Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 14 December, 2020
Accepted date: 05 January, 2021
Published date: 12 January, 2021
Citation: Ame SM, Kabole F, Abuubakar S, Nanai AM, Andemichael G, Djeunga HN (2021) An Early Indication of the Rebound of W. bancrofti Infection in Zanzibar. J Appl Microb Res. Vol: 4 Issu: 1 (18-24).
Copyright: © 2021 Ame SM et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:Lymphatic filariasis (LF) is a debilitating neglected tropical disease which was targeted for elimination through coadministration of a single dose of ivermectin and albendazole to the affected population. Following implementation of such a treatment campaign over a period of years, control programmes are urged to conduct a transmission assessment survey to monitor the impact of the treatment and to ascertain whether the transmission is interrupted to a level that no more transmission occur, and hence mass drug administration (MDA) can be halted.
Objective:This study was carried out to determine the prevalence of LF infection in Zanzibar communities, 13 years after stopping MDA, so as to inform and guide the control program.
Methodology:A Finger prick blood sample was collected from each participant after obtaining informed consent. The sample was assessed for the presence of Wuchereria bancrofti circulating antigen using rapid immunochromatographic test.
Results:A total of 2555 subjects were enrolled (1231 in Pemba and 1324 in Unguja) in the study with a mean age of 23.0yrs ± SD 18.9 (95% CI = 22.3-23.8). There were more female (53.9%) than male (46.1%); and their mean age significantly differed (t-test = 7.5, p = 0.00001). Only 2478 individuals gave blood sample with a response rate of 97%. Of them, 88 (3.55%) were found to be infected with W. bancrofti. Overall, the prevalence of infection was higher in Pemba (5.1%) than in Unguja (2.1%). The prevalence of infection was similar between different older age groups, children aged 1-5yrs in Pemba had the highest prevalence indicating that transmission is ongoing.
Development of clinical manifestation as: lymphoedema and hydrocele was also assessed. Overall, only 1.1% of the individuals had lymphoedema/elephantiasis; with males presented with more of those signs (1.6%) than females (0.7%). There was no male subject found to have hydrocele, although 8.3% of males had filariasis.
The assessment of treatment history revealed that majority (64.7%) of the respondents had received at least one treatment round during their lifetime. The history of treatments with ivermectin did not correlate with current individual levels of infection but individuals who reported to have received 2 and 4 rounds of treatment were not found to be infected with filariasis.
Conclusion and Recommendation:Prevalence of LF in Zanzibar is still high to exceed the set threshold for discontinuation of MDA campaign. Children as young as 5yrs were found infected. It is therefore important to consider continuation of MDA so as to prevent potential disease sequel which might develop.
Keywords
Lymphatic filariasis, Elimination, Preassessment survey, Zanzibar.
Abstract
Background:Lymphatic filariasis (LF) is a debilitating neglected tropical disease which was targeted for elimination through coadministration of a single dose of ivermectin and albendazole to the affected population. Following implementation of such a treatment campaign over a period of years, control programmes are urged to conduct a transmission assessment survey to monitor the impact of the treatment and to ascertain whether the transmission is interrupted to a level that no more transmission occur, and hence mass drug administration (MDA) can be halted.
Objective:This study was carried out to determine the prevalence of LF infection in Zanzibar communities, 13 years after stopping MDA, so as to inform and guide the control program.
Methodology:A Finger prick blood sample was collected from each participant after obtaining informed consent. The sample was assessed for the presence of Wuchereria bancrofti circulating antigen using rapid immunochromatographic test.
Results:A total of 2555 subjects were enrolled (1231 in Pemba and 1324 in Unguja) in the study with a mean age of 23.0yrs ± SD 18.9 (95% CI = 22.3-23.8). There were more female (53.9%) than male (46.1%); and their mean age significantly differed (t-test = 7.5, p = 0.00001). Only 2478 individuals gave blood sample with a response rate of 97%. Of them, 88 (3.55%) were found to be infected with W. bancrofti. Overall, the prevalence of infection was higher in Pemba (5.1%) than in Unguja (2.1%). The prevalence of infection was similar between different older age groups, children aged 1-5yrs in Pemba had the highest prevalence indicating that transmission is ongoing.
