Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:16 January, 2020
Accepted date:26 February, 2020
Published date:04 March, 2020
Citation: Bagheri SH, Kalantari M, Fozooni S (2020) Synthesis, Identification and Improvement of Thermal and Physical Properties of Acrylic Coatings by Nanoparticles. Int J Nano Rech Vol: 3, Issu: 1 (01-12).
Copyright: © 2020 Bagheri SH et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The polymer matrix in the organic-mineral hybrid nanocomposites is flexible and lightweight, and inorganic nanoparticles are responsible for high thermal stability and improved their mechanical properties. In this study, Methyl Methacrylate-Butyl Acrylate copolymer (BA-MMA) was synthesized by conventional emulsion polymerization with conversion percentage of 96.15%. In order to synthesis of nanocomposite, nanoparticles of silver, titanium dioxide, iron oxide (Fe3O4), unmodified silica, and modified silica with 3-(tri-methoxycyclic) propyl methacrylate (MPS) and dichloromethyl vinylsilane DMVS) modifiers were used. Based on the results of the thermal gravimetric analysis, nanocomposite prepared from BA-MMA copolymer-containing 0.3 g of modified silica with 0.183 milliliters of chloro-methyl vinyl-silane cmodifier showed the highest thermal stability. The physical strength of this copolymr was also evaluated using a nanoscratch device. The results showed that the coefficient of friction is low (0.724) and the scratch coefficient is 0.267 µN−1/2 , and therefore, the scratch resistance is high. The chemical structureswas confirmed by Fourier transform infrared spectrometry.
Keywords: Coating, Nanocomposite, Emulsion Polymerization, Organic–inorganic Hybrid, Thermal Stability, Scratch Resistance.
Abstract
The polymer matrix in the organic-mineral hybrid nanocomposites is flexible and lightweight, and inorganic nanoparticles are responsible for high thermal stability and improved their mechanical properties. In this study, Methyl Methacrylate-Butyl Acrylate copolymer (BA-MMA) was synthesized by conventional emulsion polymerization with conversion percentage of 96.15%. In order to synthesis of nanocomposite, nanoparticles of silver, titanium dioxide, iron oxide (Fe3O4), unmodified silica, and modified silica with 3-(tri-methoxycyclic) propyl methacrylate (MPS) and dichloromethyl vinylsilane DMVS) modifiers were used. Based on the results of the thermal gravimetric analysis, nanocomposite prepared from BA-MMA copolymer-containing 0.3 g of modified silica with 0.183 milliliters of chloro-methyl vinyl-silane cmodifier showed the highest thermal stability. The physical strength of this copolymr was also evaluated using a nanoscratch device. The results showed that the coefficient of friction is low (0.724) and the scratch coefficient is 0.267 µN−1/2 , and therefore, the scratch resistance is high. The chemical structureswas confirmed by Fourier transform infrared spectrometry.
Keywords: Coating, Nanocomposite, Emulsion Polymerization, Organic–inorganic Hybrid, Thermal Stability, Scratch Resistance.
Introduction
Due to the excellent film formation, durability and resistance, optical clarity, UV stability and color retention, the polyacrylic latex coatings are widely used in coating production [1]. The most popular areas of applications of acrylic coatings are architecture, protection of metal and wood, decorative objects and automobile glossy coatings [2]. In the building materials sector, acrylic latex is widely used for protecting the wall, consolidating the monuments, and cultural heritage sites. These protective materials should be transparent, stable against the oxidation and light-weighted. Furthermore, this industry demands new treatments with high adhesive power and good thermal and mechanical properties [3].
In order to decrease the volatile organic compounds (VOCs), the coating industry is developing. These VOCs lead to atmospheric pollution and raised concerns on environment. One approach to lower VOCs is to replace the solvent-based coatings by water-based coatings [4-7]. Due to their excellent durability, toughness, optical clarity, UV stability, color retention, lack of environmental problems, ease and safety, waterbased coatings are applied more than solvent-based coatings, and their synthesis approach is mostly via emulsion polymerization [2,8].
Water-based emulsion polymerization is one of the most important polymerization methods that used to produce various types of the polymers, including poly acrylates, poly methyl methacrylates and their copolymers. The properties of the polymers produced in this way (polymer latexes), in addition to the type of monomers, depend on the type and concentration of the initiator, the particle stabilizers and the reaction temperature [9]. Acrylic polymer such as poly methyl methacrylate, usually, behaves fragile technique, polybutyl acrylate also is considered as a colourless transparent rubbery polymer at ambient temperature. Thus, it is usually used in copolymer systems to alleviate the brittleness of the final product [4]. Methyl methacrylate - butyl acrylate emulsion copolymers often in acrylic formulations, despite its commercial importance are used widely as paints [10]. This type of copolymer has been widely used in the coatings industry to promote adhesion, particulary to metal or old substrates, to improve the rheological properties of coatings [11].
In recent years, with regards to the excellent mechanical, thermal, optical, electrical and magnetic properties of polymer nanocomposite/ inorganic nanoparticles and their applications in the field of plastic materials, coatings and electronic industries, a lot of attention has been paid to the preparation of these nanocomposites [12]. Generally, they are organic polymer composites combined with an inorganic filler. Their properties are a combination of inorganic fillers (e.g. toughness, thermal stability) and organic polymer (e.g. ductility, elasticity and processability). If the size of filler particles is reduced from micro to nano, the unique properties of polymer nanocomposites with small-sized filler particles considerable is increased in surface area than the ordinary composites [3].
Different approaches are being investigated for the production of nanocomposites: chemical which involves in-situ polymerization or physical which involve blending of components in solution or molten state. In-situ polymerization, which involves the grow of polymer chains in the presence of nanoparticles, is an attractive approach as dispersion of nanoparticles can be initially attained by use of appropriate conditions (e.g., solvents, surfactants). In suspension, emulsion and mini-emulsion polymerization techniques, the nanocomposites are formed by carrying out polymerization in the presence of inorganic particles which seem the ideal route to achieve good dispersion in the base polymers [1,2,13]. Among these processes, emulsion polymerization is the most frequently used. In emulsion polymerization, the principal locus of particle nucleation is either in the aqueous phase or in the monomer-swollen micelles [1,12]. The use of inorganic particles in the nanoscale range is particularly attractive since it allows to improve the properties of the polymers by controlling the degree of interaction between the polymer and the nano fillers via a top-down approach [14].
