Journal Name: International Journal of Nano Research
Article Type: Research
Received date: 15 October, 2020
Accepted date: 11 November, 2020
Published date: 18 November, 2020
Citation: Barhoumi Z, Amdouni N, Pal A, Stumbe JF (2020) Investigation on the Interaction between Sodium Dodecylsulfate and Cationic Polymer by Using Conductometric, Densimeter and Zeta Potential Technique. Int J Nano Rech Vol: 3, Issu: 2 (01-06).
Copyright: © 2020 Barhoumi Z et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The interaction of solution of poly (diallyldimethylammonium) chloride, PDADMAc and sodium dodecylsulfate, SDS has been studied using conductometric, densimeter and zeta potential methods. The critical micellization concentration (cmc) and degree of dissociation (α) were determined from electrical conductivity measurements. The cmc values increased with increase (0.5-1.5 g/L) PDADMAc concentrations. The free energy of micellization, ∆G0m and enthalpy of micellization, ∆H0m has been obtained. Our results show that ∆G0m is always negative, signifying the micellization of SDS/PDADMAc mixed systems. The values ∆H0m of are all negative, micellization of SDS in PDADMAc systems (0.5- 1.5 g/L) is an exothermic process. The limiting apparent molar volume, V0φ and limiting adiabatic compressibility, K0φ have been determined of SDS/PDADMAc solutions from density and sound speed measurements. The data have been evaluated in terms of solute-solvent and solute-solute interactions. The Zeta potential results contribute to the understanding of the complex SDS/PDADMAc interactions.
Keywords:
Ionic surfactant; Polymer solution, Critical micellization concentration, Zeta Potential
Abstract
The interaction of solution of poly (diallyldimethylammonium) chloride, PDADMAc and sodium dodecylsulfate, SDS has been studied using conductometric, densimeter and zeta potential methods. The critical micellization concentration (cmc) and degree of dissociation (α) were determined from electrical conductivity measurements. The cmc values increased with increase (0.5-1.5 g/L) PDADMAc concentrations. The free energy of micellization, ∆G0m and enthalpy of micellization, ∆H0m has been obtained. Our results show that ∆G0m is always negative, signifying the micellization of SDS/PDADMAc mixed systems. The values ∆H0m of are all negative, micellization of SDS in PDADMAc systems (0.5- 1.5 g/L) is an exothermic process. The limiting apparent molar volume, V0φ and limiting adiabatic compressibility, K0φ have been determined of SDS/PDADMAc solutions from density and sound speed measurements. The data have been evaluated in terms of solute-solvent and solute-solute interactions. The Zeta potential results contribute to the understanding of the complex SDS/PDADMAc interactions.
Keywords:
Ionic surfactant; Polymer solution, Critical micellization concentration, Zeta Potential
Introduction
The effect of salt or polyelectrolyte on the properties of aqueous surfactant solutions has been a subject of intense research due to their extensive commercial applications and researcher viewpoints [1-7]. The surfactant-polymer mixtures have become potential for domestic, industrial and technological applications such as, foods, paints, drug delivery, coatings and laundry products [8,9]. The interaction of oppositely charged polymers with ionic surfactant is more complicated comparing with nonionic polymers- surfactants. It has been shown in the literature that at certain surfactant concentrations, interaction leads to precipitation of a polymer– surfactant complex. This precipitation was characterized for various oppositely charged polyelectrolyte– surfactant systems [10-15]. Phase separation could be prevented by addition of more nonionic groups to the polymer or to the surfactant, as reported by Bronich et al. and Dubin et al. [16-18]. The polyelectrolytesurfactant interactions are interpreted by hydrophobic and electrostatic interactions and are modulated by temperature and ionic strength.when surfactant starts to form aggregates with polyelectrolyte as the surfactant concentration as a critical concentration aggregation, the counter ion of polyelectrolyte is replaced by the surfactant ion and the surfactant form a neutral ion pair [19].This surfactant binding mechanism related cooperative and non-cooperative steps as well. This cooperative binding depends on a range of factors, such as the length of carbon chain of surfactant, the salt concentration and the polyion charge density [20].