Development of clinical manifestation as: lymphoedema and hydrocele was also assessed. Overall, only 1.1% of the individuals had lymphoedema/elephantiasis; with males presented with more of those signs (1.6%) than females (0.7%). There was no male subject found to have hydrocele, although 8.3% of males had filariasis.
The assessment of treatment history revealed that majority (64.7%) of the respondents had received at least one treatment round during their lifetime. The history of treatments with ivermectin did not correlate with current individual levels of infection but individuals who reported to have received 2 and 4 rounds of treatment were not found to be infected with filariasis.
Conclusion and Recommendation:Prevalence of LF in Zanzibar is still high to exceed the set threshold for discontinuation of MDA campaign. Children as young as 5yrs were found infected. It is therefore important to consider continuation of MDA so as to prevent potential disease sequel which might develop.
Keywords
Lymphatic filariasis, Elimination, Preassessment survey, Zanzibar.
Background
The World Health Organisation (WHO) has set an ambitious goal of elimination of a number of neglected tropical diseases (NTDs) through a combination of strategies including mass drug administration (MDA), often recognised as preventive chemotherapy. The MDA strategy has been demonstrated as an effective controlling measure for some of the NTDs, though its effectiveness depends on various factors such as a compliance and coverage [1]. Lymphatic filariasis (LF) is one of the NTDs targeted for elimination [2]. During the global era of elimination of LF (Global Program for Elimination of Lymphatic Filariasis [GPELF]), preventive chemotherapy using a combination of oral single dose of ivermectin (IVM) and albendazole (ALB) was administered in communities across endemic areas [3]. Such treatment campaigns eliminated or interrupted LF in most, if not all, countries after at least 5-6 rounds of its distribution [4]. Consequently, many endemic countries halted or scaled down distribution of ivermectin. Despite the earlier reports which suggested termination of drug distribution following 5-6 rounds (WHO, 2011), in fact the exact number of treatments rounds needed to implement such decision was unclear. For example some endemic countries have implemented up to 14 rounds of MDA [5,6]. The prematurely termination of drug distribution has in part, contributed to rebounce of infection in some endemic countries [7].
Zanzibar has been long recognised as endemic for LF and so embarked in its elimination using the above-mentioned strategy similar like other LF endemic countries. Because of the stipulated number of treatment rounds (5-6), Zanzibar provided IVM and ALB for 6yrs from 2001-2006 [8-11]. As a result, LF infection was remarkably reduced to reach the elimination threshold (<1%) [11]. Following that achievement, again similar to other countries, Zanzibar stopped administration of IVM, and ALB directed towards LF. Ever since, there was no other intervention specifically targeted against LF, however, the continued efforts in malaria prevention/control measures and morbidity control for soil transmitted helminthiasis (STH) through provision of ALB, carried out in the Islands, indirectly benefited the LF control as demonstrated elsewhere [4, 12-15].
As a part of the monitoring and evaluation, LF control programmes was urged to conduct transmission assessment survey (TAS) , to understand if transmission has been completely interrupted and to demonstrate that there was no new LF cases in the communities. Zanzibar conducted its first, and indeed the only one to date, TAS in 2012 [16]. In that assessment the overall prevalence of infection was above 1% [16] and so warranted repeating of MDA with IVM. However, this was irregularly carried out between (2013, 2014 and 2017). This present survey was conducted to assess the current status in terms of transmission of LF in communities on Pemba and Unguja islands to guide the NTD program on implementation of its control strategies and to understand if there is any early indication of a rebound of LF infection.
