced tumor homing and tumor regression in a mouse model of human TNBC compared to free drug | [43] |
| Melittin peptide | Carbon | Preclinical | Drug-loaded perfluorocarbon nanoparticles improved circulation and therapeutic efficacy against human breast cancer mouse xenograft tumors and syngeneic graft mouse melanoma tumors compared to free drug | [45] |
| Bcl2L12 siRNA | Gold | Preclinical | Gold-based spherical nucleic acid [SNA] delivery crossed BBB and accumulated in human glioma tumors in mice to silence target gene and reduce tumor burden | [46] |
| Proprietary siRNA | Carbon | Preclinical | Multiwalled carbon nanotubes loaded with siRNA inhibited tumor growth and prolonged overall survival in human lung cancer xenografted mice compared to siRNA alone or liposomaldelivery of siRNA | [44] |
Table 2 : Nanoparticles applications in drug delivery and cancer therapy.
| Type of cancer therapy | Materials | Translational status | Key details of study | Ref. |
|---|---|---|---|---|
| Immunotherapy, vaccine | Protein | Phase 2, clinical trial | Viral-like protein shell delivery of CpG-rich oligodeoxynucleotide [CpG-ODN] and melanocyte differentiation antigen resulted in enhanced CD8+T cell response in melanoma patients compared to antigen alone [NCT00651703] | [54] |
| Photothermal vascular | Silica, gold | Phase 1, clinical trials | Endothelial growth factor [VEGF]-targeted nanoshellꞌs for thermal ablation and vessel disruption in mouse glioma model Currently in clinical trials for head and neck cancer[NCT00848042] and primary and/or metastatic lung tumors [NCT01679470] | [55] |
| Radiotherapy | Hafnium oxide crystals | Phase 1, clinical trials | Nanoparticles for radio-induced tumor cell killing in mesenchymal and epithelial tumor xenograft mouse models Phase 1 clinical trials are ongoing as of 2011 [NCT01946867, NCT01433068] | [56] |
| Immunotherapy, Vaccine | Polysaccharide | Phase 1 clinical trial | Her2 antigen cholesteryl mannan and cholesteryl pullulan nanoparticle vaccines previously tested in Her2- expressing murine sarcomas Currently being evaluated in patients with advanced cancer with Her2-expressing tumor | [57, 58,59] |
| Magneto-responsive | Iron/cobalt, PLGA | Phase 1 clinical trial | Magnetically guided polymer carriers loaded with Fe\\ Co nanoparticle and doxorubicin for drug-nanoparticle deposition in right or left liver lobe of rabbits. | [57] |
| Immunotherapy,RNA adjuvant | PEG-PLGA | Preclinical | Ovalbumin-loaded PEG-PLGA nanoparticles displaying RGD peptide for targeted M cell uptake and vaccination in mice. | [61] |
| Immunotherapy, RNA adjuvant | Lipid | Preclinical | Ovalbumin-loaded PEG-PLGA nanoparticles displaying RGD peptide for targeted M cell uptake and vaccination in mice. | [62] |
| Immunotherapy,Vaccine | PLG | Phase 1 clinical trial | PLG matrices that co-deliver granulocyte-macrophage colony-stimulating factor [GM-CSF], CpG-ODN, and tumor lysate antigen for recruitment of DCs to PLG matrices, potent local and systemic antitumor CD8+ T cell response in mouse melanoma model. Starting phase 1 study of implantable vaccine to treat melanoma [NCT01753089] | [60] |
Table 3 : Types of cancer therapy with the materials used.
Experimental
For the synthesis of SWCNTs, CO is used as carbon source and Fe (CO)5 as the iron-containing catalyst precursor. The formation of carbon is described by the equilibrium (equation 3), which is dependent on reaction temperature as well as the pressure. The equilibrium shifted to higher yields of carbon for temperatures less than 980 K, whereas above 980 K the CO decomposition is decreased. In addition the yield of carbon is increased by raising the CO pressure (principle of Le Chatelier).
The reaction rate is generally extremely low, but a suitable catalyst can affect it. This will be used favorably at the HiPCO-process. The way of formation of catalyst particles in the gas phase has a dominating influence on their size, yield, diameter and diameter distribution of SWCNTs and finally on the purity of the produced nanotubes. Therefore the injection of catalyst precursor into the hot reaction zone was systematically investigated besides the influence of reaction temperature and pressure. Figure 1a shows the reactor that consists of a quartz tube with an inside diameter of 32 mm and a length of 480 mm, which is heated on a tube length of 300 mm by a Kanthal resistance heater. Over the heater a steel tube is arranged for the preheating of CO, this is called the hot CO line (COh).
The facility allows working at pressures from 1 to 40 bars as high and temperatures up to 1450 K.
No SWCNTs were formed with increasing the transport rate by about a factor of five, whereas corresponding with results of Bronikowski et al. [27] a reduction of the transport rate only decreases the yield of the deposited SWCNTs. The mixture of Fe (CO)5 and CO is injected by means of an interchangeable copper nozzle through a ceramic insulated water-cooled steel tube into the hot reaction zone. The gas cooling is necessary to avoid the decomposition of Fe(CO)5 at temperatures above 323 K. The COc nozzle is positioned 9 cm inside the heated reaction zone.
The injection velocity into the hot reaction zone and therefore the spontaneous heating of the cooled CO/Fe(CO)5 mixture essentially influences the formation of SWCNTs. Different nozzle geometries can result in considerable changes of the gas flow rate and the temperature gradient between the CO/Fe(CO)5 cooled gas and the reaction zone. Rapid heating and a broad injection jet enhanced the formation of SWCNTs. Therefore the influence of the nozzle geometry and the gas flow rate of COc on the yield; purity, diameter and diameter distribution of SWCNTs were investigated. Nozzles were tested with different hole diameters (from 0.4 to 1mm) and different number of holes (up to 5 holes) and for a constant hole diameter of 0.8 mm but variable slot as shown in figure 1b.
Around the COc nozzle a shield with holes is arranged for the injection of the preheated COh into the reaction zone. By aid of the nozzle system an intensive mixing of the gas flows was achieved. The COh gas flow rate varied between 100 and 500 sccm. The produced material consists of a black felt.
The aim of our investigations was the production of SWCNTs with high purity; reduced diameter distribution and with different mean diameter. It is possible to estimate diameter and diameter distribution as well as the size of the nanotubes bundles [74,75] and to study theirs electronics character.
Results and Discussion
The growth of nanotubes was analyzed for samples obtained under different reaction pressure, temperature and CO gas flow rate. In addition, the study of the effect of the nozzle geometry (hole diameter, number of holes and slot width) and its role on the injection velocity was examined.
Yield of SWCNT in dependence on pressure and temperature
Figure 2 shows the dependence of the total yield of the produced material and the yield of SWCNTs on the reaction pressure at two different reaction temperatures profile (Tr) in the reactor figure1(a).
At low pressures the reaction product mainly consists of graphite-like carbon. The SWCNT are dominating with increasing reaction pressure. Figure 3a and 3b show HRTEM images of two samples produced at the real temperature 1320 K at injection nozzle with pressure of 1 bar (The resulting therapeutic molecule in the form of core-shell nanoparticles with iron nanoparticle of diameter 20 nm embabed in graphite thickness of 4 nm; and bundles of single walled carbon nanotubes of 14 nm, after functionalization by iron encapsulation (filling) can be use as therapeutic nanomaterials in nanomedicine in diagnosis and treatment of cancer tumor); at 10 bars, respectively. Carbon nanotubes (CNTs) provide a smart carrier system on the nanometer scale. The system can be used as a template for ferromagnetic fillers. Such a molecular hybrid is a promising potential candidate for the controlled heating of tumour tissue at the cellular level. This is a key reason why it is important to optimize the synthesis route of metal filled carbon nanotubes with regards bulk scale synthesis and purity. In the current study we present multiwalled carbon nanotubes filled with α-iron phase (Fe-MWCNT) [76].
