Journal Name: International Journal of Nano Research
Article Type: Research
Received date: 29 January, 2021
Accepted date: 05 March, 2021
Published date: 12 March, 2021
Citation: Begum Q, Mahboob T (2021) Silver Nanoparticles Protects Streptozotocin-Induced Hepatotoxicity: A Biochemical and Histopathological Approach. Int J Nano Rech Vol: 4, Issu: 1 (01-09).
Copyright: © 2021 Begum Q et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Silver nanoparticles (AgNPs) are recently used for their biomedicinal applications as an alternative in many industries. Particularly biologically synthesized silver nanoparticles have proven to be an effective source in the treatment of diabetes. Whereas diabetic hepatopathy is perhaps less common where oxidative stress plays a key role in its pathogenesis. Therefore, the present study focused on the role of biologically synthesized silver nanoparticles (AV-AgNPs) on hepatic toxicity in diabetic rats induced by streptozotocin (STZ). Wistar male albino rats (200 ± 20 g) were categorized into five groups (n=10) and designated as, Group I-Control (no treatment); Group II-Diabetic control (35 mg/kg single dose of streptozotocin, IP); Group III-Diabetic treated with AVLE (100 mg/kg); Group IV-Diabetic treated with AV-AgNPs (10 mg/kg); Group V-Diabetic treated with glibenclamide (600 μg/kg) orally. Rats were euthanized after 28 days of treatment, blood and liver specimens were collected to perform biochemical, antioxidant, and histological examinations. Results exhibited that STZ persuades diabetes and hepatic impairments indicated by significantly raised (p<0.05) levels of blood glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, (ALP), and malondialdehyde (MDA) with decreased catalase (CAT), superoxide dismutase (SOD), and glutathione reductase (GSH) enzymes activities. AV-AgNPs treatment reversed and restored the liver enzymes, antioxidant status, and histological changes but also impede deleterious effects on hepatocellular damages induced by STZ. In general, these outcomes suggested that AV-AgNPs may have antioxidant potentials and proved to be hepatoprotective therefore; they could be used for the treatment of diabetic hepatopathy and other liver injuries.
Keywords:
Silver nanoparticles, Oxidative stress, Hepatic tissue injury, Antioxidant, Histology.
Abstract
Silver nanoparticles (AgNPs) are recently used for their biomedicinal applications as an alternative in many industries. Particularly biologically synthesized silver nanoparticles have proven to be an effective source in the treatment of diabetes. Whereas diabetic hepatopathy is perhaps less common where oxidative stress plays a key role in its pathogenesis. Therefore, the present study focused on the role of biologically synthesized silver nanoparticles (AV-AgNPs) on hepatic toxicity in diabetic rats induced by streptozotocin (STZ). Wistar male albino rats (200 ± 20 g) were categorized into five groups (n=10) and designated as, Group I-Control (no treatment); Group II-Diabetic control (35 mg/kg single dose of streptozotocin, IP); Group III-Diabetic treated with AVLE (100 mg/kg); Group IV-Diabetic treated with AV-AgNPs (10 mg/kg); Group V-Diabetic treated with glibenclamide (600 μg/kg) orally. Rats were euthanized after 28 days of treatment, blood and liver specimens were collected to perform biochemical, antioxidant, and histological examinations. Results exhibited that STZ persuades diabetes and hepatic impairments indicated by significantly raised (p<0.05) levels of blood glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, (ALP), and malondialdehyde (MDA) with decreased catalase (CAT), superoxide dismutase (SOD), and glutathione reductase (GSH) enzymes activities. AV-AgNPs treatment reversed and restored the liver enzymes, antioxidant status, and histological changes but also impede deleterious effects on hepatocellular damages induced by STZ. In general, these outcomes suggested that AV-AgNPs may have antioxidant potentials and proved to be hepatoprotective therefore; they could be used for the treatment of diabetic hepatopathy and other liver injuries.
Keywords:
Silver nanoparticles, Oxidative stress, Hepatic tissue injury, Antioxidant, Histology.
Introduction
Diabetes mellitus (DM) is the most common chronic metabolic disease nowadays, characterized via hyperglycemia due to insulin deficit, insulin insensitivity, or both [1,2]. Dysregulation of insulin may develop abnormalities and complications, including nephropathy, peripheral neuropathy, retinopathy, and hepatopathy [3,4]. Although among all, hepatopathy is possibly less common and least concern area in diabetes complications. Many studies provide evidence that the complication of diabetes may involve the generation of free radicals, thus producing oxidative stress through several paths [5]. To overcome the diabetic complications, new therapeutic drugs are demands of time. WHO reported that about 80 percent of the people ponder folk medicines for the treatment of several diseases, including diabetes and its complications worldwide, predominantly by considering the use of medicinal plants [6-9]. The use of medicinal plants gaining more interest due to owing bioactive constituents; phytochemicals, which hostile oxidative stress [10-17]. Aloe vera is an attractive plant that belongs to the Liliaceae family that has many medicinal properties. The leaves of Aloe vera have greater antioxidant activity due to the presence of phytochemicals [18].