Poly(diallyldimethylammonium chloride) (PDADMAC), an important commercial water-soluble cationic functional polymer, is useful in water treatment as a flocculant or coagulant for the removal of organic and mineral contaminants such as arsenic, etc. [21-22]. It is used in the textile and paper industries for making antibacterial fiber and to improve the wet strength of papers [23]. Many efforts have studied the thermodynamic properties in binary mixtures of PDADMAc and SDS because it is important in determining the properties of these systems. Kong et al have investigated the properties of SDS/PDADMAc mixed solutions using conductometric and rheometric techniques. Maroi et al. have applied the potentiometric and fluorimetric methods to the SDS/PDADMAc mixed solutions to evaluate the critical aggregation concentration [24].
In this work, we reported physico chemical properties (conductance, densities and speed sound and zeta potential) for the following SDS-PDADMAc mixed solutions at T= (298.15, 308.15, and 318.15 K). The thermodynamic parameters ∆G0m , ∆H0m and T ∆S0m have been calculated.
Experimental Section
Poly(diallyldimethylammonium)chloride PDADMAc of average Mw 100,000–200,000 (21.8% aqueous solution) purchased from Sigma-Aldrich Co, and sodium dodecylsulfate SDS (minimum 98.5% by GC) purchased from Sigma Co. were used without additional purification. All other reagents were of analytical grade. Table 1 summarizes all chemicals used in this work, along molecular weight, mass fraction purity. The mixed polymer/SDS have been prepared by using an electronic analytical balance with a precision of ±0.0001 g (Shimadzu, model AY220).
The electrical conductivity measurements were carried out using a digital conductivity meter CM-183, and the conductivity was taken when its value less than 4%. After ensuring thorough mixing and temperature equilibration of (298.15, 308.15 and 318.15 K), the electrical conductivity was considered.
Densities and speeds of sound of pure SDS and their mixtures with PDADMAc were measured by using an Anton Paar DSA 5000 (oscillating U-tube density and speed of sound analyzer) instrument and the temperature was controlled to ±1×10−2 K by a built-in solid-state thermostat. Before each series of measurements, the densimeter was calibrated with doubly distilled, degassed water and with dry air at atmospheric pressure. The maximal error in the measurements of density and speed of sound relative to water (997.050 kg m−3 and 1496.687 m s−1) is expected to be less than 5×10−3 kg m−3 and 5×10−2m s−1.
The Zeta potentials and size of the polymer-surfactant aggregates in aqueous solutions were determined using a Nano Zs Zetasizer 3000 (Malvern Instruments). A He-Ne laser beam of wavelength 632.8 nm was used. Each run was duplicated to check the reproductibilty.
Results and Discussion
Conductance measurements
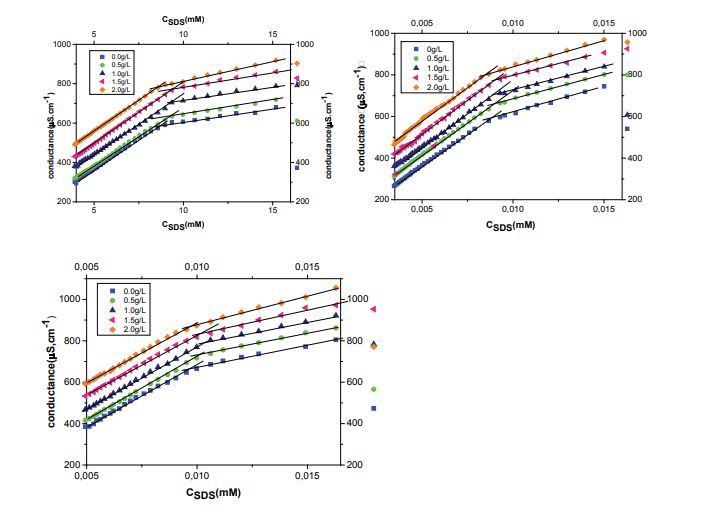
The variation of electrical conductivities of SDS/ PDADMAc mixed at temperatures ranged from 298.15 to 318.15 K is shown in Figure 1 (a,b and c). From this figure, it can be observed an increase in electrical conductivity with increasing SDS molalities and having a gradual decrease in slope value. According to the Onsager theory of electrolyte conductivity, two linear regimes were expected in conductivity graph, one corresponding to pre-micellar region while the other corresponding to the post-micellar region [25].The cmc was determined from the intersection of the tangent lines before and after break in conductivity. The cmc value of pure SDS was found to be at 8.7 mM at 298.15 K, it was found in good agreement with the literature value [26]. The degree of counter ion dissociation (α) was taken as the ratio of slopes of the lines of post to pre-micellar region. The cmc and α values for SDS/PDADMAc mixtures at different concentrations of PDADMAc and temperatures are listed in table 2. As table 2 shows, at fixed temperature the cmc values increases with increasing concentration of PDADMAc (0.5 -1.5 g/L) and higher than pure SDS solution. The increase in cmc values upon the addition of PDADMAc is attributed to the fact that there in an increase in the binding sites available for the SDS monomers or micelle like aggregate (not the true micelles) where they can bind with the polymer. Thus increasing the solubility of SDS in its monomeric form and leading to an increase of the cmc. The cmc value of SDS upon the addition of PDADMAc at 2 g/L decrease compared with the cmc value of pure SDS, suggesting that surfactant micelles may form part of the polymer -surfactant complex bound at the interface.