Methodology
Selection of study sites
To ensure there is representation from each Island, the two Islands (Unguja and Pemba) were purposively selected, and each Island was considered as an independent evaluation unit (EU). The participated Shehias were selected based on the population size (~500 individuals/Shehia as determined in 2012 national population census) and their previous history of high prevalence of infection observed in the TAS conducted in 2012 [16]. Efforts were made to ensure at least one site (shehia) was selected from each district. However, for various logistical reasons, that was not always possible.
Enrolment of participants and sample collection
The study field team in collaboration with shehia leaders, conducted a community mobilisation meeting and requested community members to turn-up to the designated sample collection area. The interested individuals were asked for their consent for participation. The consent was administered orally. For participants <18yrs, parents/ guardian were asked to consent on their behalves. Attempts were made to ensure reasonable number of males and females of all age groups are enrolled. From each participant, a finger prick blood sample was collected and immediately analysed for the presence of Wuchereria bancrofti antigen using a rapid immunochromatographic test (ICT), Filariasis Test Strip (Alere™, (Alere Scarborough, Inc)). The test was performed as per manufacturer’s instruction. Briefly, a finger prick was made and a whole blood sample was drawn using a capillary micropipette provided in the test kit. The sample was placed on the sample window and left uninterrupted at room temperature (RT) for 10 minutes. Thereafter, the test result was recorded. The sample was considered positive if visible purple lines were observed at the control and test windows. The sample was considered negative if only the control purple line was observed.
Once the blood sample was collected, each participant was requested to enter an examination room where physical examination and questionnaire were administered. Specifically, the questionnaire was meant to collect demographic information, treatment history, residency and travel history.
Data management and analysis
Data were double entered using an Epidata version 3.1 (Epidata Association, Odense, Denmark), the data were then cleaned and imported to excel and later imported to Stata version 13 (StataCorp, College Station) for analysis. The Chi Square test (χ2) was used to assess association of categorical data and the probability of ≤0.05 was considered as significant.
Result
During the month of March 2019, a survey to investigate the prevalence of filariasis following intermittent provision of treatment in communities was carried out in 8 Shehias in both Unguja and Pemba (Table 1). A total of 2555 subjects were enrolled in the study. Although, for some reasons, the demography of some of the subjects was not recorded. Nevertheless, the participants had a mean age (23.0 yrs. ± SD 18.9 [95% CI = 22.3-23.8]). The age distribution of the participants is shown in figure S1. Albeit it appeared that the number of participants in some of the age-groups (6-10 and 11-14) was higher in Unguja (Figure S2). There was slightly higher proportion of female (53.9%) than male (46.1%) enrolled in the study. Moreover, the mean age between the sexes significantly differed (25.6yrs vs. 20).
Unfortunately, not all the enrolled subjects gave blood sample for testing. Thus for determination of the prevalence of infection, only 2478 subjects were considered. Overall, 88 (3.55%) individuals were infected with W. bancrofti as determined by the detection of filarial antigen. Interestingly there was no association of infection between sexes (3.5 vs. 3.6% for female and male respectively); p = 0.8.
Table 1: Purification Table for Pectinase from Bacillus subtilis.