A highly efficient and simple methodology based on wet chemistry to fill single-wall carbon nanotubes (Fe@SWCNTs) with iron, and thus create quantum wires in a bulk [7]. The research shown is unique in that it is the first experimental single-wall carbon nanotubes that have iron continuously within their core for extended length scale. While in sample figure 3(a), large Fe magnetic nanoparticles encapsulated in graphite are seen forming core shell like nanostructure; the bundles of SWCNTs formed at 10 bars are shown in figure 3(b). The observed decrease of the yield figure 2 with the increase of reaction pressure is inconsistent with the principle of Le Chatelier. This is not surprising, because the deposition is a strongly kinetically controlled process by the measurement of CO2 concentration to estimate the amounts of SWCNTs.
On the other hand the rise of the deposition rate of carbon with increasing temperature is in agreement with a thermodynamic equilibrium process.
Figure 2: HiPCO reactor with different injection nozzle geometry used in our studies. (a) Schematic drawing of the HiPCO reactor with the temperature profile;(b) Principle of the gas injection.
Yield in dependence on the COc gas flow rate
Different deposition rates are also observed at variation of the cold CO gas flow in the turbulent flow regime. An increasing flow leads to an enhancement of the formation of SWCNTs. Almost no SWCNTs were deposited at gas flows less than 500 sccm COc . By using this low gas flow rate, the catalyst decomposed in front of the reaction zone and formed large iron particles. The formation of SWCNT starts at flow rates above 500 sccm and the yield increases as shown in figure 4. The higher the COc gas flow rates the higher the SWCNTs quantity. The reaction product consists mainly of SWCNTs from COc gas flows up to 1500 sccm. The yield of SWCNTs decreases again at very high gas flows due to the reduced stay in the reaction zone.
Diameter and diameter distribution function of the pressure
OAS is a good and express method to determine the diameter and diameter distribution of the SWCNTs. A detailed analysis of the optical absorption spectra is performed using the fitting of the tight binding model [75] in comparison with the results of the Raman spectroscopy measurement. Optical absorption spectroscopy (OAS) of SWCNTs is a efficiency method to determines the diameter and diameter distribution of the as prepared SWCNTs figure 4. Figure 5 shows the optical absorption spectra after subtracting the background as described figure 4. Previously [77]. The OAS intensity of the first two peaks (IS11, IS22) is related to the transitions between DOS van Hove singularities (vHs) in semiconducting nanotubes and the third (IM11) one in the metallic nanotubes. The intensity of the (IS22) peak describes the relative yield of the individual SWCNT as compared to amorphous carbon species [77]. The fine structure of the (IS22) peak determines the diameter and diameter distribution.
In the laser ablation process the amount of catalyst particles is between 0 and 2 wt. % and the dielectric screening is independent from the process parameters.
In addition, from the peak position of corresponding second vHs (IS22) one can calculate the diameters of the semi- conducting SWCNT according to the following equation [77,78]:
(IS22) = 4a0g0/d
with γ0 = 3 eV (overlap integral) and a0 = 0.142 nm (nearest-neighbor C-C distance).
As can be seen in the figure 5 for the HiPCO process the spectra show a shift of the peaks positions to higher photon energies. This means a shift to smaller diameters with increasing reaction pressure as shown in figure 5. The diameter distribution is relatively broad. Irrespective to the optimized preparation conditions the diameter distribution was found to shift downwards with increasing pressure.
The distribution is broader at higher pressure but the mean diameter of SWCNTs decreases with pressure figure 6, which is in agreement with Nikolaev et al. [25].
Iron-content in the deposited material
Figure 7 shows the energy dispersive spectroscopy of the deposit raw nanomaterials in dependence on the Fe content. The iron content was determined by AAS after removing of the carbon by oxidation. Since the OAS measurements were always performed with the same thickness films on the KBr or NaCl single crystal and the same preparation procedure, the intensities of the (IS22) transition or (IS11) can be directly compared and calculated. In the HiPCO process the amount of amorphous carbon species is low and the main contribution of the intensity variation of the (IS22) peak is due to different dielectric screening of the Fe catalyst nanoparticles. When the amounts of iron contents are lower the intensity becomes higher. The OAS can be used to determine the iron content in the different SWCNTs containing materials considering certain conditions. The spectra in figure 8 can be used as additional information about the raw nanomaterial by electron energy loss spectroscopy curves for the determination of the Fe concentration.
Figure 3: HRTEM micrograph: (a) – Carbon coated iron nanoparticles at pressure of 1 bar with max. (20 nm) in graphite with thickness of 4 nm ,(b) - SWCNTsbundles and iron nanoparticles at 10 bars.
Figure 4: Total yield of deposited material in dependence on the reaction pressure (solid line square, doted line triangle) and of SWCNTs (circle and open circle) at reaction temperature 1173 K and 1323 K, respectively
Figure 5: OAS signatue of SWCNTs for estimation of their mean diameter and diameter distribution meanly by pic fitting of intensities of the two first semiconducting pics E11s, E22s (Im11, Im22). The metallic pic E22m (Im22) is not used.
Effect of nozzle injection geometries
The geometries of injection nozzle used can affect the injection velocity and thereafter the yield of SWCNTs by varying the dimension of the catalyst nanoparticles and the volume of the CO in the reaction zone.
The different types of nozzles were tested to optimize the injection of the COc into the reaction zone. An optimized injection stream should increase the SWCNTs yield and reduce the nanotube diameters. If the injection is defocused small catalyst particles are formed, whereas a focused stream promotes the formation of large iron nanoparticles. The changing of the injection into the hot reaction zone is performed by variation of the slot width figure 1(b), on the other hand by using of nozzles with different holes diameters and number of holes. Table 4 contains the observed yields of as grown SWCNTs raw material and the highest intensities IS22, which correspond to the Fe content in comparison to the mean diameters for different slots. The best results are achieved at a slot width equal to 21 µm, both the purity (lowest Fe content) and yield have a maximum value. The yield of the SWCNTs is estimated by the maximum intensity of the diameter distribution of the second transition peak (IS22). The purity I(IS22) of 0.068 and 0.056 (arb. units) , yield of 8.3 and 4.8 (mg/h) and mean diameter of 1.13 and 1.02 (nm) are calculated at pressure 5 and 10 bars, respectively.
Table 5 shows a comparison between one and five holes nozzle with a hole diameter 0.6 mm. The number of holes seems to have a large influence on the diameter and the diameter distribution of the SWCNTs. At the same deposition conditions the mean diameter is reduced from 1.13 to 0.92 nm if a nozzle with five holes is used. Also a smaller diameter distribution can be observed in comparison with one hole injection nozzle. The maximum yield has a value of about 3.4 (mg/h) and mean diameter 1.13 nm at reaction pressure 10 bars and one hole of diameter 0.6 mm. The optimized nozzle geometry versus SWCNTs yield is given at one hole diameter 0.8 mm and slot width equal to 21 mm.
Tables 1 and 2 show that the nozzle geometry (hole diameter, number of holes and slot width) has a primordial effect on the injection velocity.
Figure 6: Diameter distribution and Mean diameter (dmean) of SWCNTs versus reaction pressure calculated from the optical absorbance spectra (reaction condition see Figure. 5)
| P v (bars) | Slot width (mm) | Imax(IS22)(a.u.) | dmean(nm) | Yield (mg/h) |
|---|---|---|---|---|
| 10 | 7 | 0.015 | 1.01 | 3.3 |
| 14 | 0.029 | 1.01 | 2.7 | |
| 21 | 0.056 | 1.02 | 4.8 | |
| 42 | 0.006 | 1.14 | 1.7 | |
| 5 | 21 | 0.068 | 1.12 | 8.3 |
| 42 | 0.004 | 1.12 | 2.8 |
Table 4: Observed yields of as grown SWCNTs raw material and the highest intensities IS22, which correspond to the Fe content in comparison to the mean diameters for different slots.
| P (bars) | Holes number (f=6 mm) | Imax(IS22)(a.u.) | dmean(nm) | dmean(nm) | Yield (mg/h) |
|---|---|---|---|---|---|
| 10 | 1 | 0.021 | 1.12 | 0.4 | 3.4 |
| 5 | 0.009 | 0.92 | 0.06 | 1.5 |
Table 5: Comparison between one and five holes nozzle with a hole diameter 0.6 mm. The number of holes seems to have a large influence on the diameter and the diameter distribution of the SWCNTs.
| Raman shift (cm-1) | Diameter (nm) |
|---|---|
| 337.352 | 0.7000 |
| 327.397 | 0.7225 |
| 307.421 | 0.7724 |
| 278.452 | 0.8484 |
| 264.225 | 0.9239 |
| 211.121 | 1.1370 |
Table 6: Diameter distribution calculated from the RBM (figure.11a) feature using Eq. (5).