On the other hand, at a fixed PDADMAc concentration the cmc increases with temperature because of two opposite processes. First, the degree of hydration of the hydrophilic head group usually decreases when the temperature increases which favors micellization. Second, high temperature causes the disruption in water structure and results in the increase solubilisation of surfactant molecules, along with delaying of aggregation process. The α decreases with increase in polymer concentration due to lesser dissociation of ions and therefore results in more head group repulsions which further delays the micellization process.
The thermodynamic parameters for the micelle formation of SDS were calculated with Eqs.1 to 3 and the results are summarized in Table 2. According to mass action model the standard free energies of micellization, ∆G0m of SDS in the solutions were obtained by using the equation Eq.1 [27]:
-----(1)
Where R is the gas constant, T is temperature and α is degree of counter ion.
From the temperature dependence of cmc, the standard enthalpy of micellization process was obtained using the equation Eq.2 [27,28]:
-----(2)
∆H0m values were obtained by application of equation 2 to the fitted values of Ln (Xcmc) with temperature. However d (LnXcmc)/dT was determined as the slope of the straight line obtained by plotting Ln (Xcmc) curve against temperature and subjecting the data to least squares treatment [29]
Further, the standard entropy of micelle formation ∆S09m is obtained using Eq.3 [28]:
-----(3)
It can be seen from table 1 that the free energy of micellization values are more negative in SDS/PDADMAc mixtures than pure SDS over the experimental temperature range. These results demonstrated the addition of PDADMAc promote micelle formation more spontaneously compared with pure SDS. The ∆G0m values increase with increasing concentration of PDADMAc, which suggests that the micellization becomes less favorable at 2 g/L concentration PDADMAc.
From table 2, we find that the values of ∆H0m are negative in SDS/PDADMAC mixtures at concentration (0.5-1.5 g/L) of PDADMAc, micellization of SDS in mixed system is an exothermic process. It can be seen that the micellization enthalpy ∆H0m values increase as a function of increasing PDADMAc concentration at all temperatures. From these results, the micellization enthalpy consists of SDS-SDS interactions, PDADMAc-SDS interactions, these interactions may be broken down into a hydrophobic portion as well as electrostatic contribution due to the mixing of the surfactant and PDADMAc in the head group region of the micelle. In table 2, the values of ∆H0m are positive in SDS/ PDADMAc mixed solution at 2g/L PDADMAc content. There is a contribution from the interaction of the hydrophobic surfactant and PDADMAc chains with water, which results in the formation of structured water in solution. We also find in table 2 that T∆S0m are positive for SDS/PDADMAc systems at concentration (0.5, 1.0 and 1.5 g/L) of PDADMA, indicating that disorder increases due to micellization. In these systems, 0 ∆Hm is much less than T∆S0m. This means that the micellization process is entropy driven. Thus when the mixed micelles are formed in 2 g/L concentration of polymer, ∆H0m and T∆S0m values increase, this suggests that both the polymer and surfactant are contributing to the hydrophobic effects.