Silica particles are used in various of theme such as rubber, plastics, acrylic coatings and paints, due to their excellent properties, such as non-toxic, stability, high thermal resistance, hardness, scratch resistance [15,16]. Titanium dioxide is also inexpensive, with thermal and optical stability and non-toxic. It is used in industry of ceramic, paint, catalytic, gas sensors and membrances [17]. Nano crystalline silver has exceptional antibacterial properties. The antimicrobial activity of silver is much higher than other metals such as copper, mercury, tin, chromium and lead [18]. This feature nano silver makes that used to prevent microbial infection in coating. Antibacterial coating is a kind of functional coatings that uses in clothing, textiles and medical instruments [19]. Amang magnetic nanoparticle, magnetate (Fe3O4) nanoparticle have drawn most interest due to their magnetic, catalytic, non-toxic, ease of synthesis and biological properties which could find applications in many theme including magnetic storage devices, ferro fluids, medical and magnetic resonance imaging [20-22]. The modification of nanoparticles surface by organic modifiers can reduce the number of hydroxyl groups on the nanoparticle surface and change the particles from hydrophilic to hydrophobic [16].
Polymer coatings and thin films which are used in paint and coating industry, automobiles, and microelectronics, in addition radiant appearance need to have a thermal stability and scratch resistance [23]. Polymer behavior in nanoscale is different from those in micro- and macro- scales. For example, the observation that surface coefficient of friction is lower than that of the bulk, due to the reduction of real area of contact and subsequent effects caused by such reduction when very low loads were being applied to the polymer. To investigate the surface properties in small scale and low forces, nano-scratch devices are used [24,25].
Yazdi-mamaghani et al synthesized silica nanoparticles encapsulated in acrylic copolymer by emulsion polymerization. They modificated silica nanoparticles using non-ionic surfactant and compared properties of the resulting nanocomposite with pure copolymer. The results showed that were improved the mechanical properties and thermal stability often nanocomposites in compared with pure copolymer [26]. Diacano et al Methyl methacrylate - butyl acrylate copolymer/sodium-montmorillonite nanocomposite by emulsion polymerization. They compared the effect of different concentrations of sodiummontmorillonite on the properties of nanocomposites in compared with pure copolymer. The results showed nanocomposite containing sodium-montmorillonite with concentrations <1.5%wt monomer, have mechanical and thermal properties and better permeability in compared with pure copolymer [27].
The main goal of this study is synthesis, identification and improvement of thermal and physical properties of acrylic coatings by nanoparticles
Experimental
Materials
methyl methacrylate (MMA, 99% purity), butyl acrylate (BA, purity>99%), ammonium persulfate (APS), azobisisobutyronitrile (AIBN) and benzoyl peroxide (BzO) sodium dodecyl sulfate (SDS) Silicon nanoparticles (SiO2, with average diameter of 20 nm, 99.5% purity), titanium dioxide nanoparticles (TiO2, with particles sizes < 25 nm and 99.7% purity), silver nitrate (AgNO3), thiourea, FeCl2.4H2O and FeCl3.6H2O, magnesium sulfate (MgSO4), absolute ethanol (C2H5OH), hydrochloric acid (HCl, 37-38%) and dichloromethylvinylsilane (DCMVS, 98% purity) were purchased from Merck Co. Triton X-100, 3-(trimethoxysilyl) propyl methacrylate (MPS, 98% purity) were purchased from Sigma Aldrich. Sodium hydroxide (NaOH) prepared from Kiankavehazma Company, Iran).
Experimental procedure
Copolymer synthesis: The copolymer latex was prepared by semi-continuous emulsion polymerization. In the first stage, deionized water (85mL) and SDS and Triton X-100 (ionic and non-ionic surfactant, respectively) were added in a four-neck glass flask equipped with a mechanical stirrer, nitrogen inlet, condenser, and dropping funnel. Reactor was immersed in an oil bath. The system was thoroughly purged for 30 minutes with nitrogen to remove dissolved oxygen from the reaction medium, while mixture of MMA and BA monomers was added into the reactor by dropping funnel after purification (MMA and BA were purified for several times with 10 wt% of sodium hydroxide in water solution and deionized water by extraction and then dried by MgSO4 and kept in the refrigerator till using). After the reactor temperature was stabilized, the thermal initiator ie. APS or AIBN or BzO in water solution (5mL) was rapidly added to the reactor, and reaction continued for 8 hours. The reaction was then cooled to room temperature.
Modification of Nano-SiO2 Particles: To modify the silicon nanoparticle two modifiers were used: 3-(Trimethoxysilyl)propyl methacrylate (MPS) and dichloromethylvinylsilane (DCMVS). Each modifier was selected with three different quantities of 10, 20 and 30 wt% from silicon nanoparticle. About 1g of nano-silica in a 100mL reactor was added to a mixture of ethanol absolute (19mL), deionized water (1mL) and 37-38% HCL (0.2mL). The mixture was sonicated in order to dispersion of the silica nanoparticles for 10 minutes in the ultrasound machine. Then, the given modifier was added with a special quantity and the mixture again was sonicated for 14 minutes in order to disperse the modifier. Finally, the reaction tempreture was set at 80°C for 24h under reflux conditions. After the reaction terminated, the modified silica was washed with absolute ethanol and deionized water and then dried completely in the oven at 50°C for 24h.
Preparation of magnetite nanoparticle (Fe3O4): A solution of FeCl2.4H2O (0.05 M) and of FeCl3.6H2O (0.025 M) were prepared in HCl (25mL, 2 M) separately. Then, these two solutions were added to each other and stirred under mechanical stirrer for 15 minutes. The produced solution was titrated with 25 mL of ammonia solution (0.7 M). The formed iron nanoparticles were collected by magnet and washed with deionized water [28].