Silver nanoparticles gaining more interest among researchers for their potential therapeutic activities due to their minimal toxicity, and higher biodegradability, and bioavailability [19,20]. The pharmacokinetics of AgNPs shows that the small size nanoparticles deftly interact with the biological system [21]. Silver nanoparticles are synthesized in several ways, in which green synthesis considered most advantageous among other methods since it is cost-effective and provides environmentally-friendly approaches [22-24]. The use of a plat and plant extract to synthesize nanoparticles has become the most viable approach recently in the green synthesis that provides greater potency to nanoparticles for their various uses [25,26]. Silver nanoparticles synthesized by plant and plant extract have been known to possess antimicrobial, hepatoprotective, antidiabetic, and antiinflammatory properties [27]. However, there are no reports on the protective effects of biologically synthesized silver nanoparticles against diabetic complications specifically liver injury.
Taking this as an initiative, we intended to study the protective effects of biologically synthesized AgNPs against liver injuries in STZ induced diabetic rat model. Therefore, an aqueous leaf extract of aloe vera and aloe vera derived AgNPs were used as a test agent. The procedure for synthesis and characterization of aloe vera derived AgNPs has been published earlier [28]. The results of the present research help to determine the role of the biologically synthesized silver nanoparticles in liver injuries and could serve as a basis for further studies especially to treat diabetes-related complications.
Materials and Methods
Synthesis and characterization of AV-AgNPs
Silver nanoparticles were synthesized by the method of Chandran et al. (2006) using aqueous aloe vera leaves extract and synthesized silver nanoparticles (AV-AgNPs) were characterized using analytical techniques via Shimadzu UVvisible spectrophotometer (UV-1900, Japan) and scanning electron microscopy (JEOL-Japan-JSM 6380A) described in detail in our previously published report [28].
Chemicals
All chemicals used in this study were analytical grade and purchased from the local suppliers of Pakistan although silver nitrate was acquired from Sigma-Aldrich (USA) and streptozotocin was obtained from Calbiochem (Germany).
Animals
Healthy, male adult Wistar albino rats (n=50) of the identical age group with a bodyweight of 200 (±20 g) procured by the animal care facility of ICCBS, University of Karachi. Procured animals accustomed to laboratory environments one-week before the start of the experimentation and caged in a moderate temperature-controlled room at 23 ± 4ºC and 12 hours light-dark cycles. Rats were fed on rodent pellet food and water ad libitum with free access. The experimental procedures were designed by following the world-wide accredited health research extension act (1985).
Induction of diabetes
To make the animal model of diabetes, rats were injected (once) IP, freshly prepared STZ (35 mg/ml/kg in citrate buffer, pH; 4.5) to overnight-fasted rats [29] then, 2% glucose solution (once) was given to the rats via orally to overcome the hypoglycemic shock and confirmed diabetes after 72 hours of STZ injection (IP) by blood glucose levels>250 mg/ dl and proceed for the further experimental protocol.
Study design
Experimental animals were segregated into five (05) groups, each comprised (n=10), and received the belowmentioned treatment.
Group-II (DC): Diabetic Control group received Streptozotocin (STZ) freshly prepared in chilled citrate buffer at pH:4.5, given i. p (once) 35 mg/ml/kg body weight [29].
Group-III (D+AVLE): Diabetic group received freshly prepared AV-aliquot orally 100 mg/ml/kg bodyweight for 28 days daily after induction of diabetes.
Group-IV (D+AV-AgNPs): Diabetic group received freshly prepared green synthesized silver nanoparticles (AV-AgNPs) orally 10 mg/ml/kg body weight dissolved in aqueous media for 28 days daily after induction of diabetes.
Group-V (D+GLB): Diabetic group received freshly prepared Glibenclamide orally 600 µg/kg body weight dissolved in aqueous media for 28 days daily after induction of diabetes [30].
During the experiment, animals from all experimental groups were weighed, monitored individually and regularly while the % Change in body weight of each rat was determined via formula [31].
% Change in body weight = {(Final body weight - Initial body weight) ÷ Final body weight} ×100
Sample collection
Samples of blood and liver were collected later 24 hours of the last dose of administration by decapitating the animals from the neck wound. Plasma and serum separated via centrifugation at 2000 rpm for 20 minutes while after excised liver, trimmed then rinsed with ice-chilled saline, dried, weighed, and a piece of tissue immersed in 10% formalin for histological examination. Whereas, the remaining part was kept at -80ºC for biochemical analysis.
Liver homogenate preparation
Liver (1:10 w/v) were minced and homogenized with sodium phosphate buffer (10% of 0.1 M at pH-7.4) and centrifuged (1000 rpm for 10 min at 4◦ C) to get supernatant and subsequently added 10 µl BHT (0.5 M in acetonitrile) in a fraction to prevent further oxidation whereas other part recentrifuged (12000 rpm for 20 min at 4◦C) for further estimations [32].