Figure 1: Specific conductance (κ) of aqueous SDS solutions in the presence of different concentrations of PDADMAc at temperatures (a) 298.15 K; (b) 308.15 K; (c) 318.15 K
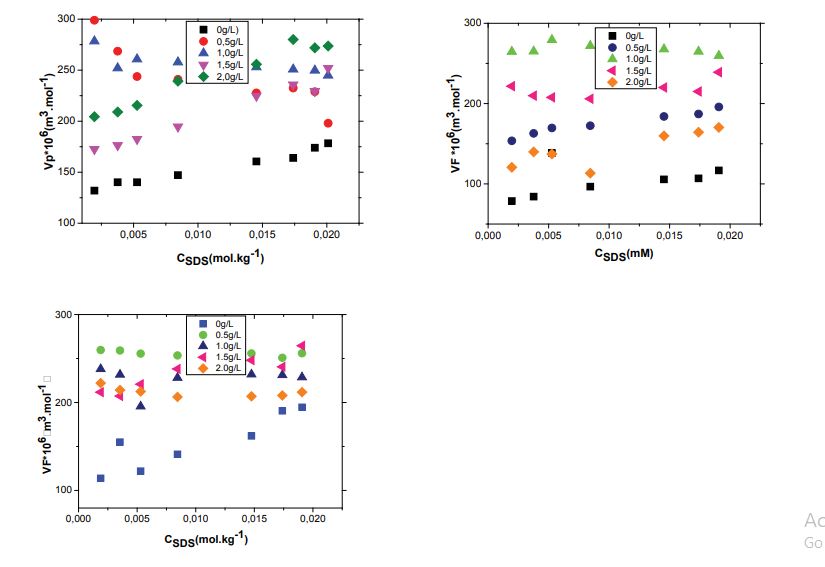
Figure 2: Plot of Vφ for surfactant in aqueous solutions of PDADMAc against molalities m of SDS at different temperatures; (a) 298.15, (b) 308.15 and (c)318.15K
| Chemical name | Provenance | Mass fraction purity (%) |
|---|---|---|
| Sodium dodecylsulphate (SDS) | Sigma Aldrich,USA | ≥ 98.5 |
| Polydiallyldimethylammonium chloride (PDADMAc) | Sidma Aldrich | 21.8% in aqueous solution |
Table 1: Specifications of chemicals.
| PDADMAc (g.L1) | Cmc (mol.Kg-1) | α | ∆G0m (kJ.mol-1) |
∆H0m (kJ.mol1) |
T∆S0m (kJ.mol-1) |
|---|---|---|---|---|---|
| T=298.15K | |||||
| 0 | 0.0086541 | 0.536 | -42.304 | --7.188 | 35.116 |
| 0.5 | 0.009300 | 0.533 | -42.129 | -6.982 | 35.147 |
| 1 | 0.0094360 | 0.517 | -42.515 | -6.885 | 35.63 |
| 1.5 | 0.009488 | 0.509 | -42.745 | -5.832 | 36.913 |
| 2 | 0.008540 | 0.496 | -43.510 | 1.110 | -44.62 |
| T=308.15K | |||||
| 0 | 0.008950 | 0.689 | -39.041 | -5.329 | 33.712 |
| 0.5 | 0.00909 | 0.668 | -39.409 | -5.110 | 34.299 |
| 1 | 0.00914 | 0.620 | -40.907 | -4.795 | 36.112 |
| 1.5 | 0.00924 | 0.605 | -41.309 | -4.665 | 36.644 |
| 2 | 0.00856 | 0.583 | -42.359 | 1.128 | -41.231 |
| T=318.15 | |||||
| 0 | 0.009748 | 0.7701 | -37.540 | -4.350 | 33.19 |
| 0.5 | 0.01010 | 0.76 | -37.728 | -4.118 | 33.61 |
| 1 | 0.01020 | 0.745 | -38.152 | -3.991 | 34.161 |
| 1.5 | 0.01030 | 0.530 | -44.650 | -3.885 | 40.765 |
| 2 | 0.00952 | 0.473 | -46.700 | 2.751 | -49.451 |
Table 2: The values of critical micelle concentration (cmc), degree of dissociation (α), free energy of micellization (∆G0m), enthalpy of micellization (∆H0m) and entropy of micellization T∆S0m ) of aqueous SDS solutions in different concentration of PDADMAc at different temperatures
Density and speed sound measurements:
The apparent molar volumes and apparent molar isentropic compressibility have been determined from the experimental density and sound speed data. The values of apparent molar volumes, Vφ for SDS/PDADMAc mixtures were calculated using Eq. 4:
-----(4)
Where, ρ and ρ0 are the densities of the mixed solutions of SDS and reference solvent (water, PDADMAc) respectively and m, M, denote the molality of the solution of SDS, and molecular weight of PDADMAc, respectively.