Figure 7: EDX spectra of the as grown HiPCO heronanocarbon.
Figure 8: EELS spectra of the as grown HiPCO heronanocarbon.
Figure 9(a): HRTEM imagines, EDX and EELS spectra of SWCNTs raw material, (b)- After purification. The optical absorption spectra of the purified sample have a higher intensity as the raw material (figure 11) indicating the much higher purity of the purified sample. The mean diameter does not change significantly.
Figure 10: Optical absorption spectra of a purified sample (green line), oxidized SWCNTs (blue line) and optimized raw material (black line). In insert is represented the fine structure of the (IS22) of the semiconducting pic Es22.
Figure 11(a): RBM spectra (Laser excitation 514 nm). The regions indicate groups of metallic (200-250 cm-1) and semiconducting (250-400 cm-1) nanotubes with different diameter, diameter distribution and synthesis pressure.
Figure 11(b): D and G bands (laser excitation 514 nm). The fine structure in between (800-1200 cm-1) and (1680-1800 cm-1) regions indicate the vibration frequency of the core shell like structure heteronanocarbon groups.
The optimal deposition condition and purification procedure
Summarizing the results from above, one can derive the optimal conditions for the synthesis of SWCNTs by HiPCO process as follows:
• a reaction pressure of 1 or 10 bars;
• a reaction temperature of 1250 K;
a COc flow rate of 2000-2500 sccm CO included Fe(CO)5 +500 sccm Ar and COh flow rate of 100 sccm, an onehole nozzle diameter of 0.8 mm with 21 µm slot. Under these conditions the yield of SWCNTs amounted to about 10 mg/h.
In figure 5 typical optical absorption spectra of material grown under such conditions are shown with the same synthesis parameters. The Fe concentration of the product is less than 5 % and the mean diameter decreased from 1.12 to 1.02 nm with increasing pressure from 5 to 10 bars. With increasing pressure the diameter distribution also decreases figure 5.
In order to remove the iron catalyst and carbon-like impurities the purification of the raw material is realized in two steps:
oxidation followed with acid treatment in HCl (36 %). The raw materials were purified by oxidation in high vacuum at 523 K in a wet Ar/20 vol. % O2 mixture to remove SWCNT carbon-like impurities and to oxidize the iron catalyst nanoparticles. at atmosphere pressure according to [79,80]. The oxidation time amounted to 6 hours. Subsequently the remaining iron oxide nanoparticles were solved and removed by chemical treatment in concentrated HCl/ alcohol, C2H5OH mixture solution in ratio 1/1 by sonication for 2 h at 353 K.
Figure 9 shows HRTEM imagines, EDX and EELS spectra of raw and purified SWCNTs. The purification effect is obvious. However in all purified samples few Fe nanoparticles can be still observed which are covered with a relatively thick carbon layer (core shell nanostructure). A complete removal of the catalyst leads to an undesirable burning of grown SWCNTs during the oxidation step. This degree of purification is also confirmed by the increasing of the optical absorbance intensity area of the first peak (IS11) and second peak (IS22) in the optical signature of the SWCNTs figure 10. The absorption peaks of the purified samples have higher intensity than the raw material figure 10 indicating the much higher purity of the purified sample. The mean diameter and diameter distribution do not change significantly by the oxidation treatment (see the inset in figure 10). This observation confirms that the oxidation treatment has no detrimental effect on the nanotube crystallinity.
Purification Procedure and Separation of the Core Shell Nanotructure
The soot-like products, obtained in HiPCO parameters processes, have to be subjected to purification of the product carbon nanotubes for functionalization, separation and sterilization of the therapeutic-molecule (core-shell iron magnetic nanoparticle encapsulated in graphite-like heteronanocarbon) in order to remove amorphous carbon, non-encapsulated metal and carbide. The purification procedures have to remove, such as impurities. In order to remove amorphous carbon in the samples it is necessary to anneal the resulting reaction products for 6 hr in 50% nitric acid HNO3 at 323K, and then to flush by distilled water until complete removal of the acid is achieved.
In order to remove noncoated iron or carbides, the samples have to be boiled in 2M HCl (24 h), and then washed in distilled water and subsequently in ethanol and annealed in dry air atmosphere at 350K. In order to also remove amorphous carbon, the chemical oxidation by KMnO4 dissolved in 50% sulfuric acid could be used [81]. Again the sample has to be washed thoroughly in distilled water and annealed in dry air atmosphere.
Characterization of Carbon Nanotubes and Core Shell like Nanostructure Molecule by Raman Spectroscopy
The Raman spectra present different features being all sensitive to chiral indices (n,m) specifying the perimeter vector (chiral vector), such as the radial breathing mode (RBM) where all the carbon atoms are moving in-phase in the radial direction, the G-band where neighbouring atoms are moving in opposite directions along the surface of the nanotube as in 2D graphite, the dispersive disorder induced D-band and its second-order related harmonic G′-band. From these five features figure 11a and b, the RBM is the one which appears more sensitive to the nanotube diameter (d) [82], according to the expression RBM.
ωRBM= A/ d + B (5)
Where ω is the vibration frequency, and A and B are constants and vary between individual nanotubes and bundle nanotubes [83, 84]. Some authors consider only the constant A in determination of the diameter [85, 86, 87].
Bat there’s no formula for calculation of the diameter of nanocapsule (iron /graphit) or core shell nanostructure in the literature.
Determination of Diameter and Diameter Distribution
Four characteristic features of a HIPCO/SWCNT sample may be found in Raman spectra presented in figure 11(a). An analysis of RBM band is visible in the red laser spectra. (Laser excitation 514 nm) for typical HIPCO/SWCNT bundles of the diameter d = 1.13±0.4 nm, A = 224 cm−1 and B = 14cm−1 have been calculated [82], B is an up shift in ωRBM due to nanotube–nanotube interactions. The calculated diameter distribution was obtained using Eq. (5) table 6, figure 11(a). The range of diameters varies between 0.7 nm and 1.13 nm. According to the Kataura plot, we have only obtained signals from semiconducting and small signal of metallic nanotubes with the green laser with different chirality (n, m). We indicate in future the use of various lasers energies analysis is very important, because each experiment can give different data on optical and physical properties. The ratio between D and G band and the RBM and its relation to the diameter distribution are very important factors allowing one to distinguish between carbon nanotubes and core shell nanostructured therapeutic molecule.
It was found that the diameter and diameter distribution obtained by optical absorbance spectroscopy (OAS) figure 5,6 and table 4,5 are in good agreement with that calculated from Raman scattering spectra.
Commercialisation Challenges
Continuous advancements in nanomedicine have opened up opportunities for application of new generation of multifunctional therapeutic nanoparticles in a variety of medical disciplines, but still we are facing some basic challenges. There is a huge gap between nanobiotechnology researches in labs and the production of commercialized therapeutic nanoparticles. This gap contains a whole litany of challenges that must be tackled. One of the most important challenges lies in scaling up processes for production commercially. For a viable scale-up, laboratory processes must be consistent with current manufacturing capabilities.
Another challenge is navigating an often mystifying regulatory perspective. Although nanoparticle based therapies refer to more complicated requirements than conventional medical treatments [in that both require clinical trials that demonstrate safety and efficacy, more complicated technology needs more evidence of effectiveness. The human, environment and animal safety that related to the life cycle issue also are the main point for discuss. Detect and determine the toxicity of engineered nanomaterials within next 5 to 15 years is an important challenge for researchers, though, describes models for predicting effects of nanomaterials on human health and the environment would be an inevitable issue. Finally, researchers should take a proactive role in translating laboratory discoveries into feasible medical technology.
Such an important role requires researchers to consider future commercialization early in their research and act accordingly.