Estimation of blood glucose
Blood glucose levels of rats were determined on alternate seven days for 28 days. Rats fasted for 12 hours, blood samples were obtained from the tail vein, and levels of glucose were measured using a Glucometer (On-Call EZ II ACON Laboratories, Inc., USA). In-addition, oral glucose tolerance tests (OGTT) were performed on Day 28. Briefly, rats fasted overnight and, after blood glucose concentrations had been determined in all groups, the OGTT was performed by administering 2g/kg of glucose to rats by gavage. Blood glucose concentrations were assessed after the 30-minute interval for 2 hours using a glucometer (On-Call EZ II ACON Laboratories, Inc., USA).
Biochemical analysis
Biochemical indices of liver function such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) were analyzed using a commercially prepared assay kit (LABKIT, Chemelex, S.A., Canovelles-Barcelona, Spain) as described and resulting values expressed as U/L.
Lipid peroxidation
The Malondialdehyde (MDA), is the measure of lipid peroxidation determined by the scheme of Okhawa et al. based on thiobarbituric acid (TBA) reacting substance interaction with MDA thus produced pink color which was determined by measuring the absorbance at 530 nm on Shimadzu Spectrophotometer (UV-1900, Japan) and resulting values were expressed as nmol/gm of tissue [33].
Superoxide dismutase
The activity of superoxide dismutase (SOD) in liver homogenate was measured by the method of Kono in the cell-free supernatant [34]. Percent inhibition in the rate of NBT reduction in the reaction mixture was recorded per minute on Shimadzu Spectrophotometer (UV-1900, Japan) at 560 nm and resulting values expressed as U/gm of tissue.
Catalase
The activity of Catalase (CAT) in liver homogenate was assayed by the method of Sinha et al. [35]. In brief, the sample was added to Hydrogen peroxide and phosphate buffer (pH;7) at 100℃ and subsequently added dichromate acetic acid reagent into a fraction of reaction mixture then allowed to boil for 10 minutes, cooled, note the absorbance on Shimadzu Spectrophotometer (UV-1900, Japan) at 570 nm and resulting values expressed as nmol/gm of tissue.
Glutathione reductase
The activity of Glutathione reductase (GSH) in liver homogenate was assayed by the method of Carlberg and Mannervik [36]. Briefly, the sample was mixed with BSA, β-NADPH, potassium phosphate buffer, and oxidizedGlutathione. Note the absorbance of each reaction mixture at 340 nm for 5 min at 25℃on Kinetic-Spectrophotometer (PRIM-500, Germany) and resulting values expressed as U/ gm of tissue.
Histopathology
Rat liver was removed quickly, washed with phosphatebuffered saline to remove debris, and a piece of tissue immersed in 10% formalin. About 5 μm tissue slices embedded in molten paraffin wax, stained with hematoxylin and eosin (H&E), and tissue morphology were analyzed by light microscopy at 10x.
Statistical analysis
All the data were presented as mean ± SD (n=10) considered significant when p < 0.05 using ANOVA (Oneway) followed by post hoc test (Tukey’s) using IBM-SPSS version 22 (IBM, Anmork, NY, USA).
Result
Change in body weights (%), liver weight, and relative liver weight
Results in table 1 displayed the change in body weight, liver weight, and relative liver weight in all groups. A considerable decrease in percent change in body weight was found in streptozotocin-induced diabetic rats than that in the control group (p<0.05). Likewise, a significantly improved percent change in body weight was also observed in all diabetic treated groups (D+AV-AgNPs, D+AVLE, and D+GLB) compared with diabetic control groups. Further, a significant gain in liver weight and relative liver weight (P<0.05) was observed in the streptozotocin-induced diabetic control group as compared with the control group. However, a significant decrease (P<0.05) in liver weight and relative liver weight was observed in all treated groups like D+AV-AgNPs, D+AVLE, and D+GLB in contrast with the diabetic control group.
Fasting and random blood glucose
Results in table 2 showed the AV-AgNPs treatment on fasting and random blood glucose levels in all groups. Both fasting and random blood glucose level was markedly elevated (p<0.05) in streptozotocin-induced diabetic rats and all diabetic treated groups (D+AV-AgNPs, D+AVLE, and D+GLB) compared with control (p<0.05). However, all diabetic treated groups significantly declined (p<0.05) fasting and random blood glucose compared with the diabetic control group.
Glucose tolerance test
Results in table 3 exhibited the AV-AgNPs treatment on glucose tolerance tests in all groups. The result revealed oral glucose administration at 30 min intervals for 2 hours (0-120 min) for tolerance test, glibenclamide improved glucose tolerance in the diabetic treated group. Likewise, diabetic treated groups (D+AV-AgNPs, D+AVLE, and D+GLB) significantly reduced blood glucose levels (p<0.05) at 120 min compared with control and diabetic control. Moreover, the result showed better tolerance in D+AV-AgNPs compared with D+AVLE.
Hepatic function markers
Results in table 4 present the AV-AgNPs treatment on hepatic functions in all groups. In the diabetic control and diabetic treated groups (D+AV-AgNPs, D+AVLE, and D+GLB) hepatic function markers like ALP, AST, and ALT were remarkably elevated compared with control (p<0.05). However, the diabetic treated groups (D+AV-AgNPs, D+AVLE, and D+GLB) restored the ALP, AST, and ALT levels (p<0.05) in contrast with diabetic control.