Preparation of silver nanoparticle (Ag): A specific volume of AgNO3 was added to 30 mL of deionized water and put on the magnetic stirrer in 50°C for 20 minutes, during which the thiourea was gradually added to it [18].
Preparation of nanocomposite: In order to investigate the effect of nanoparticle, all parameters were kept fixed based on the copolymer with highest conversion percent. Nanocomposites were prepared with different weight percent (based on the total weight of monomers) of modified and unmodified nanoparticles of SiO2, TiO2, Ag and Fe3O4 by semi-continuous emulsion polymerization. Polymerization was carried in a four-neck glassy flask equipped with condenser, mechanical stirrer, nitrogen gas inlet and dropping funnel. In the first stage, the deionized water and a special amount of a nanoparticle were sonicated in the ultrasound for 6 minutes in order to disperse the nanoparticles. Then the mixture was transferred into the Reactor and ionic and nonionic surfactants were added. Reactor was immersed in an oil bath. The system was thoroughly purged for 30 minutes with nitrogen to remove dissolved oxygen from the reaction medium, while mixture of pure MMA and BA monomers was added into the reactor by dropping funnel. After the reactor temperature was stabilized in 80°C, the thermal initiator in water solution was rapidly added to the reactor and reaction continued for 8 hours and then cooled to the room temperature.
Change in type and amount of nanoparticles in nanocomposite synthesis: Various amount of nanoparticles TiO2, Fe3O4 and Ag without modifier, and different amount of modified and unmodified nanoparticles SiO2 with different amount and type of modifier were used for nanocomposite synthesis. These amount are presented in tables 1,2.
Properties
Thermogravimetric analysis (TGA) was determined using the thermo-gravimetric device model PC Luxx 409 from NETZSCH Company, made in Germany, under the N2 within the temperature range 25 – 650°C with heat rate of 7°C/min. In order to confirm the chemical structure, the Fourier-transform infrared spectroscopy (FT-IR) model TENSOR 27 manufactured by Bruker Co. USA, was utilized. The related spectrum was fixed on the frequency range of 400-4000 cm-1. Mechanical resistance of nanocomposites such as scratch was studied by nano-scratch test, using the Tribo Scope nano-mechanical test instrument. This device has a three-sided diamond indenter with tip radius 200 nm from type of Berkovich.
Results and discussion
Calculation of copolymer conversion percent
The quantity of acted monomers are from among the important and effective factors in determination of reaction conversion percent [8]. Conversion can be calculated from three different measurements: 1) gravimetric data 2) unreacted monomer measured by gas chromatography (GC), and 3) estimated online from calorimetric data [29].
In this study, the conversion percent (x) is obtained by gravimetric technique and using Relation (1):
Where, T is the total weight of latex removed from reactor, m1 is the weight of sample copolymer latex before drying, m2 is the weight of sample latex after drying, N is the weight of nonvolatile compounds of polymerization such as emulsifier and initiator, Wm is the total weight of monomers that were added to the reactor [30].
Investigation of effect of various factors on conversion percent: In this section, in order to obtain the highest conversion percent of copolymer, was investigated of effect of different variables involve weight ratio of monomers, round of mechanical stirrer, type and amount of initiator, total amount and ratio of consumed emulsifiers and reaction temperature, on the conversion percent which the results are given in table 3.
| Amount of nanoparticle (g) | Content ** nanoparticle (Wt%) | Amount of modifier (mL) | Content* modifier (Wt%) | Modifier | Nanoparticle | Sample |
|---|---|---|---|---|---|---|
| 0.1 | 1 | - | - | - | SiO2 | MBS1 |
| 0.3 | 3 | - | - | - | SiO2 | MBS3 |
| 0.5 | 5 | - | - | - | SiO2 | MBS5 |
| 0.3 | 3 | 0.191 | 20 | MPS | SiO2 | S3MPS20 |
| 0.1 | 1 | 0.287 | 30 | MPS | SiO2 | S1MPS30 |
| 0.3 | 3 | 0.287 | 30 | MPS | SiO2 | S3MPS30 |
| 0.5 | 5 | 0.287 | 30 | MPS | SiO2 | S5MPS30 |
| 0.3 | 3 | 0.091 | 10 | DCMVS | SiO2 | S3DMV10 |
| 0.5 | 5 | 0.091 | 10 | DCMVS | SiO2 | S5DMV10 |
| 0.3 | 3 | 0.183 | 20 | DCMVS | SiO2 | S3DMV20 |
| 0.5 | 5 | 0.183 | 20 | DCMVS | SiO2 | S5DMV20 |
| 0.1 | 1 | 0.275 | 30 | DCMVS | SiO2 | S1DMV30 |
| 0.3 | 3 | 0.275 | 30 | DCMVS | SiO2 | S3DMV30 |
| 0.5 | 5 | 0.275 | 30 | DCMVS | SiO2 | S5DMV30 |
| *Relative to the amount of SiO2.** Relative to the total amount of monomers. | ||||||
Table 1: Various amount of SiO2 and different amount and type of modifier used for nanocomposite synthesis.
| Thiourea(g) | AgNO3(g) | Content * nanoparticle (Wt %) | Nanoparticle | Sample |
|---|---|---|---|---|
| - | - | 0.25 | TiO2 | TiO2-1 |
| - | - | 0.5 | TiO2 | TiO2-2 |
| - | - | 0.25 | Fe3O4 | Fe3O4-1 |
| - | - | 0.5 | Fe3O4 | Fe3O4-2 |
| 0.0024 | 0.01 | - | Ag | Ag-1 |
| 0.0048 | 0.02 | - | Ag | Ag-2 |
| 0.0072 | 0.03 | - | Ag | Ag-3 |
| *Relative to the total amount of monomers. | ||||
Table 2: Various amount of different nanoparticles used in nanocomposite synthesis.