The Future of Nanomedicine
In spite of a huge number of researches about nanomedicine, unmet medical demands in cancer diagnosis and therapy remain substantial.
The potential for nanotechnology in medicine, in the future of cancer diagnosis and therapy is unprecedented. Continuously drives advances in diagnostic nanobiotechnological devices design, with diameters of hundreds of atoms is a considerable progress that can revolutionized the ability of cancer diagnosis especially about medical data collection, detect chemical changes in the body, the ability of closer real-time tracking of a patient’s status and the build of nanoscale microscopic cameras. All of these advancements can provide a complete map of most of the tissue in the human body, in a level of detail that’s impossible with X-ray or MRI. With the application of quantum dots as an optical barcode may indeed gene sequencing and chemical analysis be speed up and finally it provides the possibility of faster, cheaper and more reliable diagnostic tests outside the body. The outcome of these progressions at cellular and molecular levels will be result in the potential for diagnosing and treating many conditions preemptively, before they have the opportunity to proliferate.
Toxicity of Core-Shell Iron Magnetic Nanoparticle Encapsulated in Graphite-like Heteronanocarbon
Safety is the first requirement of any material used in medicine. A large number of studies have been performed in the past several years to explore the potential toxic effects of carbon nanotubes. The conclusions of these reports varied drastically, showing a large dependence on the type of nanotube materials as well as functionalization approaches.
Cell culture experiments and in vivo pilot studies conducted by various groups observed no obvious toxicity of properly functionalized carbon nanotubes [88,89]. On the other hand, raw carbon nanotubes were shown to be toxic to mice after inhalation into the lung [90,91]. Recent research showed that unfunctionalized, long MWNTs may pose a carcinogenic risk in mice. As a result of the wide variety of reports, both the public and research communities are currently concerned about using carbon nanotubes for biomedical applications. It is thus critical and urgent to clarify the toxicity issue of carbon nanotubes. The current status is that toxicity appears to be dependent on the material preparation, especially geometry and surface functionalization. Wellfunctionalized CNTs with biocompatible surface coatings have been shown to be nontoxic in vitro to cells and in vivo in mice.
In vitro toxicity of core-shell iron magnetic nanoparticle encapsulated in graphite-like heteronanocarbon
Even in cell culture experiments, the issue of toxicity of the molecule is still controversial. While inhibition of HEK 293 cell proliferation after exposure to SWNTs was observed by Cui et al. [92], Ding et al. observed that MWNTs induce cell cycle arrest and increase apoptosis/necrosis of human skin fibroblasts [93]. However, neither of these studies used functionalized carbon nanotubes in the experiments. Apoptosis of T lymphocytes induced by oxidized MWNT was observed by Bottini et al. [94]. However simple oxidization is not enough to render carbon nanotubes soluble and stable in saline and cell medium, and thus does not represent a biocompatible functionalization. Sayes et al. further reported that the toxicity of CNTs was dependent on the density of functionalization on nanotubes, with minimal toxicity for those heavily functionalized with the highest density of phenyl-SO3X groups on nanotubes [95]. These results are understandable because CNTs without proper functionalization have a highly hydrophobic surface, and thus may aggregate in the cell culture and interact with cells by binding to various biological species including proteins via hydrophobic interactions, to induce certain cell responses such as cell toxicity.
Other factors may also contribute to the observed toxicity of CNT samples in vitro. Excess surfactants in the CNT suspensions are known to be highly toxic to cells [96].
The metal catalyst content in CNTs should also be considered when the toxicity of carbon nanotubes is investigate [97]. Moreover, proper assays must be employed in toxicity tests to avoid interference of carbon nanotubes with the assay reagents [98,99]. For these reasons, in vitro toxicity assays of carbon nanotubes should be carefully designed and performed with well prepared and characterized materials, as well as suitable assay methods.
We and many other groups successfully used well functionalized, serum stable carbonnanotubes for in vitro cellular uptake experiments without observing apparent toxicity [89,100-108]. Cells exposed to SWNTs, PEGylated by various PL-PEG24 amphiphiles used in our work, exhibited neither enhanced apoptosis/neurosis, norreduced proliferation of various cell lines in vitro [101,102,103]. Carbon nanotubes covalently functionalized by 1,3-dipolar cycloaddition developed by Prato et al. also appeared to be safe to their tested cell lines, including primary immune cells [89,104].
Carbon nanotubes with biomimic coating engineered by Bertozzi et al. were also nontoxic to cells [105,106]. Several other independent groups also reported that CNTs coated by DNA, amphiphlic helical peptides and serum proteins were not toxic to cells [107-109]. The latest finding by Jin et al. discovered that SWNTs taken up by cells via endocytosis exited cells through exocytosis without affecting the viability of cells [95]. It appears that raw CNTs and CNTs without serum-stable functionalization show toxicity to cells at moderate dosage, while serum-stable, functionalized CNTs show little toxicity even at high dosages.
In vivo toxicity core-shell iron magnetic nanoparticle encapsulated in graphite-like heteronanocarbon
To address the possible side effects of CNTs on human health and our environment, researchers have investigated the toxicology of CNTs in animal models. Unfucntionalized raw CNTs have been intratracheally (IT) instilled into animals, showing obvious pulmonary toxicity including unusual inflammation and fibrotic reactions due to the aggregation of hydrophobic raw CNTs in the lung airways [90, 91]. Those results suggest that aerosol exposure of raw CNTs in the workplace should be avoided to protect human health. Nevertheless, toxicities observed by intratracheal instillation of large amounts of raw CNTs may have little relevance to the toxicology profile of functionalized soluble CNTs for biomedical applications, especially when they are administered through other routes such as intraperitoneal (IP) and intravenous (IV) injections, by which lung airways are not accessible to CNTs.
In a recent pilot study, Poland et al. noticed asbestoslike pathogenic behaviors such as mesothelioma associated with exposing the mesothelial lining of the body cavity of mice to large MWNTs (length 10~50 μm, diameter 80~160 nm) following intraperitoneal injection [111]. Despite the importance of this finding in the discovery of potential negative effects of CNTs to human health, the MWNT materials used in this study are simply sonicated in 0.5% bovine serum albumin (BSA) solutions without careful surface functionalization and hence are not directly meaningful for functionalized CNTs with biocompatible coatings recommended for biomedical applications. Furthermore, length dependent pathogenicity was observed, as no obvious toxic effect was observed for shorter and smaller MWNTs (length 1~20 μm, diameter 10~14 nm), indicating that the toxicology profiles of CNTs may significantly differ between CNTs of various sizes (diameter and length). It is worth note that functionalized SWNTs used in typical biomedical research have length 50~300 nm and diameter 1~2 nm, which are entirely different from the geometry of MWNTs used by Poland et al.
The first reported in vivo toxicity study of functionalized SWNTs was conducted by the Gambhir group and our group [112,113]. Both covalently and non-covalently PEGylated SWNTs were used in this study. Mice intravenously injected with PEGyalted SWNTs (~3 mg/kg) were monitored over four months, with systolic blood pressure, complete blood counts and serum chemistry recorded every month. Careful necropsy and tissue histology examinations were performed at the end of 4 months. Normal blood chemistries and histological observations were observed in this study, suggesting that functionalized biocompatible SWNTs may be safe for in vivo biological applications. Another separate study by our group showed similar results, suggesting that PEGylated SWNTs are slowly excreted from the body following systemic distribution in mouse models, without exhibiting obvious toxicity in the process [114]. Recently, Yang et al. showed in a 3 month toxicity study that SWNTs suspended by Tween-80, which is likely not an ideal coating molecule, exhibited low toxicities to the tested mice at a very high dose (~40mg/kg) following i.v. administration. Such toxicity may be due to the oxidative stress induced by SWNTs accumulated in liver and lung [115]. The toxicity observed was dose dependent, and appeared to be less obvious at lower doses (2mg/kg and 16mg/kg). Another recent report by the same group showed that their covalently PEGylated SWNTs, with much higher aqueous stabilities and biocompatibilities, exhibited an ultra-long blood circulation half-life in mice [116]. Although the long-term toxicology of such “improved” SWNTs has yet to be determined, no acute toxicity has been reported even at a high dose (24mg/kg).