The variation of apparent molar volumes for SDS solutions at different PDADMAc concentrations (0.5, 1.0, 1.5 and 2g/L) at (298.15, 308.15 and 318.15) K is shown in figure 2(a, b and c). From figure 2, it can be seen that apparent molar volumes values increase with increasing of the molality of the surfactant; also it increases with increase of the temperature. Such behavior was also observed by Gheorghe et al. and Dubey et al., these values could suggest the presence of strong intermolecular forces between surfactant and water molecules [30-31].
Finally, the apparent molar isentropic compressibility (κφ ) is evaluated by using Eq. 5:
-----(5)
Where κs and Sk,0 are the isentropic compressibility of the mixture and reference solvent respectively. For diluted solutions, a simple linear relationship can be applied to describe the variation of the apparent molar volume,
V0=VÔÔ+Svm
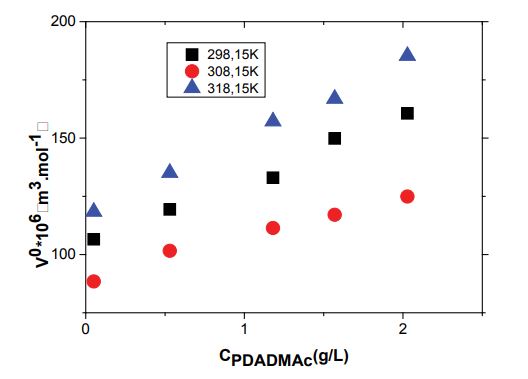
where K0Ôand K0Ô are the limiting values of apparent molar volume and apparent molar adiabatic compressibility, respectively, and often regarded equal to the infinite dilution partial molar volume and infinite dilution partial molar adiabatic compressibility. Figure 3 shows the variation of limiting apparent molar volume, V0Ô of SDS solutions as function of PDADMAc concentration. It can be observed that V0Ô with the increase of temperature and PDADMAc concentration this indicates an increase in solute–solvent interactions and a decrease in solute–solute interactions. The values of K0Ô become less negative with increase in temperature (as shown in Figure 4). The negative values of K0Ô indicate that the water molecules surrounding SDS are less compressible. The more negative values of K0Ô for surfactant at low temperature indicate prominence of strong attractive interactions between water and neighbouring species in medium. With increase in temperature the K0Ô values become less negative which means decrease in electrostriction and release of some water molecules to bulk. The release of water molecule into bulk by the hydration of ions occurring due to dissociation of SDS micelles results in strong attractive interactions [32].
Zeta potential
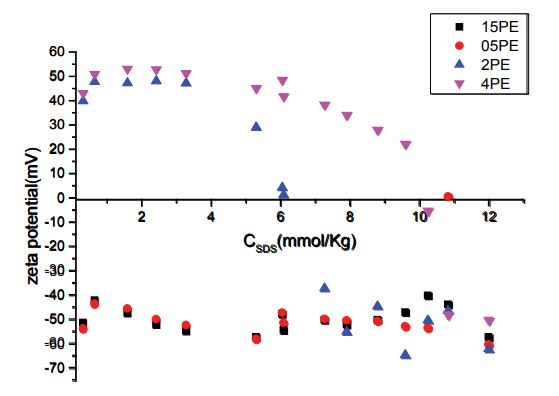
The zeta potential of PDADMAc-SDS complex as function of SDS concentration is shown in figure 5. Our results show that with an increase in SDS concentration, the zeta potential of some mixtures undergoes a charge reversal, going from positive to negative values. The sign of potential becomes more negative with the addition of PDADMAc concentrations (0.5 and1.5 g/L), when the surface negative charge increased, more surfactant adsorbed. These results demonstrated that SDS/PDADMAc complex is governed by electrostatic interactions. So, the polymer configuration tended to become compact by the electrostatic interaction with DS- at a number of its positive Me2N+ centers. The zeta potential increases up to 1.5 g/L of PDADMAc in solution where ζ = 0 and then decreases as the concentration of surfactant increases further. The positive value of the zeta potential (ranged at 6Mm of SDS) may be attributed to the existence of electrostatic interactions and hydrophobic interactions between surfactant and PDADMAc. At some concentration of the added SDS, with (ζ = 0 ), the electrical forces of repulsion are lowered sufficiently that the forces of attraction dominate. Under these conditions, the particles may approach each other more closely and form loose aggregates termed floccules.