Hepatic oxidative stress and antioxidant enzymes
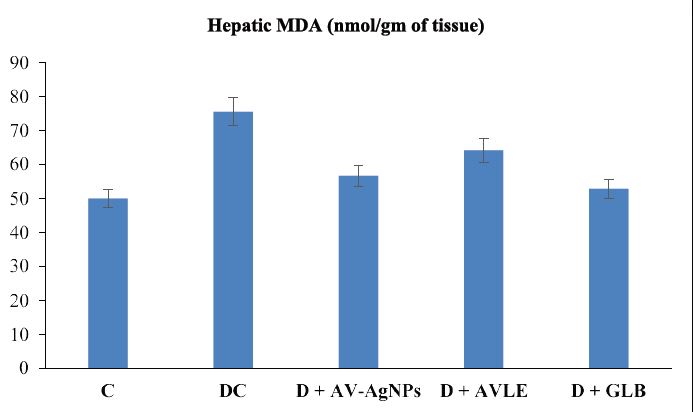
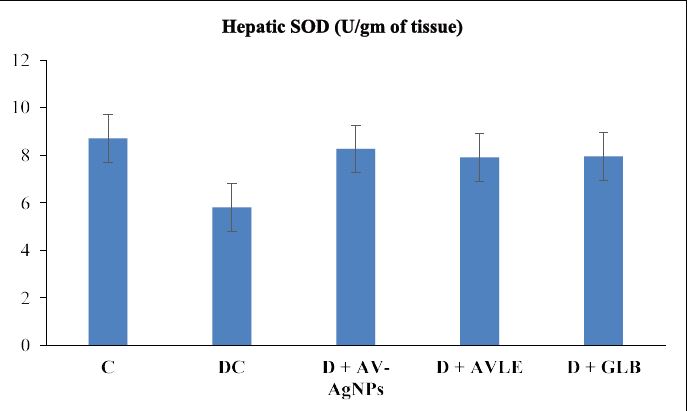
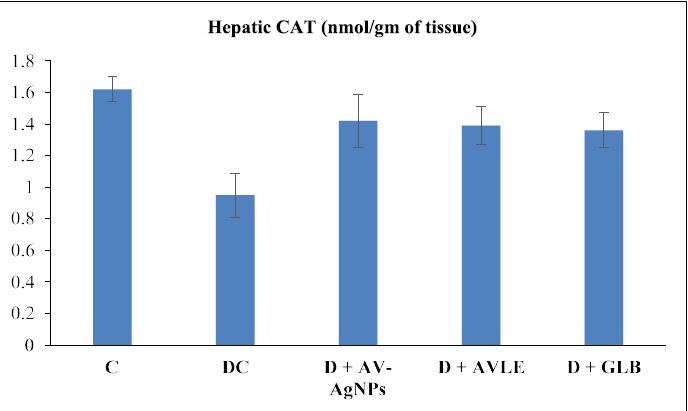
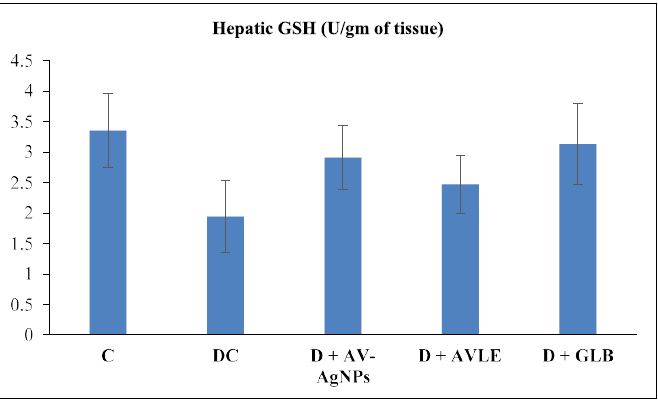
Figures 1 to 4 illustrated the hepatic oxidative stress and antioxidant enzymes (MDA, SOD, CAT, and GSH) in control and all treated groups. Hepatic tissue MDA level markedly raised (p<0.05) in the diabetic control group as compared with the control. However, diabetic rats treated with AVAgNPs, AVLE, and GLB markedly reduced MDA (p<0.05) as compared with diabetic control. On the other hand, a significant decrease (p<0.05) was observed in the level of SOD in the hepatic tissues of diabetic rats. Further, the administration of AV-AgNPs, AVLE, and GLB to diabetic rats significantly improved (p<0.05) antioxidant enzyme SOD as compared with diabetic control. In a similar trend, the level of CAT remarkably reduced significantly (p<0.05) in the hepatic tissues of diabetic rats. While, all treated diabetic rats (D+AV-AgNPs, D+AVLE, and D+GLB) significantly improved (p<0.05) antioxidant enzyme CAT as compared with diabetic control. Likewise, a remarkably reduced (p<0.05) level of GSH was observed in the hepatic tissues of diabetic rats. But the diabetic treated rats with AV-AgNPs, AVLE, and GLB significantly improved (p<0.05) antioxidant enzyme GSH as compared with diabetic control.