| S/no. | Shehia name | No. of subjects | District | Island |
|---|---|---|---|---|
| 1 | Finya | 294 | Wete | Pemba |
| 2 | Maziwa Ng’ombe | 309 | Micheweni | Pemba |
| 3 | Mkokotoni | 353 | Kaskazini A | Unguja |
| 4 | Mzuri | 307 | Kusini | Unguja |
| 5 | Ngwachani | 303 | Mkoani | Pemba |
| 6 | Nungwi | 324 | Kaskazini A | Unguja |
| 7 | Ole | 325 | Chake-chake | Pemba |
| 8 | Pangawe | 340 | Magharibi B | Unguja |
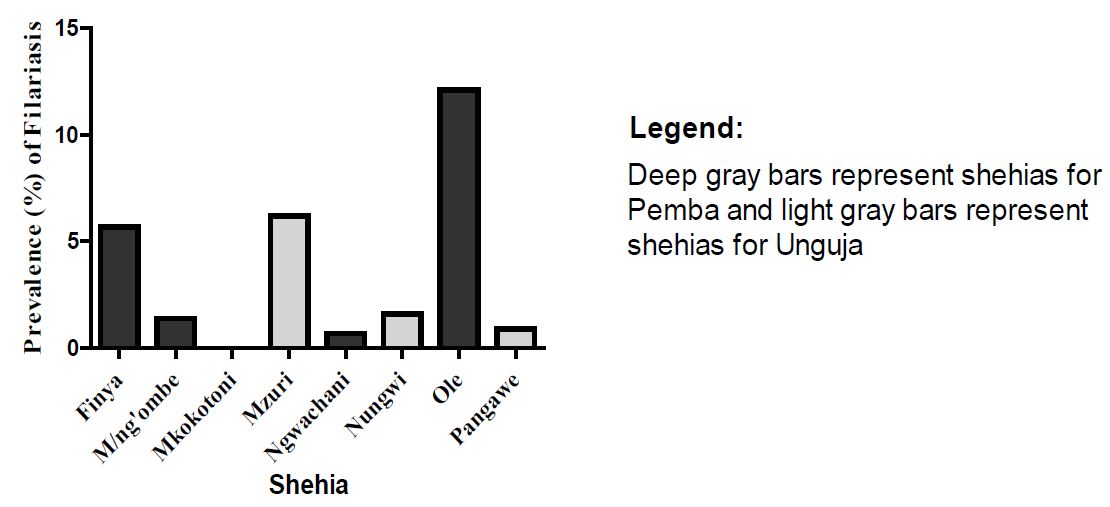
Figure 1: Prevalence of filariasis across different Shehias.
The analysis of filariasis infection in Shehias revealed a varying prevalence; with Ole having by far the highest (12.1%) prevalence and with no infection in Mkokotoni (0.0%) (Figure 1), though, most of the surveyed Shehias had evidence of filariasis. The proportion of the infected individuals observed in Ole alone, equated to 44.3% of the combined infection across the whole sample.
With regards to the prevalence of infection across the Islands, it showed that Pemba had higher prevalence (5.1%) than Unguja (2.1%).
With regards to the prevalence of infection across the Islands, it showed that Pemba had higher prevalence (5.1%) than Unguja (2.1%).
Prevalence of filaria infection in different age groups.
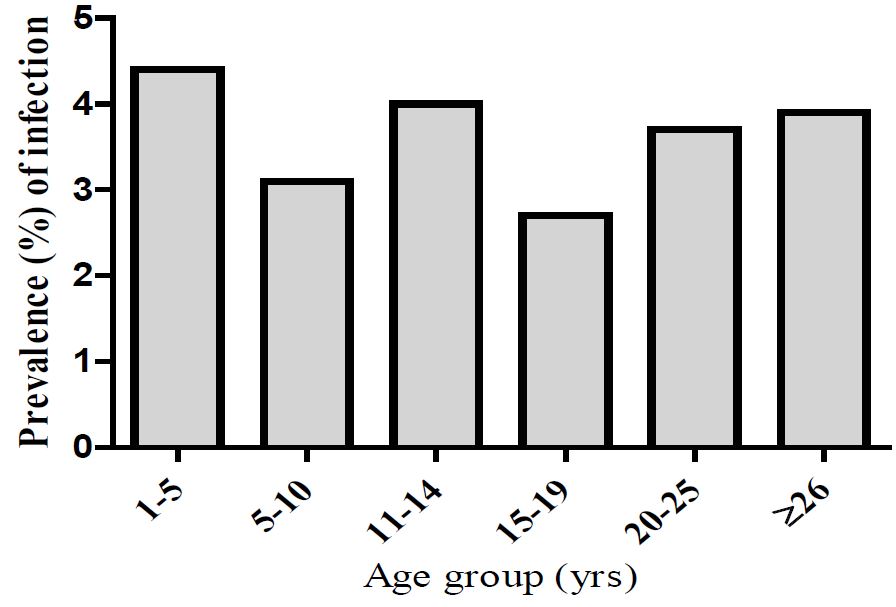
The prevalence of filariasis infection in different age groups is shown in figure 2. Generally, the prevalence of infection was similar between the different age groups, although younger children (aged between 1-5yrs) had a slightly higher infection rate.