Figure 3: Plot of Vφ for surfactant SDS in aqueous solutions of PDADMAc at different temperatures.
Figure 4: The variation in limiting apparent adiabatic compressibility, K0φ as a function of concentration of PDADMAc at different temperatures.
Figure 5: The variation in limiting apparent adiabatic compressibility, K0φ as a function of concentration of PDADMAc at different temperatures.
Conclusion
This paper has examined the effects of polymer concentration and temperature on the physico chemical properties of aqueous SDS solutions by conductance, Density, Speed of sound and Zeta Potential methods. The obtained (cmc) using conductivity method increase in the presence of the polyelectrolyte which confirms the complexation between PDADMAc and SDS monomers. Using the cmc and α values obtained from conductance, and it is observed that and values are negative for all the studied systems indicating the spontaneous. Large and positive values of T∆S0m indicates the crucial role of hydrophobic forces in micellization. densities and sound speed data were experimentally determined for SDS mixed solutions at temperatures from 298.15 to 318.15 K. from the experimental data for the density and sound sound, it was possible calculate the limiting values of apparent molar volume (V0Ô) and apparent molar isentropic compressibility( κ0φ ). Positive values for V0Ô Ô and negative values for κ0φ have been observed, which increase upon increasing the temperature. It depicts decrease in electrostriction with increase in temperature. Further, Zeta Potential method help in bettter understand of the interaction between SDS and PDADMAc.
Goddard ED (1986) Polymer—surfactant interaction Part I. uncharged water-soluble polymers and charged surfactants. Colloids and Surfaces 19: 255-300.[ Ref ]
Li Y, Xia J, Dubin PL (1994) Complex formation between polyelectrolytes and oppositely charged mixed micelles: static and dynamic light scattering. Study of the effect of polyelectrolyte molecular weight and concentration. Macromolecules 27: 7049-7055.[ Ref ]
Ghoreishi SM, Fox GA, Bloor DM, Holzwarth JF, Wyn-Jones E (1999) EMF and microcalorimetry studies associated with the binding of the cationic surfactants to neutral polymers. Langmuir 15 : 5474-5479.[ Ref ]
Matulis D, Rouzina L, Bloomfield VA (2000) Thermodynamics of DNA binding and condensation: isothermal titration calorimetry and electrostatic mechanism. J Mol Biol 296: 1053-1063.[ Ref ]
Chakraborty T, Chakraborty I, Ghosh S (2006) Sodium carboxymethylcelluloseCTAB interaction: a detailed thermodynamic study of polymer-surfactant interaction with opposite charges. Langmuir 22: 9905-9913.[ Ref ]
Bain CD, Claesson PM, Langevin D, Meszaros R, Nylander T, et al. (2010) Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv Colloid Interface Sci 155: 32-49.[ Ref ]
J€onsson B, Lindman B, Holmberg K, Kronberg B (1998) Surfactants and Polymers in Aqueous Solution. John Wiley & Sons Ltd, UK.[ Ref ]
Chilkoti A, Christensen T, Mackay JA (2006) Stimulus responsive elastin biopolymers: applications in medicine and biotechnology. Curr Opin Chem Biol 10: 652-657.[ Ref ]
Campbell AR, Ash PA, Bain CD (2007) Dynamics of Adsorption of an Oppositely Charged Polymer−Surfactant Mixture at the Air−Water Interface: Poly(dimethyldiallylammonium chloride) and Sodium Dodecyl Sulfate. Langmuir 23: 3242-3253.[ Ref ]
Muzzalupo R, Infante MR, Pérez L, Pinazo A, Marques EF, et al. (2007) Interactions between Gemini Surfactants and Polymers: Thermodynamic Studies. Langmuir 23: 5963-5970.[ Ref ]
Chakraborty T, Chakraborty I, Ghosh S (2006) Sodium Carboxymethylcellulose−CTAB Interaction: A Detailed Thermodynamic Study of Polymer−Surfactant Interaction with Opposite Charges. Langmuir 22: 9905-9913.[ Ref ]