To fully address the toxicity concern of CNTs, further investigations, including animal models other than mice, and at larger scales, are still required. Moreover, the interactions between administered CNTs and the immune complement system, whose activation is an important first defense line against foreign species, especially microbes, requires more attentions [117]. Moreover, increased efforts are needed not only from the chemical approach, i.e. further optimizing CNT surface chemistry and geometry for improved biocompatibility, but also from those with biological expertise, to systematically study the complete CNT toxicology profile in different animal models with different routes of administration.
In vitro delivery of biomolecules by core-shell iron magnetic nanoparticle encapsulated in graphitelike heteronanocarbon
The work of using carbon nanotubes for drug delivery in our group was triggered by an unexpected finding that functionalized CNTs are able to enter cells by themselves without obvious toxicity [88]. Similar results were published by the Prato group around the same time [118]. The CNT cellular uptake mechanism may differ depending on the functionalizations and sizes of the CNTs, including endocytosis as reported by us and several other groups [88,107,109,110,119], or passive diffusion as observed by the Prato group when CNTs are functionalized by 1,3-dipolar cycloaddition [119]. CNTs have been used to efficiently shuttle various biological cargoes, ranging from small drug molecules to bio-macromolecules, such as protein and DNA/ RNA, into different types of cells. Once taken up by cells via endocytosis, SWNTs are able to exit cells through exocytosis [120].
Summary
Our systematic study of the HiPCO process shows that several parameters strongly affect the properties of the deposited material (purity, yield and diameter) e. g. the reaction pressure, the temperature at injection nozzle and the injection velocity of the CO/Fe (CO) 5 gas mixture in the reaction zone. These parameters are important for nucleation and deactivation of the iron nanoparticles/ SWCNT growth to be dealt with lightly.
With increasing of the CO through Fe(CO)5 pressure the diameter of SWCNTs decreases. The new contribution in our work on the synthesis of SWCNTs by CVD (HiPCO method) is given by varying of the injection nozzle geometry to optimize the dimension of the iron catalyst nanoparticles and the volume of the gas phase in the reaction zone where the SWCNTs nucleate. This has produced parameters nanotubes as small as 1.0 nm in diameter. The purity and yield of the deposited material are increased with increasing of COc gas flow by means of rapid heating of the gas mixture and using an optimum injection profile. The optimal conditions to produce highly pure SWCNTs were found at 1250 K pressures between 1, 10 bars and a gas flow in the cold line of 2000-2500 sccm CO and 500 sccm Ar. Higher gas in turbulent flows regime at adjusted injection nozzle geometry should represent a new interesting result for instance. We continue to synthesizing this therapeutic molecule at low pressure (1 bar) with variation of other parameters in order to obtain uniform and fine molecules according to the size of the cell of human tissues. The HiPCO process is one of the best-investigated procedures, for carbon nanotubes synthesis and functionalization of single walled carbon nanotubes after by filling of iron can be use as therapeutic nanomaterials in nanomedicine in diagnosis and treatment of cancer tumor. The resulting therapeutic molecule in the form of core-shell nanoparticles with iron nanoparticle of diameter 20 nm embabed in graphite thickness of 4 nm; and bundles of single walled carbon nanotubes of 14 nm, after functionalization by iron encapsulation (filling) can be use as therapeutic nanomaterials in nanomedicine in diagnosis and treatment of cancer tumor; at 1 and 10 bars, respectively.
The magnetic measurement and Raman scattering measurements will be performed in the near future according to their nanomedicine application by photothemal vascular cancer therapy (hyperthermia) and cellular dimension in the body after purification, separation and sterilization. By this process we have obtained at low pressure metal ferromagnetic in core shell structure by injection of nanocatalyst Metalcene, Me(CO)5 (Me=Fe, Ni and Co).
This information is not only for the special equipment used but also can be generalized to large industrial-scale of the HiPCO process with high yield and uniformed therapeutic molecule diameter iron nanoparticle and heteronanocarbon thickness. There is a huge gap between nanobiotechnology researches in labs and the production of commercialized therapeutic nanoparticles. This gap contains a whole litany of challenges that must be tackled. One of the most important challenges lies in scaling up processes for production commercially. For a viable scale-up, laboratory processes must be consistent with current manufacturing capabilities.
Finally, researchers should take a proactive role in translating laboratory discoveries into feasible medical nanotechnology. Such an important role requires researchers to consider future commercialization early in their research and act accordingly.
Acknowledgments
We acknowledge support of PHC-Tassili project (2019-2020) between LASEA-UBMA-Algeria and DOLPHIN-Nanobio-materials for live-University of Lorraine-France.
The partner Pr. A. LANKAR from the (Laboratoire Central de Cytologie Pathologiques CHU Annaba, Annaba, 23000, Algeria) are also acknowledge for the nanoparticles applications in cancer diagnosis and therapy.
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature 363: 603-605.[ Ref ]
Sinha N (2005) Carbon nanotubes for biomedical applications. IEEE Transactions on Nanobioscience 4:180-195.[ Ref ]
Saito S (1997) Carbon Nanotubes for Next-Generation Electronics Devices. Science 278: 77-78.[ Ref ]
Saito R, Dresselhaus G, Dresselhaus MS (1998) Physical properties of carbon nanotubes. (London, Imperial College).[ Ref ]
Borowiak-Palen E, Pichler T, Fuentes GG, Bendjemil B, Liu X, et al. (2003) Infrared response of multiwalled boron nitride nanotubes. Chem Commun 1:82-84. [ Ref ]
