Journal Name: J Clinical Pharmacy
Article Type: Research
Received date: 18 June, 2019
Accepted date: 05 July, 2019
Published date: 12 July, 2019
Citation: Asmaul-Husna, Sailaja AK (2019) Preparation and Evaluation of Mefenamic Acid Loaded BSA Nanoparticles by Desolvation Technique Using Acetonitrile as Desolvating Agent. J Clin Pharm Vol: 1, Issu: 1 (46-49).
Copyright: © 2019 Sailaja AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of the present investigation is to prepare mefenamic acid nanoparticles by desolvation technique using acetonitrile as desolvating agent by intermittent addition method. To 1% aqueous solution of mefenamic acid the desolvating agent was added intermittently at the rate of 1 mL per every five minutes till the solution become turbid. Then a cross linking agent was added and kept for stirring for a period of 8 hours. The obtained nanoparticles were evaluated for product yield, Drug content, entrapment efficiency and loading capacity. The Scanning Electron Microscope (SEM) images clearly reveals that the particles were found to be spherical in shape. The product yield, drug content, entrapment efficiency and loading capacity was found to be 93.9%, 63.7%, 65.43% and 28.4% respectively.
Keywords
Rheumatoid arthritis, Cytokines, Inflammation, Corticosteroids.
Abstract
The aim of the present investigation is to prepare mefenamic acid nanoparticles by desolvation technique using acetonitrile as desolvating agent by intermittent addition method. To 1% aqueous solution of mefenamic acid the desolvating agent was added intermittently at the rate of 1 mL per every five minutes till the solution become turbid. Then a cross linking agent was added and kept for stirring for a period of 8 hours. The obtained nanoparticles were evaluated for product yield, Drug content, entrapment efficiency and loading capacity. The Scanning Electron Microscope (SEM) images clearly reveals that the particles were found to be spherical in shape. The product yield, drug content, entrapment efficiency and loading capacity was found to be 93.9%, 63.7%, 65.43% and 28.4% respectively.
Keywords
Rheumatoid arthritis, Cytokines, Inflammation, Corticosteroids.
Introduction
Rheumatoid arthritis is a systemic, chronic, long-term autoimmune disorder that leads to inflammation of the synovial joints, and surrounding tissues gradually leading to joint destruction. The pattern of joints affected is usually symmetrical which effects the small joints of the hand and feet [1-2]. It can also affect other organs including heart, lungs and eyes. Activated macrophages are responsible for production of inflammatory cytokines which results in inflammation, swelling, bone erosion leading to chronic pain, stiffness and functional impairment.
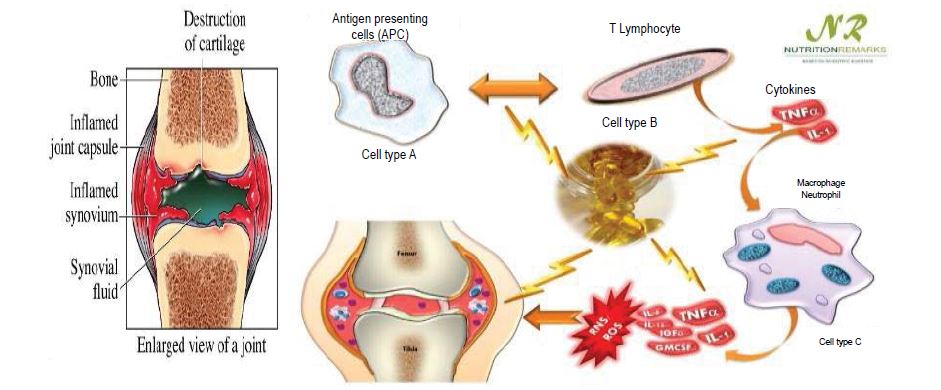
The drugs which are employed for the treatment of rheumatoid arthritis are Non-Steroidal Anti-Inflammatory Drugs (NSAIDS), corticosteroids, DMARD’S etc., Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are most commonly used to reduce inflammation and pain. NSAIDs acts by cyclooxygenase enzyme inhibition resulting in antiinflammatory action, the main factor limiting the oral use of NSAIDs is the development of Gastrointestinal (GI) adverse events, ranging from dyspepsia to serious life-threatening events. Several studies have shown the effectiveness of topical NSAIDs in treating acute and chronic soft tissue conditions [3]. Mefenamic acid is a widely prescribed NSAID and used as first line therapy for the treatment of ailments such as Arthritis and Dysmenorrhoea and its half-life t1/2 1.5-2 hrs. It is considered to be a BCS Class II drug (low soluble and high permeable) (Figure 1).