Identification of chemical structure
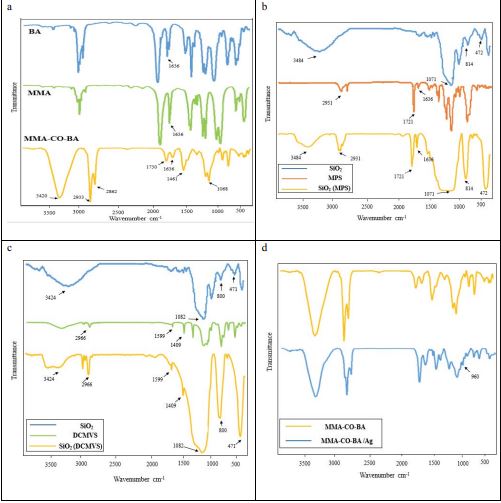
In this part, the FT-IR analysis has been used for investigation and Confirmation of copolymerization. In figure (1-a), the bands at 2933 cm-1 and 2862 cm-1 is related to stretching vibration of C-H groups of methyl and methylene. The absorption band of 1461 cm-1 and 1068 cm-1 respectively is related to bending vibration of C-H bond and C-O bond. Also, it can be seen C=O bond in 1730 cm-1 corresponded to carbonyl groups. Furthermore, there is a small band at 1636 cm-1 which corresponds to the bond C=C and this band enables the free radical polymerization. Reduction in C=C bond in spectrum MMA-CO-BA than the spectrums MMA and BA indicates that the covalent bond of acrylic chains is created and stir250 sample is copolymerized.
According to figure (1-b), in spectrum (MPS) SiO2 and SiO2 is shown the symmetric and asymmetric stretching vibration and bending vibration of bond Si-O-Si respectively at 1071 cm-1 , 814 cm-1 and 472 cm-1 these peaks are feature silica particle. In spectrum (MPS) SiO2, the peak of the stretching vibration of C-H at 2931 cm-1, C=O at 1721 cm-1, and C=C at 1636 cm-1 indicating that SiO2 was successfully modified by MPS. In spectrum (MPS) SiO2 , the relative intensity of the adsorption at 3484 cm-1 corresponded to the functional group OH is weaker than that of spectrum (SiO2). This shows that due to the reaction between OH groups on the surface of SiO2 and Si–OCH3 bond related to MPS, a number of Si-OH are converted into Si-O-Si. On the other hand, an increase in absorption bands intensity corresponded to Si-O-Si bond in spectrum (MPS) SiO2, confirms that the SiO2 surface has been modified by MPS.
According to figure (1-c), in spectrum (DCMVS) SiO2 and SiO2 is shown the symmetric and asymmetric stretching vibration and bending vibration of bond Si-O-Si respectively at 1082 cm-1 , 800 cm-1 and 471 cm-1 these peaks are feature silica particle. In spectrum (DCMVS) SiO2, the peak of the stretching and bending vibration of bond C-H respectively at 2966 cm-1 and 1409 cm-1, C=C at 1599 cm-1 indicating that SiO2 was successfully modified by DCMVS. In spectrum (DCMVS)SiO2, the relative intensity of the absorption at 3424 cm-1 corresponded to the functional group OH is weaker than that of spectrum (SiO2). This shows that by performing the reaction between OH groups on the surface of SiO2 and Si–Cl bond related to DCMVS, a number of Si-OH are converted into Si-O-Si. On the other hand, an increase in absorption bands intensity corresponded to Si-O-Si bond in spectrum (DCMVS) SiO2, confirms that the SiO2 surface has been modified by DCMVS.
In this part, nanocomposite produced from methyl methacrylate- butyl acrylate copolymer and one of the nanoparticles such as silver nanoparticle (sample Ag-3), was investigated and confirmed using the FT-IR analysis. According to figure (1-d), in spectrum MMA-CO-BA/Ag, the absorption band in wave number 960 cm-1 was corresponded to Ag-O bond and indicates that nanocomposite of MMACO-BA/Ag is synthesized properly. On the other hand, displacement of peaks and difference in size of peaks confirms this subject with compared to the bands existing in both spectrums.
| Conversion (%) | Temperature(ºC) | stirrer speed(rpm) | APS (g) | SDS+TX100 (g) | SDS/TX100 (Wt) | MMA:BA* (Wt%) | Sample |
|---|---|---|---|---|---|---|---|
| 47.09 | 80 | 200 | 0.12 | 1 | 1:3 | 100:0 | PMMA |
| 89.50 | 80 | 200 | 0.12 | 1 | 1:3 | 70:30 | MB7030 |
| 93.07 | 80 | 200 | 0.12 | 1 | 1:3 | 60:40 | MB6040 |
| 83.39 | 80 | 200 | 0.12 | 1 | 1:3 | 55:45 | MB5545 |
| 90.13 | 80 | 200 | 0.12 | 1 | 1:3 | 0:100 | PBA |
| 87.53 | 80 | 200 | 0.12 | 1 | 1:3 | 60:40 | stir300 |
| 96.15 | 80 | 250 | 0.12 | 1 | 1:3 | 60:40 | stir250 |
| 73.60 | 80 | 250 | 0.06 | 1 | 1:3 | 60:40 | APS1 |
| 86.01 | 80 | 250 | 0.10 | 1 | 1:3 | 60:40 | APS2 |
| 94.78 | 80 | 250 | 0.14 | 1 | 1:3 | 60:40 | APS4 |
| 82.78 | 80 | 250 | 0.2 | 1 | 1:3 | 60:40 | APS5 |
| 74.44 | 80 | 250 | 0.12 | 1 | 1:0 | 60:40 | SDTX1-1 |
| 94.37 | 80 | 250 | 0.12 | 1 | 1:2 | 60:40 | SDTX1-2 |
| 84.39 | 80 | 250 | 0.12 | 1 | 1:5 | 60:40 | SDTX1-3 |
| 76.86 | 80 | 250 | 0.12 | 1 | 0:1 | 60:40 | SDTX1-4 |
| 95.28 | 80 | 250 | 0.12 | 0.5 | 0:0.5 | 60:40 | SDTX2-1 |
| 94.41 | 80 | 250 | 0.12 | 0.5 | 1:3 | 60:40 | SDTX2-2 |
| 91.88 | 80 | 250 | 0.12 | 0.5 | 0.5:0 | 60:40 | SDTX2-3 |
| 34.54 | 70 | 250 | 0.12 | 1 | 1:3 | 60:40 | T1 |
| 89.16 | 90 | 250 | 0.12 | 1 | 1:3 | 60:40 | T2 |
| * MMA+BA=10g | |||||||
Table 3: Investigation of effective factors on the conversion percent of the copolymer
| Sample | Td-10%*(̊C) | Td-50%**(̊C) | Mass of residue in ~ 500°C (wt%) |
|---|---|---|---|
| stir250 | 370.938 | 398.874 | 4.939 |
| MBS1 | 370.903 | 399.320 | 3.749 |
| MBS3 | 370.679 | 399.373 | 6.421 |
| MBS5 | 369.539 | 400.371 | 5.977 |
| S3MPS20 | 382.505 | 408.056 | 4.233 |
| S1MPS30 | 384.770 | 409.042 | 6.009 |
| S3MPS30 | 384.818 | 410.866 | 5.191 |
| S5MPS30 | 384.350 | 407.925 | 4.773 |
| *Temperature at which a 10% weight loss occurs. ** Temperature at which a 50% weight loss occurs. | |||
Table 4: Thermal properties pure copolymer and nanocomposites of Unmodified SiO2 nanoparticles, and modified with MPS.