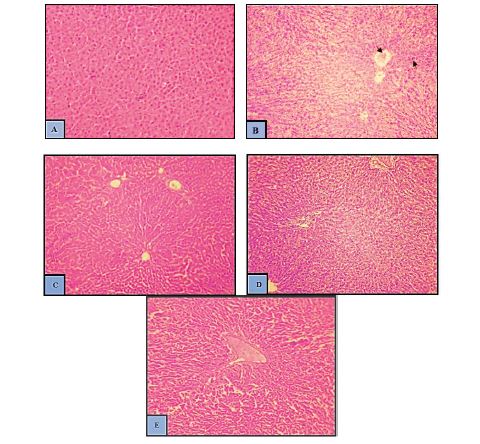
Histology of hepatic tissues in control and treated groups
The histopathological screening of hepatic tissues of the normal control group displayed normal hepatic tissue architecture with arranged hepatocytes and central vein (CV) along with the sinusoidal spaces (Figure 5A). In STZ induced diabetic control group, hepatic tissues indicated congestion in a central vein (CV), loss of normal central to the portal relationship, and Infiltration of inflammatory cells (Figure 5B). Administration of AV-AgNPs and AVLE respectively after induction of diabetes induced by STZ, protected against STZ induced hepatotoxicity (Figure 5C-E) thereby suggesting a protective role of AV-AgNPs against diabetic induced hepatotoxicity.
Discussion
The present study was conducted to investigate the role of AV-AgNPs against hepatic tissue injury in streptozotocininduced diabetic rats. Present results exhibited that rats treated with streptozotocin (STZ) caused various pathophysiological consequences such as derangements of body weight, liver weight, liver functional markers, elevated blood glucose (both fasting and random), elevated MDA, depletion of antioxidant enzymes, and disturbed liver histology. These observations corroborate by earlier reports on streptozotocin-induced diabetes29. Streptozotocin (STZ) is extensively used to persuade diabetes in investigational rats by targeting β-cell of islets of Langerhans through GLUT2 (glucose transporter) leads to DNA alkylation, and necrosis consequently declined insulin, and progress hyperglycemia [37].
In the present study, streptozotocin persuaded diabetes significantly induced a marked reduction in percent change in body weight compared with control (Table 1). The effects of silver nanoparticles on rats reliant on AgNPs concentration, the route by which it is administered, and duration of treatment [25]. Earlier studies found that the AgNPs did not have any adverse effects on the body weights of the animals following its oral administration [26,27]. The possible mechanism associated with AV-AgNPs antioxidant ability which counteracting the generation of free radicals via STZ in the pancreas [28]. Also, it can diminish the STZ persuaded toxicity and potentiates β-cells to proliferate more insulin secretion. The augmented insulin secretion leads to promote glucose consumption through extrahepatic tissues thus decreases blood glucose.
The liver is the primary organ, accounting for the metabolism, detoxification, storage, and elimination of byproducts and their metabolites [38,39]. It also aids in sustaining blood glucose levels via metabolic pathways like glycogenolysis and gluconeogenesis also in the postabsorptive state. Hyperglycemic condition distresses the liver and liable them to damage. ALT, ALP, and AST are the reliable indicators of liver function, which remain within the cells under normal physiological conditions whereas they are leaking into the serum upon cell damage [40,41]. In our study, STZ persuaded hepatic toxicity consequently alleviated the levels of ALT, ALP, and AST in serum [42,43]. These markers were found to significantly improve after AVAgNPs administration which is consistent in our study (Table 3) [31,44]. The decrease in serum transaminases near to normal after the treatment of AV-AgNPs indicates a possible revival of hepatocytes and a possible healing effect on the hepatic parenchyma [45]. The overall restoration observed in AV-AgNPs treated group represents its therapeutic effect. Besides this, lipid peroxidation may also attribute to the extreme ROS production which is mechanistically involved in hepatic impairment [46].
It has been well documented that STZ induced diabetes is associated with ROS generation and lipid peroxidation [47]. While the extreme formation of free-radical controls by antioxidant enzymes that protect cell membranes in all tissues to divergent oxidative injury including hepatic tissues (reference). Noticeable increment in the level of MDA (Figure 1) followed with substantial diminution in activities of SOD, CAT, and GSH in hepatic tissues in STZ persuaded toxicity (Figures 2-4) revealed in the present study which was overcome by administration of AV-AgNPs. Though, these findings are consistent with the earlier findings which exhibited that STZ imposed liver dysfunction via oxidative stress followed by lipid peroxidation and silver nanoparticles can deal with it [48,49].
Evidence suggested that the cell follows an adaptive mechanism to normalize stress exposure by altering oxidative stress and antioxidant enzymes [50]. An earlier study reported that micro-sized silver nanoparticles were the most effective antioxidant at smaller doses and prevent toxicity via decrease accumulations [51,52]. In the present study, we use a smaller dose (10mg/kg) of 20-24 nm particle size, which supports our findings by exhibiting beneficial therapeutic effects. The mechanism of silver nanoparticles being antioxidant is due to the presence of active compounds present in AVLE which capped silver nanoparticles [53]. Evidence could account for the AV-AgNPs antioxidant potency in restoring the oxidative stress-induced hepatic injury.