Mefenamic acid binds the prostaglandin synthetase receptors COX-1 and COX-2, inhibiting the action of prostaglandin synthetase. As these receptors have a role as a major mediator of inflammation, the symptoms of pain are temporarily reduced. Ethyl cellulose is non-biodegradable, bio-compatible, non-toxic synthetic polymer and widely used in oral and topical formulation. Sodium alginate is a biodegradable natural polymer [4]. It is used in the preparation of sustained release oral formulations since it can delay the dissolution of a drug. The main objective of this work as mefenamic acid has less biological life t1/2 1.5-2 hrs. Hence frequent administration of drug 200-400 mg twice daily is required to maintain the desired steady state level so, formulating such drug into controlled drug delivery system i.e., nanoparticles using different polymers is expected to increase the sustain release action and to improve patient compliance by reducing the dosing frequency.
Figure 1:Factors responsible for causing rheumatoid arthritis.
Nanoparticles are drug loaded particles with diameter ranging from 1 to 1000 nm [5]. Nanoparticles are defined as solid, sub micro-sized drug carrier that may or may not be biodegradable. Nanoparticle is a collective term used for both nanospheres and nanocapsule. Nanospheres have a matrix type structure and the drug may be adsorbed at the sphere surface or encapsulated within the particle. Nanocapsules are the vesicular system in which the drug is confined to a cavity consisting of an inner liquid core surrounded by a polymeric membrane. In this case the drug is usually dissolved in the inner core but may also be adsorbed to the capsule surface. In this research work attempts were made to prepare mefenamic acid nanoparticles by desolvation technique using acetonitrile as desolvating agent by intermittent addition method.
Materials and Methods
Mefenamic acid was obtained as a gift sample from Dr. Reddy Labs. Ltd, Hyderabad. BSA acetone and glutaraldehyde were supplied from S.D Fine Chemicals Ltd, Mumbai.
Preparation of BSA nanoparticles by continuous and intermittent addition of Acetone as desolvating agent
Requisite amount of Bovine Serum Albumin (BSA) was dissolved in 100 mL water. In Intermittent addition method acetonitrile (desolvating agent) was added at the rate of 1 mL per every five minutes. The solution was stirred at a speed of 500 rpm until the solution become slightly turbid. pH of the solution was maintained at 7. One Drop of 6% glutaraldehyde was added for cross linking of resultant nanoparticles.
Solvent was removed by rota evaporator [6]. The pH was the most important factor to control the coagulation of BSA molecules during the desolvation process. The isoelectric point (pI) of BSA is about 4.7. When the pH of solution was closed to the pI, the enhanced protein-protein reaction leads to increased coagulation among BSA molecules. At pH 7, BSA molecules possessed a negative charge and coagulation of BSA molecules reduced which allowed the formation of small particles.
Characterization and evaluation
The obtained nanoparticles were evaluated for Particle size, product yield, Drug content, entrapment efficiency and loading capacity.
Determination of particle size
Study of surface morphology of nanoparticles by scanning electron microscope (SEM): The prepared amorphous nanoparticles were dispersed in deionised water and sonicated for 30 minutes. A circular metal plate is taken on to which carbon double tape (1 mm×1 mm) is stickered; a drop of the resultant nano dispersion is placed on to the tape and allowed to dry for a while. Then it is scanned under SEM for morphology. The obtained nanoparticles were found to be spherical in shape [7] (Figure 2).
Product yield
The yields of the prepared nanoparticles were calculated. The dried nanoparticles were weighed and the yield of nanoparticles was calculated using the formula:
The product yield was observed as 93.9%.