Rümmeli MH, Borowiak-Palen E, Gemming T, Knupfer M, Biedermann K, et al. ( 2005) On the formation process of silicon carbide nanophases via hydrogenated thermally induced templated synthesis. Appl Phys A 80: 1653-1656.[ Ref ]
Borowiak-Palen E, Mendoza E, Bachmatiuk A, Rümmeli MH, Gemming T, et al. (2006) Iron filled single-wall carbon nanotubes – A novel ferromagnetic medium. Chem Phys Lett 421: 129-133.[ Ref ]
Wang R, Zhang D, Zhang Y, Liu C (2006) Boron-doped carbon nanotubes serving as a novel chemical sensor for formaldehyde. J Phys Chem B 110: 18267-18271.[ Ref ]
Peng-Xiang H, Man S, Jin-Cheng L, Chang L, Shi-Sheng L (2015) Synthesis of high quality nitrogen-doped single-wall carbon nanotubes. Science China Materials 58: 6603-6610.[ Ref ]
Mohammadi F, Tavakol H (2018) Synthesis of phosphorus doped carbon nanotubes using chemical vapor deposition. Journal Fullerenes, Nanotubes and Carbon Nanostructures 26: 218-225.[ Ref ]
Chico L, Crespi VH, Benedict LX, Louie SG, Cohen ML (1996) Pure Carbon Nanoscale Devices: Nanotube Heterojunctions. Phys Rev Lett 76: 971- 974.[ Ref ]
Wong EW, Sheehan PE, Lieber CM (1997) Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 277: 1971-1975.[ Ref ]
Salvetat JP, Bonard JM, Thomson NH, Kulik AJ, Forró L, et al. (1999) Mechanical properties of carbon nanotubes. Appl. Phys. A 69: 255–260.[ Ref ]
Mimitmire JW, Dunlop BI, White, CT(1992) Are fullerene tubules metallic? Phys Rev Lett. 68: 631-636.[ Ref ]
Hamada N, Sawada SI, Oshiyama A (1992) New one-dimensional conductors: Graphitic microtubules. Phys Rev Lett 68: 1579-1589.[ Ref ]
Dresselhaus MS, Dresselhaus G, Eklund PC (1996) Science of fullerenes and carbon nanotubes: their properties and applications, San Diego, Academic Press.[ Ref ]
Thess, Lee R, Nikolaev P, Dai H, Petit P, et al. (1996) Crystalline Ropes of Metallic Carbon Nanotubes. Science 273: 483-487.[ Ref ]
Rinzler G, Liu J, Nikolaev P, Huffmann CB, Rodriguez- Macias FJ, et al. (1998) Large-scale purification of single-wall carbon nanotubes: process, product, and characterization. Appl Phys A 67: 29-37.[ Ref ]
Guo T, Nikolaev P, Thess A, Colbert DT, Smalley RE (1995) Catalytic growth of single-walled manotubes by laser vaporization. Chem Phys Lett 243: 49-54.[ Ref ]
Journet, Masser WK, Bernier P, Loiseau A, Lamy de la chapelle M, et al. (1997) Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 388: 756-758.[ Ref ]
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56-58. [ Ref ]
Hafner JH, Bronikowski MJ, Azamian BR, Nikolaev P, Rinzler AG, et al. (1998) Catalytic growth of single-wall carbon nanotubes from metal particles. Chem Phys Lett 296: 195-202.[ Ref ]
Colomer JF, Bister G, Willems I, Konya Z, Fonsera J, et al. (1999) Synthesis of single-wall carbon nanotubesby catalytic decomposition of hydrocarbons Chem Comm 14: 1343-1350.[ Ref ]
Yan H, Li Q, Zhang J, Lin Z (2002) Possible tactics to improve the growth of single-walled carbon nanotubes by chemical vapor deposition. Carbon 40: 2693-2698.[ Ref ]
Bronikowski MJ, Nikolaev P, Bradley RK, Rohmound F, Colbert DT, et al. (1999) Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chemical Physics Letters 313: 91-97.[ Ref ]
Bronikowski MJ, Willis PA, Colbert DT, Smith KA, Smalley RE (2001) Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J Vac Sci Technol A 19: 1800-1814.[ Ref ]
Selbmann D, Bendjemil B, Leonhardt A, Pichler T, Täschner C, et al. (2008) A parametric study of the synthesis and purification of singlewalled carbon nanotubes using the high-pressure carbon monoxide process. Applied Physics A 90: 637-643.[ Ref ]
Cheng HM, Li F, Sun X, Brown SDM, Pimenta MA, et al. (1998) Bulk morphology and diameter distribution of single-walled carbon nanotubes synthesized by catalytic decomposition of hydrocarbons. Chem Phys Lett 289: 602-610.[ Ref ]
Colomer J, Stephan C, Lefrant S, Tendeloo GV (2000) Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem Phys Lett 317: 83-89.[ Ref ]
Gökçen T, Dateo ChE, Meyyappan M (2002) Modeling of the HiPco Process for Carbon Nanotube Production, II. Reactor-Scale Analysis. Journal of Nanoscience and Nanotechnology 2: 535-544.[ Ref ]
Nie S, Xing Y, Kim GJ, Simons JW (2007) Nanotechnology applications in cancer. Annu Rev Biomed Eng 9: 257-288.[ Ref ]
Srinivasan M, Rajabi M, Mousa SA (2015) Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomatherials 5: 1690-1703.[ Ref ]
Wen CY, Xie HY, Zhang ZL, Wu LL, Hu J, et al. (2016) Fluorescent/ magneticmicro/nano-spheres based on quantum dots and/or magnetic nanoparticles: preparation, properties, and their applications in cancer studies. Nanoscale 8: 12406-12429.[ Ref ]
Yu MK, Park J, Jon S (2012) Targeting strategies formultifunctional nanoparticles in cancer imaging and therapy. Theranostics 2: 3-44.[ Ref ]
Nie S, Xing Y, Kim GJ, Simons JW (2007) Nanotechnology applications in cancer. Annu Rev Biomed Eng 9: 257-288.[ Ref ]
Wang Y, Gao S, Ye WH, Yoon HS, Yang YY (2006) Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater 5: 791-796.[ Ref ]
Zhang L, Radovic-Moreno AF, Alexis F, Gu FX, Basto PA, et al. (2007) Co-delivery of hydrophobic and hydrophilic drugs from nanoparticleaptaer bioconjugates. ChemMedChem 2: 1268-1271.[ Ref ]
Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, et al. (2013) Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther 21: 1096-1103.[ Ref ]
Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, et al. (2012) Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 4: 128-139.[ Ref ]
Ajima K, Murakami T, Mizoguchi Y, Tsuchida K, Ichihashi T, et al. (2008) Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano 2: 2057-2064.[ Ref ]
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, et al. (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68: 6652- 6660.[ Ref ]
Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, et al. (2011) Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3: 73-121.[ Ref ]
Moore L, Chow EK, Osawa E, Bishop JM, Ho D (2013) Diamond-lipid hybrids enhance chemotherapeutic tolerance and mediate tumor regression. Adv Mater 25: 3532-3541.[ Ref ]
Podesta JE, Al-Jamal KT, Herrero MA, Tian B, Ali-Boucetta H, et al. (2009) Antitumor activity and prolonged survival by carbon-nanotubemediated therapeutic siRNA silencing in a human lung xenograft model. Small 5: 1176-1185.[ Ref ]
Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, et al. (2009) Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice. Reducing tumor growth. J Clin Invest 119: 2830-2842.[ Ref ]
Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, et al. (2013) Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med 5: 209-219.[ Ref ]
Chaudhuri P, Paraskar A, Soni S, Mashelkar RA, Sengupta S (2009) Fullerenol-cytotoxic conjugates for cancer chemotherapy. ACS Nano 3: 2505-2514.[ Ref ]
Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, et al. G.M. (2009) Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest 119: 2830-2842.[ Ref ]
Ajima K, Murakami T, Mizoguchi Y, Tsuchida K, Ichihashi T, et al. (2008) Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano 2: 2057-2064.[ Ref ]
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, et al. (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68: 6652- 6660.[ Ref ]
Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, et al. (2011) Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3: 21-73.[ Ref ]
Huang H, Pierstorff E, Osawa E, Ho D (2007) Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett 7: 3305-3314.[ Ref ]
Pouponneau P, Leroux JC, Soulez G, Gaboury L, Martel S (2011) Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 32: 3481-3486.[ Ref ]
Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, et al. (2012) Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur J Immunol 42: 3049-3061.[ Ref ]
Day ES, Zhang L, Thompson PA, Zawaski JA, Kaffes CC, et al. (2012) Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine 7: 1133-1148.[ Ref ]
Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, et al. (2012) Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol 8: 1167-1181.[ Ref ]
Kageyama S, Kitano S, Hirayama M, Nagata Y, Imai H, et al. H. (2008) Humoral immune responses in patients vaccinated with 1–146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci 99: 601-607.[ Ref ]
Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, et al. (2006) HER2- specific T-cell immune responses in patients vaccinatedwith truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res 12: 7397-7405.[ Ref ]
Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, et al. (2008) Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02, Clin Cancer Res 14: 797-803.[ Ref ]
Ali OA, Emerich D, Dranoff G, Mooney DJ (2009) In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med 1: 8-19.[ Ref ]
Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, Rieux Ad, et al. (2007) PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release 120: 195-204.[ Ref ]
Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, et al. (2012) Lipidderived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci U. S. A 109: 797-803.[ Ref ]
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, et al. (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464: 1067-1070.[ Ref ]
Heidel JD, Liu JY, Yen Y, Zhou B, Heale BS, et al. (2007) Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin Cancer Res 13: 2207-2215.[ Ref ]
Koldehoff M, Steckel NK, Beelen DW, Elmaagacli AH (2007) Therapeutic application of small interfering RNA directed against bcr-abl transcripts to a patient with imatinib-resistant chronic myeloid leukaemia. Clin Exp Med 7: 47-55.[ Ref ]
Ren Y, Cheung HW, von Maltzhan G, Agrawal A, Cowley GS, et al. (2012) Targeted tumorpenetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci Transl Med 4: 147-158.[ Ref ]
Senior J, Crawley JC, Gregoriadis G. (1985) Tissue distribution of liposomes exhibiting long halflives in the circulation after intravenous injection. Biochim Biophys Acta 839: 1-8.[ Ref ]
Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA (2007) Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett 7: 1929-1934.[ Ref ]
Loo, Lowery A, Halas N, West J, Drezek R (2005) Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett 5: 709- 711.[ Ref ]
Hu X, Gao X (2011) Multilayer coating of gold nanorods for combined stability and biocompatibility. Phys Chem Chem Phys 13: 10028-10035.[ Ref ]
Kannadorai RK, Liu Q (2013) Optimization in interstitial plasmonic photothermal therapy for treatment planning. Med Phys 40: 103301.[ Ref ]
Chen CL, Kuo LR, Lee SY, Hwu YK, Chou SW (2013) Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 34: 1128-1134.[ Ref ]
Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, et al. (2012) Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol 8: 1167-1181.[ Ref ]
Kuzmany H, Burger B, Hulman M, Kürti J, Rinzler AG, et al. (1998) Spectroscopic analysis of different types of single-wall carbon nanotubes. Eur Phys Lett 44: 518-528.[ Ref ]
Kuzmany H, Plank W, Hulman M, Kramberger Ch, Gruneis A, et al. (2001) Determination of SWCNT diameters from the Raman response of the radial breathing mode Eur Phys J 22: 307-320.[ Ref ]
Costa S, Borowiak‐Palen E, Bachmatiuk A, Rümmeli MH, Gemming T, et al. (2007) Filling of carbon nanotubes for bio‐applications. P.S.S. 4315- 4318[ Ref ]
Jost O, Gorbunov AA, Pompe W, Pichler T, Friedlein R, et al. (1999) Diameter grouping in bulk samples of single-walled carbon nanotubes from optical absorption spectroscopy. Appl Phys Lett 75: 2217-2224.[ Ref ]
.Liu X, Pichler T, Knupfer M, Golden MS, Fink J, et al. (2002) Detailed analysis of the mean diameter and diameter distribution of single-wall carbon nanotubes from their optical response. Phys Rev B 66045411: 1-8.[ Ref ]
Chiang W, Brinson BE, Huang AY, Willis PA, Bronikowski MJ, et al. (2001) Purification and Characterization of Single-Wall Carbon Nanotubes (SWNTs) Obtained from the Gas-Phase Decomposition of CO (HiPco Process). J Phys Chem B 105: 8297-8301.[ Ref ]
Bendjemil, Borowiak-Palen E, Graff A, Pichler T, Fink J (2004) Elimination of metal catalyst and carbon-like impurities from single-wall carbon nanotube raw material. Appl Phys 78: 311-314 .[ Ref ]
Hayashi T, Hirono S, Tomita M, Umemura S (1996) Magnetic Thin Films of Cobalt Nanocrystals Encapsulated in Graphite-Like Carbon. Nature 381: 722-774.[ Ref ]
Dresselhaus MS, Dresselhaus G, Saito R, Jorio A (2005) Raman spectroscopy of carbon nanotubes. Phys Rep 409: 47-99.[ Ref ]
Takeda N, Murakoshi K (2007) Characteristics of the Raman spectra of single-walled carbon nanotube bundles under electrochemical potential control. Anal Bioanal Chem 388:103-108.[ Ref ]