Histopathology of livers tissues of the normal control group indicated normal hepatic tissue architecture with arranged hepatocytes and central vein (CV) along with the sinusoidal spaces (5A). In the STZ intoxicated group, the hepatic architecture indicated congestion in a central vein (CV), loss of normal central to the portal relationship, and Infiltration of inflammatory cells (5B). Administration of AV-AgNPs and AVLE after induction of diabetes induced by STZ was brought back to near normal architecture of hepatic tissues indicating substantial protection of the liver (Figure 5C to 5E) against diabetic induced hepatotoxicity (Figure 5C to 5E). The histological findings of this study are in harmony with biochemical results. Similar observations in liver tissues were predictable in earlier studies [31,44].
The capability of a drug to diminish injuries/damages or to reserve the normal physiological function of the liver after induction of toxicity is the index of its hepatocurative effect [54]. Plant extracts used to produce AgNPs can lead to synergistic effects, including an antioxidant effect [55]. Outcomes attained, considering the biochemical and histological parameters, imply that AV-AgNPs were effective in protection against STZ intoxication. Failure in the antioxidant system can lead to an upsurge in lipid peroxidation and loss of normal cellular functioning in the liver [46]. Thus, the antioxidant and free radical scavenging effects of AV-AgNPs might play a crucial role in the revival of the biochemical and histological parameters near to normal in the treatment groups. This makes AV-AgNPs a valuable candidate for several therapeutic purposes, including protective effects on the liver.
| Parameters | Ca | DCb | D+AV-AgNPsc | D+AVLEd | D+GLBe |
|---|---|---|---|---|---|
| Change in body weight (%) | 10.31 ± 3.81 | -16.72 ± 7.64a | 8.84 ± 1.50b | 9.61 ± 2.86b | 4.55 ± 4.14b |
| Liver weight (g) | 5.94 ± 0.34 | 7.76 ± 0.51a | 6.21 ± 0.42b | 6.67 ± 0.61ab | 6.05 ± 0.21b |
| Relative liver weight (%) | 2.72 ± 0.32 | 3.90 ± 0.23a | 2.62 ± 0.24b | 2.63 ± 0.27b | 2.55 ± 0.12b |
| Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AV-AgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s). | |||||
Table 1: Effects of AV-AgNPs Treatment on Change in Body Weights, Organ Weight, and Relative Organ Weight.
| Parameters | Ca | DCb | D+AV-AgNPsc | D+AVLEd | D+GLBe |
|---|---|---|---|---|---|
| Fasting Blood Glucose (mg/dl) | 84.45 ± 8.25 | 328.63 ± 25.09a | 119.37 ± 9.96ab | 140.37 ± 11.95abc | 128.47 ± 9.34ab |
| Random Blood Glucose (mg/dl) | 118.49 ± 8.83 | 340.3 ± 15.40a | 130.73 ± 11.11b | 149.35 ± 11.13abc | 145.52 ± 20.39ab |
| Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AV-AgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s). | |||||
Table 2:Effects of AV-AgNPs Treatment on Fasting Blood Glucose and Random Blood Glucose.
| Parameters | Ca | DCb | D+AV-AgNPsc | D+AVLEd | D+GLBe |
|---|---|---|---|---|---|
| 0 min | 78.43 ± 5.30 | 263.44 ± 10.34a | 254.9 ± 5.70a | 258.25 ± 3.61a | 262.71 ± 9.82a |
| 30 min | 91.27 ± 5.11 | 281.55 ± 13.72a | 265.48 ± 8.66ab | 279.62 ± 11.30a | 286.59 ± 6.56ac |
| 60 min | 97.78 ± 5.32 | 331.57 ± 27.24a | 283.66 ± 11.44ab | 299.11 ± 12.42ab | 295.93 ± 6.17ab |
| 90 min | 89.53 ± 4.63 | 305.72 ± 17.15a | 217.14 ± 33.83ab | 251.53 ± 6.83abc | 231.27 ± 12.31ab |
| 120 min | 83.25 ± 6.48 | 284.54 ± 17.75a | 176.63 ± 12.7ab | 196.32 ± 36.31ab | 187.41 ± 11.94ab |
| Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AV-AgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s). | |||||
Table 3: Effects of AV-AgNPs Treatment on Glucose Tolerance Test.
| Parameters | Ca | DCb | D+AV-AgNPsc | D+AVLEd | D+GLBe |
|---|---|---|---|---|---|
| ALT (U/l) | 29.81 ± 1.51 | 62.26 ± 2.39a | 36.17 ± 4.28ab | 40.17 ± 3.37ab | 33.73 ± 2.14bd |
| AST (U/l) | 39.46 ± 5.72 | 78.82 ± 6.89a | 50.74 ± 3.88ab | 59.04 ± 6.91abc | 42.74 ± 5.35bed |
| ALP (U/l) | 62.81 ± 6.21 | 149.76 ± 11.73a | 81.92 ± 8.57ab | 94.22 ± 9.13abc | 266.81 ± 10.49bcd |
| Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AV-AgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s). | |||||
Table 4: Effects of AV-AgNPs Treatment on Liver Functions.