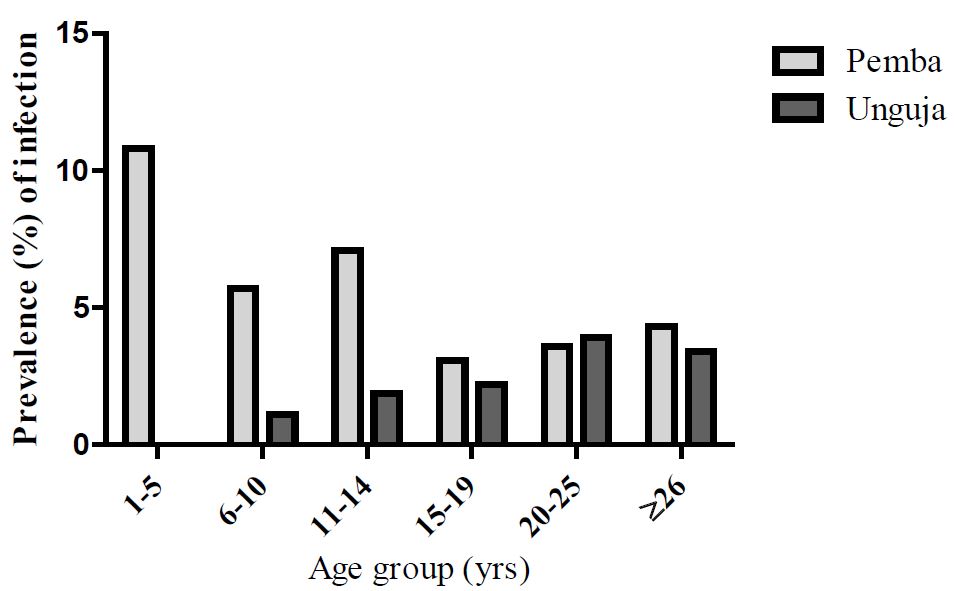
The further analysis of the data across different age groups and between Pemba and Unguja islands is displayed in figure 3 which showed a contrasting pattern. For Pemba, the pattern of filariasis was somewhat inconsistent in children (1-14yrs), though was more prominent in very young age group (1-5yrs). The lowest infection rate was observed for those aged from 15-19 ys old then the infection steadily increased in older adults age groups. Whilst for Unguja, there was more consistent pattern and the prevalence steadily increased with age with highest infection rate detected at age group of 20-25yrs (Supplementary figures S1).
Figure 2: Prevalence of filariasis infection in different age groups in Zanzibar.
Figure 3:Prevalence of filariasis infection between Pemba and Unguja among different age groups.
Assessment of clinical manifestations resulting from filariasis
In addition to collection of blood samples, development of clinical manifestation resulting from filariasis namely lymphoedema or elephantiasis and hydrocele were also observed. As our survey was community based, in some areas the environment was non-conducive to conduct thorough physical examination thus not all the subjects were examined for the presence of clinical manifestations especially hydrocele. Only 338 male subjects agreed for that. Of them, none had hydrocele, although 8.3% of them were found to be positive for filarial antigen; indicating presence of filariasis infection.