Doorn SK, Heller DA, Barone PW, Usrey ML, Strano MS (2004) Resonant Raman excitation profiles of individually dispersed single walled carbon nanotubes in solution. Appl Phys A 78: 1147-1155.[ Ref ]
Dresselhaus MS, Dresselhaus G, Jorio A, Souza AG, Pimenta MA, et al. (2002) Single Nanotube Raman Spectroscopy. Acc Chem Res 35: 1070- 1078. [ Ref ]
Dresselhaus MS, Dresselhaus G, Jorio A, Souza AG, Pimenta MA, et al. (2002) Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 40: 2043-2016[ Ref ]
Dresselhaus MS, Dresselhaus G, Jorio A, Souza AG, Saito R (2002) Raman spectroscopy on one isolated carbon nanotube. Physica B 323: 15-20.[ Ref ]
Kam NWS, Jessop TC, Wender PA, Dai H (2004) Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells. J Am Chem Soc 126: 6850-6851.[ Ref ]
Dumortier H, LacotteS, Pastorin G, Marega R, Wu W, et al. (2006) Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett 6: 3003-3003.[ Ref ]
Lam CW, James JT, McCluskey R, Hunter RL (2004) Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Lett 77: 126-134.[ Ref ]
Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, et al. (2004) Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Lett 77: 117-125.[ Ref ]
Cui DX, Tian FR, Ozkan CS, Wang M, Gao HJ (2005) Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 155: 73-85.[ Ref ]
Ding LH, Stilwell J, Zhang TT, Elboudwarej O, Jiang HJ, et al. (2005) Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett 5: 2448-2464.[ Ref ]
Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, et al. (2006) Multiwalled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett 160: 121-126.[ Ref ]
Sayes CM, Liang F, Hudson JL, Mendez J, Guo WH, et al. (2006) Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 161: 135-142.[ Ref ]
Dong L, Joseph KL, Witkowski CM, Craig MM (2008) Cytotoxicity of single-walled carbon nanotubes suspended in various surfactants. Nanotechnology 19: 255702.[ Ref ]
Plata DL, Gschwend PM, Reddy CM (2008) Industrially synthesized singlewalled carbon nanotubes: compositional data for users, environmental risk assessments, and source apportionment. Nanotechnology 19: 185706.[ Ref ]
Casey A, Herzog E, Davoren M, Lyng FM, Byrne HJ, et al. (2007) Spectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicity. Carbon 45: 1425-1432.[ Ref ]
Worle-Knirsch JM, Pulskamp K, Krug HF (2006) Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett 6: 1261- 1268.[ Ref ]
Cherukuri P, Bachilo SM, Litovsky S H, Weisman RB (2004) Nearinfrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J Am Chem Soc 126: 15638-15639.[ Ref ]
Liu Z, Sun X, Nakayama N, Dai H (2007) Supramolecular Chemistry on Water- Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 1: 50-56.[ Ref ]
Kam NWS, O’Connell M, Wisdom JA, Dai H (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102: 11600- 11605.[ Ref ]
Liu Z, Winters M, Holodniy M, Dai HJ (2007) siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew. Chem Int Ed 46: 2023-2027.[ Ref ]
Wu W, Wieckowski S, PastorinG, Benincasa M, Klumpp C, et al. (2005) Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed 44: 6358-6362.[ Ref ]
Chen X, Lee GS, Zettl A, Bertozzi CR (2004) Biomimetic engineering of carbon nanotubes by using cell surface mucin mimics. Angew Che Int Ed 43: 6111-6116.[ Ref ]
Chen X, Tam UC, Czlapinski JL, Lee GS, Rabuka D, et al. (2006) Interfacing carbon nanotubes with living cells. J Am Chem Soc128: 6292-6293.[ Ref ]
Chin SF, Baughman RH, Dalton AB, Dieckmann GR, Draper RK, et al. (2007) Amphiphilic helical peptide enhances the uptake of singlewalled carbon nanotubes by living cells. Exper Biol Med 232: 1236- 1244.[ Ref ]
Yehia HN, Draper RK, Mikoryak C, Walker EK, Bajaj P, et al. (2007) Single-walled carbon nanotube interactions with HeLa cells. J Nanobiotech 5: 8-20.[ Ref ]
Heller DA, Baik S, Eurell TE, Strano MS (2005) Single-walled carbon nanotube spectroscopy in live cells: Towards long-term labels and optical sensors. Adv Mater 17: 2793-2799.[ Ref ]
Kam NWS, Liu ZA, Dai HJ (2006) Carbon nanotubes as intracellular transporters for proteins and DNA: An investigation of the uptake mechanism and pathway. Angew Chem Int Ed 45: 577-581[ Ref ]
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, et al. (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotech 3: 423-428.[ Ref ]
Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, et al. (2007) Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotech 2: 108-113.[ Ref ]
Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NWS, Chu P, et al. (2008) A pilot toxicology study of single walled carbon nanotubes in a small sample of mice. Nat Nanotech 3: 216 -221.[ Ref ]
Liu Z, Davis C, Cai W, He L, Chen X, et al. (2008) Circulation and longterm fate of functionalized, biocompatible single-walled carbon nanotubes in mice 50 probed by Raman spectroscopy. Proc Natl Acad Sci USA 105: 1410-1415.[ Ref ]
Yang ST, Wang X, Jia G, Gu Y, Wang T, et al. (2008) Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett 181: 182-189.[ Ref ]
Yang ST, Fernando KA, Liu JH, Wang J, Sun HF, et al. (2008) Covalently PEGylated carbon nanotubes with stealth character in vivo. Small 4: 940-944.[ Ref ]
Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green MLH, et al. (2006) Complement activation and protein adsorption by carbon nanotubes. Mol Immunol 43: 193-201[ Ref ]
Pantarotto D, Briand JP, Prato M, Bianco A (2004) Translocation of bioactive peptides across cell membranes bycarbon nanotubes. Chem Commun 7: 16-17.[ Ref ]
Bianco A, Kostarelos K, Partidos CD, Prato M (2005) Biomedical applications of functionalised carbon nanotubes. Chem Commun 5: 571-577.[ Ref ]
Jin H, Heller DA, Strano MS (2008) Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH3T3 cells. Nano Lett 8: 1577-1585.[ Ref ]
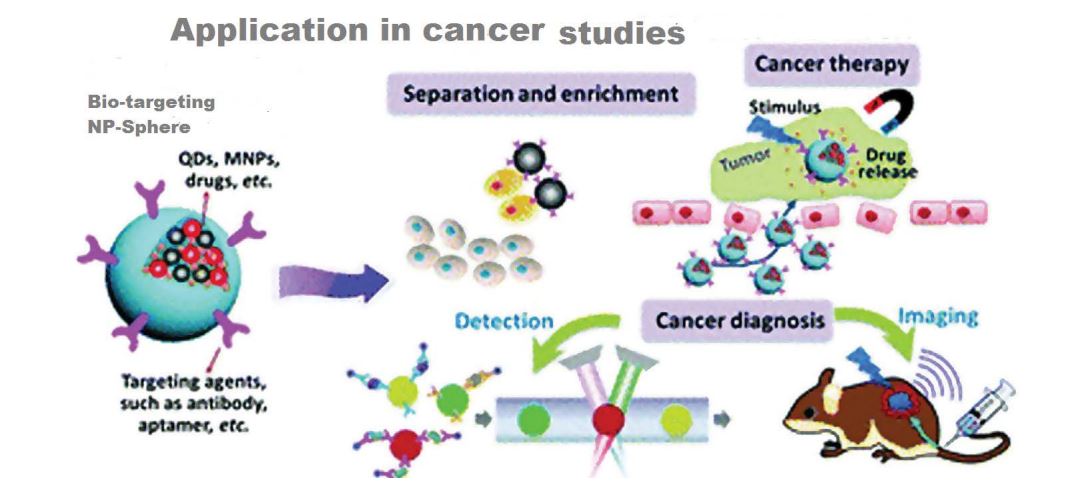
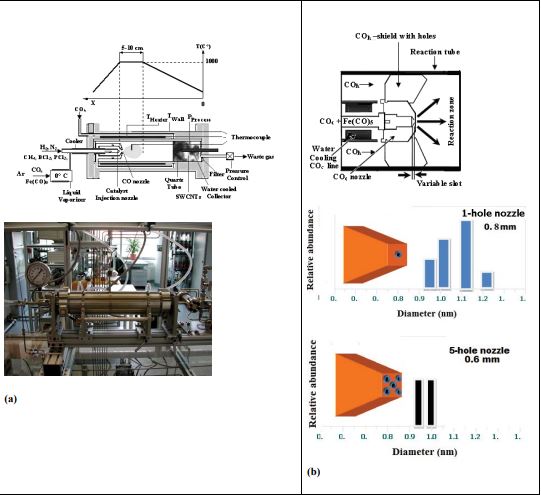
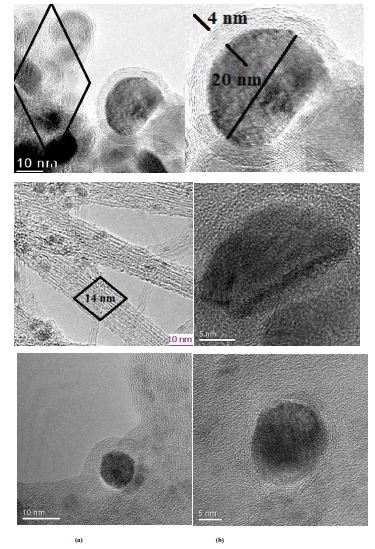
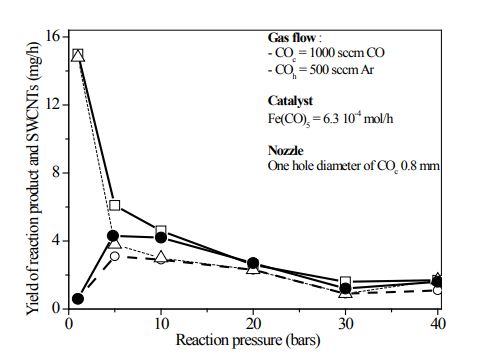
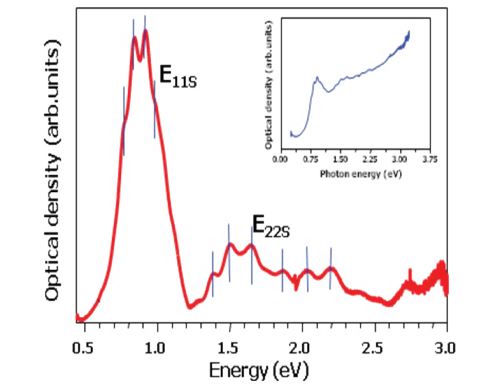
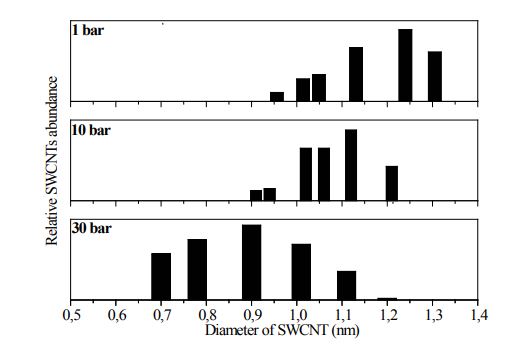
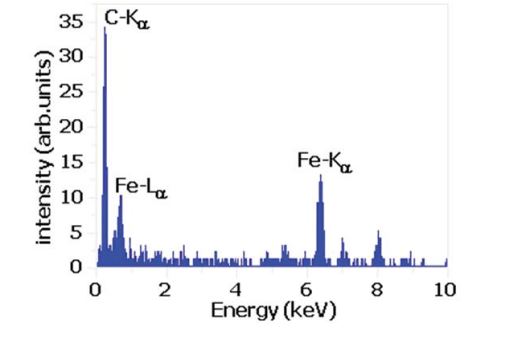
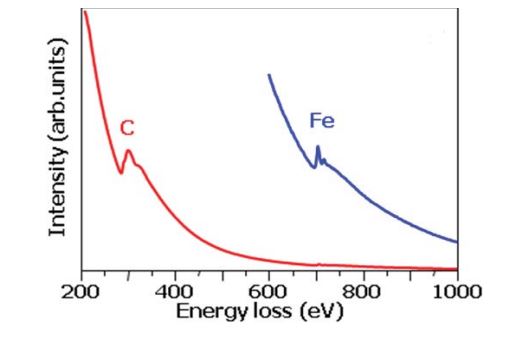
 IJNR-115.jpg)
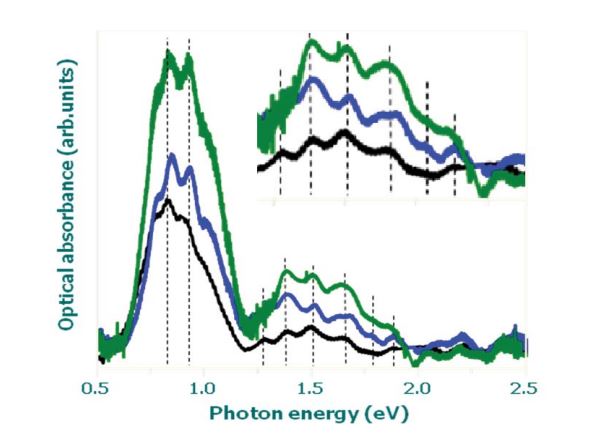
 IJNR-115.JPG)
 IJNR-115.JPG)