Figure 1: Spectrum FT-IR. a) Copolymer spectrum with highest conversion percent (stir250). b) SiO2 spectrum modified by MPS 30wt%. c) SiO2 spectrum modified by DCMVS 30wt%. d) nanocomposite spectrum of copolymer by highest conversion percent with silver nanoparticle (Ag-3).
Investigation of thermal stability
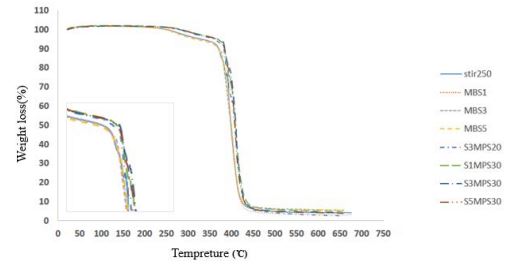
Figure 2 shows plots of mass loss as a function of temperature (TGA) for copolymer of methyl methacrylate (MMA) - butyl acrylate (BA) selected based on the highest conversion percent, and nanocomposites of this copolymer with unmodified SiO2 nanoparticles, and modified with MPS modifier in different values of this nanoparticle and modifier. According to table 4, we find that based on the comparison already made, samples of S1MPS30, S3MPS30 and S5MPS30 have higher thermal stability than the pure copolymer and nanocomposite SiO2 without modifier and modified by MPS. On the other hand, the sample S1MPS30 has the largest mass of residue in ~ 500ºC indicating the more amount of nanoparticle in synthesis. By comparing the thermal degradation temperature of these three samples, S3MPS30 sample in compared with the S1MPS30, S5MPS30 samples has a higher thermal degradation temperature and as a result higher thermal stability.
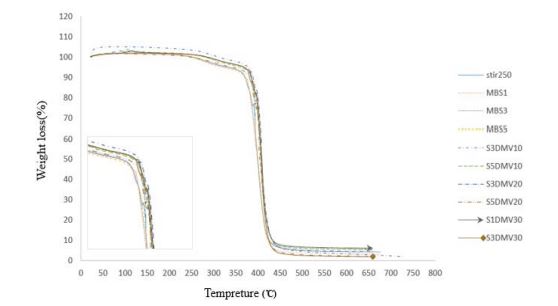
Figure 3 shows the TGA curves for copolymer of methyl methacrylate (MMA) - butyl acrylate (BA) selected based on the highest conversion percent, and this copolymer’s nanocomposites with unmodified SiO2 nanoparticles, and modified by DCMVS modifier in different values of this nanoparticle and modifier. According to table 5, samples of S3DMV20 and S1DMV30 have higher thermal stability than the pure copolymer and its nanocomposite with SiO2 nanoparticles without modifier and modified by DCMVS. On the other hand, sample S1DMV30 has the largest mass of residue in ~ 500ºC indicating the more amount of nanoparticles participating in synthesis.
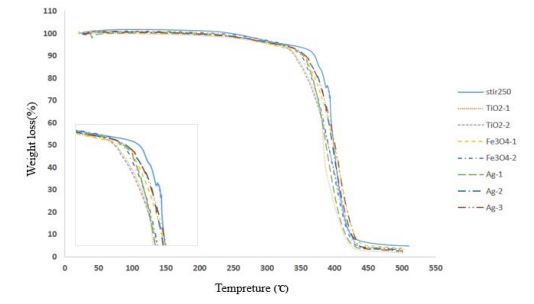
Figure 4 shows the TGA curves for copolymer of methyl methacrylate (MMA) - butyl acrylate (BA) selected based on the highest conversion percent, and this copolymer’s nanocomposites with unmodified TiO2, Ag and Fe3O4nanoparticles in different values of this nanoparticles. According to table 6, we find that the thermal decomposition temperature in 10 wt% loss occurs for nanocomposites of copolymer of MMA-BA/Ag, Fe3O4 and TiO2 shifts toward the temperature range lower than the pure copolymer which this indicates that incorporation of silica nanoparticles into as copolymer has not improved the degradation mechanisms of the polymer. On the other hand, comparation of these nanocomposites in different amount and type of nanoparticle and with pure copolymer, Ag-3 sample has higher thermal stability in 50 wt% loss occurs.
By comparing the results of the TGA test is the thermal stability of the S3DMV20 sample>S1DMV30> S1MPS30> Ag-3. By such comparison, it can be concluded that the nanocomposite of silver nanoparticle without the modifier has lower thermal stability than the nanocomposite of silica nanoparticle modified by MPS and the latter has lower thermal stability than the nanocomposite of silica modified by DCMVS. Therefore, sample of S3DMV20 is selected as the best example for thermal stability in this comparison.