Figure 1: Effects of AV-AgNPs Treatment on Hepatic MDA. Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AVAgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s).
Figure 2: Effects of AV-AgNPs Treatment on Hepatic SOD. Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AVAgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s).
Conclusion
Treatment with AV-AgNPs was found to be a potent cellular antioxidant agent to restore the hepatic toxicity induced by STZ thus, mediated the body and relative weights, hepatic biomarkers, lipid peroxidation, and antioxidant enzyme activities. Because of current outcomes, it is concluded that AV-AgNPs can be used effectively to diminish oxidative stress which is the main progressive agent for developing diabetic comorbidities and other diseases via its antioxidant property. A further mechanistic approach in this regard is required to clarify the exact mechanism.
Conflicting Interests
No potential conflicts of interest concerning the research, authorship, and/or publication of this article declared by the author(s).
Figure 3: Effects of AV-AgNPs Treatment on Hepatic CAT. Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AVAgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s).
Figure 4: Effects of AV-AgNPs Treatment on Hepatic GSH. Data presented as mean ± SD (n=10), groups compared with a control; b diabetic control; c D+AVAgNPs; d D+AVLE, e D+GLB; result considered significant when p<0.05 using ANOVA (One-way) followed by post hoc test (Tukey’s).
Figure 5: (A-E). Effects of AV-AgNPs Treatment on Hepatic morphology (A) Control (B) Diabetic Control (C) D+AV-AgNPs (D) D+AVLE (E) D+GLB.
Funding
No financial support for the research, authorship, and/ or publication for this article was received by the author(s).
Ghosh J, Das J, Manna P (2011) The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials 32: 4857-4866. [ Ref ]
Saxena A, Vikram NK (2004) Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med 10: 369-378. [ Ref ]
Ritz E, Hasslacher C, Tschöpe W (1990) Diabetic nephropathy--are there differences between type I and type II?. Miner Electrolyte Metab 16: 69-72. [ Ref ]
Hendriksen PH, Oey PL, Wieneke GH (1992) Subclinical diabetic neuropathy: similarities between electrophysiological results of patients with type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 3: 690-695. [ Ref ]
Das J, Roy A, Sil PC (2012) Mechanism of the protective action of taurine in toxin and drug induced organ pathophysiology and diabetic complications: a review. Food Funct 3: 1251-1264. [ Ref ]
World Health Organization (WHO) (2002) WHO traditional medicine strategy 2002–2005. WHO, Geneva. [ Ref ]
Dobs AS, Goldstein BJ, Aschner P (2013) Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. J Diabetes 5: 68-79. [ Ref ]
Patel DK, Prasad SK, Kumar R (2012) An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2: 320-330. [ Ref ]
Nabeel MA, Kathiresan K, Manivannan S (2010) Antidiabetic activity of the mangrove species Ceriops decandra in alloxan-induced diabetic rats. J Diabetes 2: 97-103. [ Ref ]
.Gajalakshmi S, Vijayalakshmi S, Devi RV (2012) Phytochemical and pharmacological properties of Annona muricata: a review. Int J Pharm Pharm Sci 4: 3-6. [ Ref ]
Lavinya BU, Akalya JL, Srujana KS (2014) Appraisal of the in vitro antibacterial and anti-oxidant potential of the leaf extracts of Cadaba fruticosa. Asian J Pharm Clin Res 7: 118-120. [ Ref ]
Gholami-Ahangaran M, Bahmani M, Zia-Jahromi N (2012) Comparative and evaluation of anti-leech (Limnatis nilotica) effect of olive (Olea europaea L.) with levamisol and tiabendazole. Asian Pac J Trop Dis 2: S101-S103. [ Ref ]
Singh LW (2011) Traditional medicinal plants of Manipur as antidiabetics. J Med Plant Res 5: 677-687. [ Ref ]
Malviya N, Jain S, Malviya SA (2010) Antidiabetic potential of medicinal plants. Acta Pol Pharm 67: 113-118. [ Ref ]
Chauhan A, Sharma PK, Srivastava P (2010) Plants having potential antidiabetic activity: a review. Der Pharm Lett 2: 369-387. [ Ref ]
Modak M, Dixit P, Londhe J (2007) Recent advances in Indian herbal drug research guest editor: Thomas Paul Asir Devasagayam Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr 40: 163-173. [ Ref ]
Hannan JM, Marenah L, Ali L (2007) Insulin secretory actions of extracts of Asparagus racemosus root in perfused pancreas, isolated islets and clonal pancreatic β-cells. J Endocrinol 192: 159-168. [ Ref ]
Hes M, Dziedzic K, Gorecka D (2019) Aloe vera (L.) Webb: Natural Sources of Antioxidants–A Review. Plant Foods Hum Nutr 74: 255-265. [ Ref ]
Shanmuganathan R, MubarakAli D, Prabakar D (2018) An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res 25: 10362-10370. [ Ref ]
Saravanan M, Barik SK, MubarakAli D (2018) Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog 116: 221-226. [ Ref ]