Mata J, Patel J, Jain N, Ghosh G, Bahadur P (2006) Interaction of cationic surfactants with carboxymethylcellulose in aqueous media. J Colloid Interface Sci 297: 797-804.[ Ref ]
Penfold J, Tucker I, Thomas RK, Taylor DJF, Zhang J, et al. (2007) The Impact of Electrolyte on the Adsorption of Sodium Dodecyl Sulfate/ Polyethyleneimine Complexes at the Air−Solution Interface. Langmuir 23: 3690-3698.[ Ref ]
Kong L, Cao M, Hai M (2007) Investigation on the Interaction between Sodium Dodecyl Sulfate and Cationic Polymer by Dynamic Light Scattering, Rheological, and Conductivity Measurements. J Chem Eng Data 52: 721- 726.[ Ref ]
Bronich TK, Nehls A, Eisenberg A, Kabanov AV (1999) Novel drug delivery systems based on the complexes of block ionomers and surfactants of opposite charge. Colloids Surf B Biointerfaces 16: 243-251.[ Ref ]
Bronich TK, Vinogradov SV, Kabanov AV (2001) Interaction of nanosized copolymer networks with oppositely charged amphiphilic molecules. Nano Lett 1: 535-540.[ Ref ]
Dubin PL, Oteri R (1983) Association of polyelectrolytes with oppositely charged mixed micelles. J. Colloid Interface Sci 95: 453-461. [ Ref ]
Bakshi MS, Sachar S (2004) Surfactant polymer interactions between strongly interacting cationic surfactants and anionic polyelectrolytes from conductivity and turbidity measurements. J Colloid and Polymer Sci 282: 993-999.[ Ref ]
Radeva T (2001) Physical Chemistry of Polyelectrolytes. Dekker, New York.[ Ref ]
Dautzenberg H (2000) Polyelectrolyte complex formation: Role of a double hydrophilic polymer. Macromol. Chem Phys 201: 1765-1773. [ Ref ]
Meda AM, Taboada P, Krajewska B, Willemeit M, Deml A, et al. (2010) DNA−Poly(diallyldimethylammonium chloride) Complexation and Transfection Efficiency. J Phys Chem B 114: 9356-9366.[ Ref ]
Krajcik R, Jung A, Hirsch A, Neuhuber W, Zolk O (2008) Functionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genes. Biochem Biophys Res Commun 369: 595-603.[ Ref ]
Lee J, Moroi Y (2004) Investigation of the Interaction between Sodium Dodecyl Sulfate and Cationic Polymers. Langmuir 20: 4376-4379.[ Ref ]
Jones MJ, Chapman D, Micelles (1995) Monolayers and Biomembranes. Wiley-LISS, New York.[ Ref ]
Rodriguez MP, Prieto G, Rega C, Varela LM, Sarmiento F, et al. (1998) A Comparative Study of the Determination of the Critical Micelle Concentration by Conductivity and Dielectric Constant Measurements. Langmuir 14: 4422- 4426.[ Ref ]
Rosen MJ (1988) Surfactant and Interfacial Phenomenon. John Wiley and Sons, New York.[ Ref ]
Winnik MA, Bystryak SM, Chassenieux C (2000) Study of Interaction of Poly(ethylene imine) with Sodium Dodecyl Sulfate in Aqueous Solution by Light Scattering, Conductometry, NMR, and Microcalorimetry. Langmuir 16: 4495-4510.[ Ref ]
Jolicoeur C, Philip PR (1974) Enthalpy-zntropy compensation for micellization and other hydrophobic interactions in aqueous solutions. Can J Chem 52: 1834.[ Ref ]
Gheorghe L, Stoicescu C, Sirbu F (2016) Partial molar volumes ,isentropic compressibilities and partial molar expansibilities of N-Methylglycine and d-Glucose in aqueous environments at temperatures between (298.15 and 323.15K). J Mol Liq 218: 515-524.[ Ref ]
Dubey GP, Rani S, Kumar H (2019) Acoustic, volumetric and spectral studies of binary liquid mixtures of aliphatic dialkylamine and 2-alkanols at different temperatures. J Chem Thermodyn 132: 1-8.[ Ref ]
Pal A, Kumar H, Maan R, Sharma HK (2014) Thermophysical properties of alkyl acetates in aqueous solutions of ionic liquid {1-butyl-3- methylimidazolium bromide [bmim][Br]} at different temperatures. Physics and Chemistry of Liquids 53: 390-402.[ Ref ]
Merta J, Stenius P (1999) Interactions between cationic starch and mixed anionic surfactants. Colloids Surf 149 : 367-377.[ Ref ]