Figure 2: TGA curves for pure copolymer and nanocomposites of unmodified SiO2 nanoparticles, and modified with MPS.
Figure 3: TGA curves for pure copolymer and nanocomposites of unmodified SiO2 nanoparticles, and Modified with DCMVS.
| Sample | Td-10%*(̊C) | Td-50%**(̊C) | Mass of residue in ~ 500°C (wt%) |
|---|---|---|---|
| stir250 | 370.938 | 398.874 | 4.939 |
| MBS1 | 370.903 | 399.320 | 3.749 |
| MBS3 | 370.679 | 399.373 | 6.421 |
| MBS5 | 369.539 | 400.371 | 5.977 |
| S3DMV10 | 385.266 | 407.349 | 4.414 |
| S5DMV10 | 384.059 | 406.674 | 5.882 |
| S3DMV20 | 388.911 | 408.267 | 5.035 |
| S5DMV20 | 383.722 | 405.686 | 2.825 |
| S1DMV30 | 385.665 | 408.017 | 6.675 |
| S3DMV30 | 380.566 | 407.160 | 2.428 |
| *Temperature at which a 10% weight loss occurs.** Temperature at which a 50% weight loss occurs. | |||
Table 5: Thermal properties pure copolymer and nanocomposites of Unmodified SiO2 nanoparticles, and modified with DCMVS.
| Sample | Td-10%*(̊C) | Td-50%**(̊C) | Mass of residue in ~ 500°C (wt%) |
|---|---|---|---|
| stir250 | 370.938 | 398.874 | 4.939 |
| TiO2-1 | 342.255 | 384.255 | 1.706 |
| TiO2-2 | 341.518 | 390.518 | 3.313 |
| Fe3O4-1 | 355.114 | 399.114 | 3.781 |
| Fe3O4-2 | 350.629 | 390.629 | 2.196 |
| Ag-1 | 353.991 | 386.991 | 2.632 |
| Ag-2 | 357.072 | 397.072 | 3.023 |
| Ag-3 | 357.891 | 400.891 | 2.501 |
| *Temperature at which a 10% weight loss occurs.** Temperature at which a 50% weight loss occurs. | |||
Table 6: Thermal properties pure copolymer and nanocomposites of TiO2, Ag and Fe3O4 nanoparticles.
| Sample | Td-10%*(̊C) | K | K(µN −1/2) | Scratch depth (nm) | FN( µN ) |
|---|---|---|---|---|---|
| S3DMV20 | 388.911 | 0.724 | 0.267 | 175 | 8 |
Table 7: The results of nano-scratch test.
FT-IR (sample of S3DMV20)
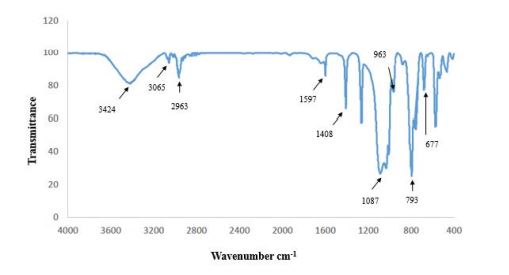
In this part, nanocomposite produced from methyl methacrylate- butyl acrylate copolymer with silica nanoparticle modified by DCMVS as the best example for thermal stability (sample of S3DMV20), was investigated and confirmed using the FT-IR analysis. According to figure 5, the observed peaks in the region of 793 cm-1 and 1087 cm-1 is related to stretching vibration of Si-O-Si and the appearing peak in the region of 963 cm-1 is related to Si-O bond. The wide absorption band in the region of 3424 cm-1 is related to stretching vibration of O-H bond. The peak of 2963 cm-1 is related to saturated C-H and the peak of 3065 cm-1 is related to stretching vibration of vinyl C-H. In addition, the presence of a double bond of C = C in the silane modified is confirmed with observed absorption band in the area 1408 cm-1 and 1597 cm-1. Also the observed peak in 677 cm-1 is related to the Si-Cl bond. The presence of these peaks shows that nanocomposite of S3DMV20 is synthesized properly.
Investigation of nanoscratch resistance (sample of S3DMV20)
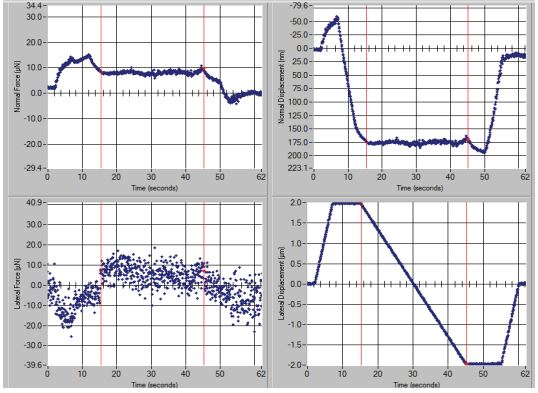
The test under force 0-40 µN with load rate 40 µN/s is performed on 4 µm length at scratch rate of 0.133 µm/s. An average of three nanoscratch tests in three different points are carried out on each sample and them average is reported. In this test, a normal force (NF) and a lateral force (LF) are applied to the sample. The lateral force usually shows more noise according to the heterogeneity of the surface. In fact, two of the four curves obtained from this test are related to these two forces after reaching to a about steady state with time. The third curve reveals the changes in scratch depth (normal displacement) and the fourth shows scratch width (lateral displacement) with time. Scratch occurs for 30 seconds at a maximum depth to the length 4 µm.
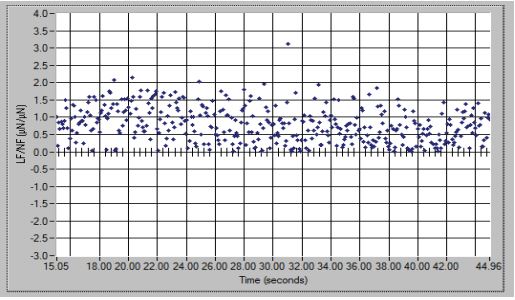
Lateral force (FL) divided by the normal force (FN) is defined as Friction coefficient (k) and obtained curve friction coefficient by drawing friction coefficient changes with time (equation 2). This parameter is used for investigating the surface scratch resistance. Very low value of friction coefficient in accordance with a good scratch resist. [23,31-35]:
Scratch coefficient (K) with unit µN-1/2 is considered as a scratch resistance criterion (equation3). This means that the lower scratch coefficient the higher scratch resistance [36].