Pugazhendhi A, Edison TN, Karuppusamy I (2018) Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm 539: 104-111. [ Ref ]
Saratale RG, Saratale GD, Shin HS (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res 25: 10164-10183. [ Ref ]
Saha J, Begum A, Mukherjee A (2017) A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain Environ Res 27: 245-250. [ Ref ]
Pugazhendhi A, Prabakar D, Jacob JM (2018) Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog 114: 41-45. [ Ref ]
Zhang XF, Liu ZG, Shen W (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17: 1534. [ Ref ]
.Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep 7: 15867. [ Ref ]
Suriyakalaa U, Antony JJ, Suganya S (2013) Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculate. Colloids Surf B 102: 189-194. [ Ref ]
Begum Q, Mahboob T (2019) Evaluation of antioxidant activity of biologically synthesized silver nanoparticles using Aloe vera. Int J Biol Biotech 16: 641-653. [ Ref ]
Gayathri M, Kannabiran K (2008) Antidiabetic and ameliorative potential of Ficus bengalensis bark extract in streptozotocin induced diabetic rats. Indian J Clin Biochem 23: 394-400. [ Ref ]
Prabhu S, Vinodhini S, Elanchezhiyan C (2018) Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin‐induced diabetic rats. J Diabetes 10: 28-42. [ Ref ]
Tabassum A, Mahboob T (2018) Role of peroxisome proliferator– activated receptor-gamma activation on visfatin, advanced glycation end products, and renal oxidative stress in obesity-induced type 2 diabetes mellitus. Hum Exp Toxicol 37: 1187-1198. [ Ref ]
Khan MS, Khan MK, Siddiqui MH (2011) An in vivo and in silico approach to elucidate the tocotrienol-mediated fortification against infection and inflammation induced alterations in antioxidant defense system. Eur Rev Med Pharmacol Sci 15: 916-930. [ Ref ]
Okhawa H, Ohishi N, Yagi K (1979) Reaction of linoleic acid hydroperoxides with thiobarbituric acids. Anal Biochem 19: 1053-1057. [ Ref ]
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamineand an assay for superoxide dismutase. Arch Biochem Biophys 186: 189-195. [ Ref ]
Sinha KA (1972) Colorimetric assay of catalase. Anal Biochem 47: 389-394. [ Ref ]
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113: 484-490. [ Ref ]
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50: 537-546. [ Ref ]
Nagalekshmi R, Menon A, Chandrasekharan DK, Nair CK (2011) Hepatoprotective activity of Andrographis paniculata and Swertia chirayita. Food Chem Toxicol 49: 3367-3373. [ Ref ]
Francis GA, Fayard E, Picard F (2003) Nuclear receptors and the control of metabolism. Annu Rev Physiol 65: 261-311. [ Ref ]
Ramaiah SK (2007) A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45: 1551-1557. [ Ref ]
Johnston DE (1999) Special considerations in interpreting liver function tests. Am Fam Physician 59: 2223-2232. [ Ref ]
Felig P, Marliss E, Ohman JL (1970) Plasma amino acid levels in diabetic ketoacidosis. Diabetes 19: 727-729. [ Ref ]
.Zhang H, Jacob JA, Jiang Z (2019) Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int J Nanomedicine 14: 3517-3524. [ Ref ]
Chatterjee S, Dey A, Dutta R (2011) Hepatoprotective effect of the ethanolic extract of Calocybe indica on mice with CCl4 hepatic intoxication. Int J PharmTech Res 3: 2162-2168. [ Ref ]
.Seif HS (2016) Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef univ j basic appl sci 5: 134-146. [ Ref ]
Akhgari M, Abdollahi M, Kebryaeezadeh A (2003) Biochemical evidence for free radical induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol 22: 205-211. [ Ref ]
Dakhil AS (2017) Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats. Journal of King Saud University-Science 29: 462-467. [ Ref ]
Afifi M, Abdelazim AM (2015) Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac J Trop Biomed 5: 874-877. [ Ref ]
Heidary T, Shayesteh FK, Ghasemi H (2014) Effects of silver nanoparticle (Ag NP) on oxidative stress, liver function in rat: hepatotoxic or hepatoprotective?. Issues Bio Sci Pharm Res 2: 40-44. [ Ref ]
.Adeyemi OS, Faniyan TO (2014) Antioxidant status of rats administered silver nanoparticles orally. J Taibah Univ Medical Sci 9: 182-186.[ Ref ]
Schluesener JK, Schluesener HJ (2013) Nanosilver: application and novel aspects of toxicology. Arch toxicol 87: 569-576. [ Ref ]
.Rajasekaran S, Sivagnanam K, Subramanian S (2005) Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J pharm Pharmacol 57: 241-246. [ Ref ]
Yadav NP, Dixit VK (2003) Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J Ethnopharmacol 86: 197-202. [ Ref ]
Nagaich U, Gulati N, Chauhan S (2016) Antioxidant and antibacterial potential of silver nanoparticles: biogenic synthesis utilizing apple extract. J pharm. [ Ref ]
Keshari AK, Srivastava R, Singh P (2020) Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med 11: 37-44. [ Ref ]