Similarly, only 936 subjects were examined for detection of lymhoedema or elephantiasis. Of these, 1.1% (10) was found to have lymphoedema/elephantiasis. Interestingly there were more male subjects (1.6%) who had lymphoedema/elephantiasis than female (0.7%). Albeit the presence of lymphoedema/elephantiasis was statistically not associated with sex (χ2 = 1.6, p = 0.2). Note 47 (3.5%) of the females were positive for LF antigen in their blood test compared to 41 (3.6%) males.
Assessment of treatment history
We have assessed treatment history based on mass drug administration (MDA) directed towards control of filariasis (for the subjects) to understand how frequency of treatment could have impacted transmission of filariasis infection in communities. Individuals aged ≦7yrs were not considered for this assessment as they were ineligible when they were <5yrs and also there was no treatment campaign in 2016 and 2017 (by this time those children could have turned to 7yrs). A total of 1199 individuals responded to the question. Of these 64.7%, 11.2%, 11.2% and 12.9% had received 1 (one), 2 , 3 and 4 rounds of treatment, respectively. Further analysis showed an inconsistent trend by detecting infection (3.0% and 5.3%) in individuals who reported receiving, respectively 1 and 3 treatment rounds. All individuals who reported to receive 2 and 4 rounds were not found to be infected with filariasis.
Discussion and Conclusion
The suffering and debilitating effect of LF is well appreciated and some initiatives have been put in place to alleviate those suffering. Indeed in responding to the outcomes of infection, the WHO has endorsed resolution WHA 50.29 to urge endemic countries to eliminate LF as a public health problem and also focus on management of clinical conditions [17,18]. The potential for elimination of the infection was boosted following advancement in disease diagnosis and the availability of the potent antimicrofilarial drug, ivermectin [3]. It was demonstrated that regular distribution of a single oral dose of ivermectin over a period of years could successful interrupt transmission of LF and possibly eliminate the infection.
We report here the finding of the assessment of LF transmission in communities which have inconsistently received ivermectin treatment after stoppage in 2006. This survey followed a similar one conducted in [16]. In this present study an overall prevalence of 3.55% was demonstrated, though, varied between the communities (Figure 1). Interestingly the prevalence of infection was very similar between males (3.6%) and females (3.5%) and so the infection was not associated with sexes. Generally the proportion of infection was higher in Pemba (5.1%) than in Unguja (2.1%). Moreover, the prevalence of infection across the different age groups was similar, although a much higher rate was among young children (1-5yrs). When the data were further stratified in relation to age-groups and islands, overall, in Pemba, the prevalence was more prominent in younger children (1-5yrs) but there was inconsistent pattern for other age groups. For Unguja the prevalence of infection steadily increased with age groups with highest infection rate detected in adult age groups. Specifically, a steadily increase of infection rate was found in individuals aged 15yrs. Our finding of overall prevalence of 3.55% is slightly higher than what has been detected previously in the same setting [16]. The reason for observation of an overall increase of and/ or decrease of infection between the two Islands has not been immediately established as there has no interventions carried out in all Islands specifically directed towards LF, other than side benefit which could result from malaria control initiatives and STH infections- through provision of ALB. All environmental factors including rainfall patterns between the Islands remained almost constant between the two surveys. Nevertheless, it should be noted that in the earlier study [16], only schoolchildren aged between 6-7yrs were investigated, as carried out in other endemic countries [19]. Whilst the present study investigated community members with a wide age range (2-86yrs). There are mixed perceptions on whether conducting a school-based or community-based TAS but other authors have drawn attention on possibility of leaving out non-school attendants some of whom might have not received the drugs [6]. For examples in American Samoa, high prevalence of filariasis was observed in community-based assessment (6.2%) than in school-based survey (0.7%) [20]. Of note, despite variation in the number of subjects (3275 for 2012 vs. 2478 for 2019) and the age range between those two studies, the actual number of infected individuals was very similar (89 (2012) vs. 88(2019)). As aforementioned, the present study has observed LF infection in wide range of age groups, though slightly higher in children 1-5yrs. This finding is very alarming as these young children haven’t been treated with ivermectin and indicates active transmission of infection in communities. Though, the study done by Witt and Ottesen showed that in LF endemic areas children become infected at very early age [21].