Fours curves related to lateral force, normal force, side displacement and vertical displacement by time and also the friction coefficient curve of these samples are given in figures 6,7. According to Figure 6 of the S3DMV20 sample, the amount of normal force is about 8 µN and maximum scratch depth (normal displacement) is 175 nm. The average friction coefficient of this sample is 0.724 according to equation2. The average scratch coefficient is obtained 0.267 µN−1/2 from equation3 table 7.
Figure 4: TGA curves for pure copolymer and nanocomposites of TiO2, Ag and Fe3O4 nanoparticles.
Figure 5: Spectrum FT-IR: nanocomposite of methyl methacrylate- butyl acrylate copolymer with silica nanoparticle modified by DCMVS as the best example for thermal stability (sample of S3DMV20).
Figure 6: Curves of the nano-scratch test (S3DMV20).
Figure 7: Curve of the friction coefficient (S3DMV20).
Conclusion
In this study, the copolymer of methyl methacrylate - butyl acrylate was synthesised by conventional emulsion polymerization aiming at achieving the high conversion percent of monomer, and the impact of various parameters was investigated on conversion percent. This copolymer with nanoparticles of titanium dioxide, iron oxide, silver, and modified and unmodified nano silica with different modifiers was nanocomposited that using as coating. The thermal stability of this coatings was compared. Coating with highest thermal stability was selected then its mechanical resistance (scratch) was investigated.
According to the results obtained, the best conditions for synthesis of copolymer of methyl methacrylate- butyl acrylate with highest conversion percentage (96.15%) were as follows: weight ratio of MMA: BA = 60:40 with a total amount of 10 g monomers, mechanical stirrer speed: 250 rpm, thermal initiator: water-soluble APS with amount of 0.12 g, emulsifiers: SDS and TX-100 at a weight ratio of 1 to 3 and a total value of 1 g, and the reaction temperature: 80°C. TGA analysis showed that the nanocomposites with 0.1, 0.3 and 0.5 g modified silica nanoparticle with 0.287 mL MPS, 0.3 g modified silica with 0.183 mL DCMVS, 0.1 g modified silica with 0.275 mL DCMVS and silver nanocomposite including 0.03 g AgNO3 and 0.0072 g thiourea have the highest thermal stability.The copolymer of methyl methacrylate - butyl acrylate nanocomposite with nanosilica modified by DCMVS has higher thermal stability than the nanocomposite of silica nanoparticle modified by MPS and the latter has higher thermal stability than the nanocomposite of silver nanoparticle without the modifier.In the present study, the nanocomposite coating of copolymer of methyl methacrylate - butyl acrylate containing 0.3 g modified silica with 0.183 mL DCMVS was selected as the best sample in terms of thermal stability. According to the results of the nanoscratch test, this coating with the lower friction coefficient 0.724 and lower scratch coefficient 0.267 µN−1/2 with normal force of 8 µN ad scratch depth of 175 nm has high scratch resistance.
Romo-Uribe A, Arcos-Casarrubias J, Reyes-Mayer A, Guardian-Tapia R (2016) Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles. In-situ semi-batch emulsion polymerization. European Polymer Journal 76:170- 187.[ Ref ]
Romo-Uribe A, Arcos-Casarrubias JA, Hernandez-Vargas ML, Reyes-Mayer A, Aguilar-Franco M (2016) Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles by in-situ batch emulsion polymerization. Progress in Organic Coatings 97: 288-300.[ Ref ]
Ramos-Fernández J, Guillem C, Lopez-Buendía A, Paulis M, Asua J (2011) Synthesis of poly-(BA-co-MMA) latexes filled with SiO2 for coating in construction applications. Progress in Organic Coatings 72: 438-442.[ Ref ]
Sirapanichart S, Monvisade P, Siriphannon P, Nukeaw J (2011) Poly (methyl methacrylate-co-butyl acrylate)/ organophosphate-modified montmorillonite composites. Iran Polym J 20: 803.[ Ref ]
Liu R, Winnik MA, Stefano FD, Vanketessan J (2001) Interdiffusion vs Cross-Linking Rates in IsobutoxyacrylamideContaining Latex Coatings. Macromolecules 34: 7306-7314.[ Ref ]
Liu Y, Schroeder W, Soleimani M, Lau W, Winnik MA (2010) Effect of Hyperbranched Poly (butyl methacrylate) on Polymer Diffusion in Poly (butyl acrylate-co-methyl methacrylate) Latex Films. Macromolecules 43: 6438-6449.[ Ref ]
Tomba JP, Portinha D, Schroeder WF, Winnik MA, Lau W (2009) Polymer diffusion in high-M/low-M hard-soft latex blends. Colloid Polym Sci 287: 367-378.[ Ref ]
Pishvaei M (2008) Synthesis of acrylic copolymer used in water-based paints by emulsion polymerization. Journal of Color Science and Technology 2: 159-169. [ Ref ]
Afghani T, Bagheri R, Nehzat M (2006) Emulsion copolymerization of methyl methacrylate and butyl acrylate: The study of effective parameters on resin formation and its physical properties, Iranian. Journal of PolymerScience andTechnology 19: 255-264.[ Ref ]
Sayer C, Lima E, Pinto J, Arzamendi G, Asua J (2000) Kinetics of the seeded semicontinuous emulsion copolymerization of methyl methacrylate and butyl acrylate. Journal of Polymer Science Part A: Polymer Chemistry 38:367-375.[ Ref ]