Drug content
To determine the drug content, 50 mg drug equivalent to formulation was weighed accurately and transferred into three necked RBF containing 50 mL of methanol. The solution was stirred at 700 rpm for 3 hrs by using magnetic stirrer. The resultant solution was filtered and the amount of the drug in the filtrate was estimated after suitable dilution by ultraviolet (UV) spectrophotometer at 285 nm. The drug content was found to be 63.7%.
Figure 2:SEM images of BSA nanoparticles by continuous addition method.
Entrapment efficiency
Entrapment efficiency indicates the amount of drug encapsulated in the formulation. The method of choice for drug content determination is separation of free drug by ultra-centrifugation, followed by quantitative analysis of the drug from the formulation. The samples were centrifuged by using ultracentrifuge at 17640 rpm for 40 min [8].
Percentage entrapment efficiency may be calculated from the following formula:
The entrapment efficiency of the formulation was found to be 65.43%.
Loading capacity
The loading capacity (L.C) refers to the percentage amount of drug entrapped in nanoparticles [9]:
The loading capacity was observed as 28.4%
Conclusion
Mefenamic acid loaded BSA nanoparticles were prepared by desolvating technique using acetonitrile as desolvating agent. Intermittent addition method was followed for the addition of desolvating agent to the aqueous solution of BSA. SEM Images reveals that the formulations was found to be in Nano range. The product yield and drug content were found to be 93.9% and 63.7% respectively. The entrapment efficiency and loading capacity were observed as 65.43% and 28.4% respectively. Mefenamic acid nanoparticles were prepared by desolvation technique successfully using acetonitrile as desolvating agent and the results reveals that the entrapment efficiency and loading capacity were found to be good and the formulation can be further studied for drug release and stability.
Dongmei Zhao, Xiuhua Zhao, Yuangang Zu, Jialei Li, Yu Zhang, et al. (2010) Preparation characterization and In vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. International Journal of Nanomedicine 5: 669-677. [ Ref ]
Zhan Yu, Min Yu, Zhibao Zhang, Ge Hong, Qingqing Xiong, et al. (2014) Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Research Letters 9: 343. [ Ref ]
Ramdhan Yadav, Dharmesh Kumar, Avnesh Kumari, Sudesh Kumar Yada (2014) Encapsulation of Catechin and Epicatechin on BSA nanoparticles improved their stable and antioxidant potential. EXCLI Journal 13: 331- 346. [ Ref ]
Bagul R, Mahajan V, Dhake A (2012) New approaches in nanoparticle drug delivery system-A Review. International Journal of Current Pharmaceutical Research 4: 29-38. [ Ref ]
Kumaresh SS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric nanoparticles as drug delivery devices. Journal of controlled release 7: 1-20. [ Ref ]
Sri Priyalakshmi S, Anjali CH, George PD, Rajith B, Ravindran A (2014) BSA Nanoparticle Loaded Atorvastatin Calcium-A New Facet for an Old Drug. PLOS ONE 9: 86-97. [ Ref ]
Sneha S, Swarnlatha S, Kaur CD, Shailendra S (2013) Biocompatible nanoparticles for sustained tropical delivery of anti-cancer phytoconstituent Quercetin. Pak J Biol Sci 16: 601-609. [ Ref ]
Aarti PN, Mukesh PR, Shilpa PC (2014) Nanoparticles- An Overview. Int. J. Res. Dev. Pharm. L. Sci 3: 1121-1127. [ Ref ]
Mahammad RS, Madhuri K, Panati D (2012) Polymers in controlled drug delivery systems. International journal of pharma sciences 2: 112-116. [ Ref ]
Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer based nanocapsules for drug delivery. International journal of pharmaceutics 385: 113-142. [ Ref ]
Mehrothra A, Pandit JK (2012) Critical process parameters evaluation of modified nanoprecipitation method on Lomustine nanoparticles and cytostatic activity study on L132 human cancer cell line. J Nanomedicine and Nanotechnology 3: 149. [ Ref ]
Mora-Huertas CE, Fessi H, Elaissari A (2011) Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification diffusion methods critical comparison. Advances in colloid and interface science 163: 90-122. [ Ref ]