With regards to observation of clinical conditions, our study has demonstrated that none of the male subjects (agreed for physical examination) had hydrocele, albeit 8.3% of them were found to have filariasis. Similarly, only small proportions (1.1%) of individuals were found to have lymphoedema/elephantiasis. The finding of the absence of development of hydrocele or low proportion of individuals with lymphoedema or elephantiasis could be possibly due to low parasite density or an acquired immunity, though microfilaria density was not established in this present study. There are limited studies investigated the relationship of development of filariasis associated morbidity and worm burden but the existing evidence and experience from other nematodes infections indicates strong association between worm burden and progression to chronic clinical manifestations [22]. Nevertheless, it should be noted that progression of those chronic stage of infection especially lymphoedema is a slow process which mostly occurs in older age and rarely found in individuals aged ~20yrs [23]. This observation might true, as in this present study, many of the infected individuals (66 [3.5%]) were aged ≤ 35yrs (this analysis was not shown). Moreover, a longitudinal study conducted in Tanzania to assess development of hydrocele and lymphoedema revealed no association of the presence of microfilaremia and development of hydrocele or lymphoedema [24].
The present study also assessed treatment history relative to LF based MDA in the population. Generally most individuals received at least one round of IVM – MDA at certain point of time, however, many of them didn’t receive subsequent treatments. The present study revealed that only 12.9% of the respondents received 4 rounds of IVMMDA, a minimum number of treatment rounds that could achieve elimination. Surprisingly, an inconsistent trend of prevalence of infection was observed in relation to treatment history. Specifically, we have found substantial proportions (3.0% and 5.3%) of infection in individuals received one or three rounds of treatment respectively but not in individuals received 2 or 4 rounds of treatment. As hypothesized that, for LF based MDA at least 5 rounds of treatment with reasonably coverage (~65%) are required to successfully interrupt transmission and eventual eliminate the disease [3,10]. It is sensible to believe that our finding of the LF infection among previously treated individuals could not be a surprising phenomenon for many reasons including (i) many individuals did not constantly receive repeated doses (sub-optimal treatment) of the drugs and that possibly had residual microfilaremia (ii) there was low drug coverage and so some members of the communities were in fact not treated (iii) possibility of treatment failure. Although, the former might may play a little role as treatment failure has only been reported with diethylcarbamazine (DEC), another drug used in MDA to combat LF, but not with IVM [25].
In view of our findings it clearly shows that the prevalence of LF in Zanzibar is still high to exceed the set threshold for discontinuation of MDA campaign. Children as young as 5yrs were found infected. It is therefore important to consider continuation of MDA so as to prevent potential disease sequel which might develop.
Study Limitation
This study investigated LF in only 8 shehias (4 in each Island i.e. Unguja and Pemba), the situation which doesn’t provide the true reflection of the prevalence of LF in Zanzibar.
Declaration
Ethics statement
The ethical approval was obtained from the Zanzibar Medical Research and Ethics Committee (ZAMREC, reference no. ZAMREC 0003/Nov/2018) in Zanzibar, United Republic of Tanzania. The study participants were informed about the study objectives and procedures. It was intended to obtain a written consent from all participants (adults and minors) prior to sample collection but this wasn’t possible due limited time in the field. However, participation was voluntary and participants could withdraw or be withdrawn.
The consent to publish this work was also granted by the ZAMREC.
Conflict of interest
The authors declared no conflict of interest exists.
Funding
This study was funded by the World Health Organisation.