Hong S, Soleimani M, Liu Y, Winnik MA (2010) Influence of a hydrogen-bonding co-monomer on polymer diffusion in poly (butyl acrylate-co-methyl methacrylate) latex films. Polymer 51: 3006-3013.[ Ref ]
Qi DM, Bao YZ, Weng ZX, Huang ZM (2006) Preparation of acrylate polymer/silica nanocomposite particles with high silica encapsulation efficiency via miniemulsion polymerization. Polymer 47: 4622-4629.[ Ref ]
Dashtizadeh A, Abdouss M, Mahdavi H, Khorassani M (2011) Acrylic coatings exhibiting improved hardness, solvent resistance and glossiness by using silica nanocomposites. Applied Surface Science 257: 2118-2125.[ Ref ]
Amerio E, Fabbri P, Malucelli G, Messori M, Sangermano M (2008) Scratch resistance of nano-silica reinforced acrylic coatings. Progress in Organic Coatings 62: 129-133.[ Ref ]
Sangermano M, Gaspari E, Vescovo L, Messori M (2011) Enhancement of scratch-resistance properties of methacrylated UV-cured coatings. Progress in Organic Coatings 72: 287-291.[ Ref ]
Bauer F, Flyunt R, Czihal K, Buchmeiser MR, Langguth H (2006) Nano/micro particle hybrid composites for scratch and abrasion resistant polyacrylate coatings. Macromolecular Materials and Engineering 291: 493-498.[ Ref ]
Sadeghi H (2012) Investigation of synthesis of boron doped titanium dioxide nano powder during steam phase synthesis process. Master’s thesis of chemical engineering, Shahid Bahonar University of Kerman 1-6.[ Ref ]
Mamaghani MY, Pishvaei M, Kaffashi B (2011) Synthesis of latex based antibacterial acrylate polymer/nanosilver via in situ miniemulsion polymerization. Macromolecular research 19: 243-249.[ Ref ]
Motlagh AL, Bastani S, Hashemi M (2014) Investigation of synergistic effect of nano sized Ag/TiO2 particles on antibacterial, physical and mechanical properties of UVcurable clear coatings by experimental design. Progress in Organic Coatings 77: 502-511.[ Ref ]
Mahdavian R, Sehri Y, Salehi-Mobarakeh H (2008) Nanocomposite particles with core–shell morphology II. An investigation into the affecting parameters on preparation of Fe3O4-poly (butyl acrylate–styrene) particles via miniemulsion polymerization. European polymer journal 44: 2482-2488.[ Ref ]
Yang Y, Mahdavian AR, Daniels ES, Klein A, El‐Aasser MS (2013) Gold deposition on Fe3O4/(co) Poly (N‐octadecyl methacrylate) hybrid particles to obtain nanocomposites with ternary intrinsic features. Journal of Applied Polymer Science 127: 3768-3777.[ Ref ]
Guin D, Manorama SV (2008) Room temperature synthesis of monodispersed iron oxide nanoparticles. Materials letters 62: 3139-3142.[ Ref ]
Javan Nikkhah S, Hojati H, Moghbeli M (2014) Self-Crosslinking Acrylate Copolymer/Organoclay Nanocomposite Emulsion Coating: Nanoindentation and Nanoscratch Behavior. Polymer-Plastics Technology and Engineering 53: 268-277.[ Ref ]
Shen W, Sun J, Liu J, Mao W, Nordstrom JD (2004) Methods for studying the mechanical and tribological properties of hard and soft coatings with a nano-indenter. Journal of Coatings Technology and Research, 1: 117-125. [ Ref ]
Wong JS, Sue HJ, Zeng KY, Li RK, Mai YW (2004) Scratch damage of polymers in nanoscale. Acta Materialia 52: 431-443.[ Ref ]
Yazdimamaghani M, Pourvala T, Motamedi E, Fathi B, Vashaee D (2013) Synthesis and characterization of encapsulated nanosilica particles with an acrylic copolymer by in situ emulsion polymerization using thermoresponsive nonionic surfactant. Materials 6: 3727-3741.[ Ref ]
Diaconu G, Paulis M, Leiza JR (2008) Towards the synthesis of high solids content waterborne poly (methyl methacrylate-co-butyl acrylate)/montmorillonite nanocomposites. Polymer 49: 2444-2454.[ Ref ]
Hamidian H, Tavakoli T (2016) Preparation of a new Fe3O4/ starch-g-polyester nanocomposite hydrogel and a study on swelling and drug delivery properties. Carbohydrate polymers 144: 140-148.[ Ref ]
Sayer, Lima EL, Pinto JC, Arzamendi G, Asua JM (2000) Molecular weight distribution in composition controlled emulsion copolymerization. Journal of Polymer Science Part A: Polymer Chemistry 38: 1100-1109.[ Ref ]
Zhang W, Mu L, Wang Y, Lin S, Pan Q (2014) Synthesis of poly (butyl acrylate)‐poly (methyl methacrylate) core–shell nanomaterials of anti‐crease‐whitening properties. Journal of Applied Polymer Science 131: 39991.[ Ref ]
Krupička A, Johansson M, Hult A (2003) Use and interpretation of scratch tests on ductile polymer coatings. Progress in Organic Coatings 46: 32-48.[ Ref ]
Yahyaei H, Mohseni M (2013) Use of nanoindentation and nanoscratch experiments to reveal the mechanical behavior of sol–gel prepared nanocomposite films on polycarbonate. Tribology International 57: 147-155.[ Ref ]
Gauthier, Durier AL, Fond C, Schirrer R (2006) Scratching of a coated polymer and mechanical analysis of a scratch resistance solution. Tribology International 39: 88-98.[ Ref ]
Sangermano M, Messori M (2010) Scratch resistance enhancement of polymer coatings. Macromolecular Materials and Engineering 295: 603-612.[ Ref ]
Gauthier, Schirrer R (2000) Time and temperature dependence of the scratch properties of poly (methylmethacrylate) surfaces. Journal of Materials Science 35: 2121-2130.[ Ref ]
Naimi‐Jamal M, Kaupp G (2008) New nanoindentation and nanoscratching parameters of thermoplastics. Macromolecular symposia 274: 72-80.[ Ref ]