Smits HL (2009) Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti Infect Ther 7: 37-56. [ Ref ]
WHO (2010) Progress report 2000-2009 and strategic plan 2010-2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. World Health Organization. [ Ref ]
Ottesen EA (2000) The global programme to eliminate lymphatic filariasis. Trop Med Int Heal 5: 591-594. [ Ref ]
Rebollo MP, Sambou SM, Thomas B, Biritwum NK, Jaye MC, et al. (2015b) Elimination of lymphatic filariasis in the Gambia. PLoS Negl Trop Dis 9. [ Ref ]
WHO (2011) Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphatic filariasis: a manual for national elimination programmes. World Health Organization. [ Ref ]
Biritwum NK, Frempong KK, Verver S, Odoom S, Alomatu B (2019) Progress towards lymphatic filariasis elimination in Ghana from 2000- 2016: Analysis of microfilaria prevalence data from 430 communities. PLoS Negl Trop Dis 13: e0007115. [ Ref ]
Boyd A, Won KY, McClintock SK, Donovan CV, Laney SJ, et al. (2010) A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis 4: e640. [ Ref ]
Michael E, Malecela-Lazaro MN, Maegga BTA, Fischer P, Kazura JW (2006) Mathematical models and lymphatic filariasis control: monitoring and evaluating interventions. Trends Parasitol 22: 529-535. [ Ref ]
Ottesen EA (1998) The global programme to eliminate lymphatic filariasis [WWW Document]. Parasitol Int 47: 46. [ Ref ]
Stolk WA, Swaminathan S, Oortmarssen GJ, Das PK, Habbema JDF (2003) Prospects for elimination of bancroftian filariasis by mass drug treatment in Pondicherry, India: a simulation study. J Infect Dis 188: 1371-1381. [ Ref ]
Mohammed KA, Molyneux DH, Albonico M, Rio F (2006) Progress towards eliminating lymphatic filariasis in Zanzibar: a model programme. Trends Parasitol 22: 340-344. [ Ref ]
de Souza DK, Ansumana R, Sessay S, Conteh A, Koudou B, et al. (2015) The impact of residual infections on Anopheles-transmitted Wuchereria bancrofti after multiple rounds of mass drug administration. Parasit Vectors 8: 488. [ Ref ]
Eigege A, Kal A, Miri E, Sallau A, Umaru J, et al. (2013) Long-lasting insecticidal nets are synergistic with mass drug administration for interruption of lymphatic filariasis transmission in Nigeria. PLoS Negl Trop Dis 7: e2508. [ Ref ]
Kelly-Hope LA, Molyneux DH, Bockarie MJ (2013) Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasit Vectors 6: 39. [ Ref ]
Pion SDS, Chesnais CB, Weil GJ, Fischer PU, Missamou F, et al. (2017) Effect of 3 years of biannual mass drug administration with albendazole on lymphatic filariasis and soil-transmitted helminth infections: a community-based study in Republic of the Congo. Lancet Infect Dis 17: 763-769. [ Ref ]
Rebollo MP, Mohammed KA, Thomas B, Ame S, Ali SM, et al. (2015a) Cessation of mass drug administration for lymphatic filariasis in Zanzibar in 2006: was transmission interrupted? PLoS Negl Trop Dis 9: e0003669. [ Ref ]
Addiss DG (2013) Global elimination of lymphatic filariasis: a “mass uprising of compassion.” PLoS Negl Trop Dis 7: e2264. [ Ref ]
Molyneux D (2003) Lymphatic filariasis (elephantiasis) elimination: a public health success and development opportunity. Filaria J 2: 13. [ Ref ]
Dewi RM, Tuti S, Ganefa S, Anwar C, Larasati R, et al. (2015) Brugia RapidTM antibody responses in communities of Indonesia in relation to the results of ‘transmission assessment surveys’ (TAS) for the lymphatic filariasis elimination program. Parasit Vectors 8: 499. [ Ref ]
Sheel M, Sheridan S, Gass K, Won K, Fuimaono S, et al. (2018) Identifying residual transmission of lymphatic filariasis after mass drug administration: Comparing school-based versus community-based surveillance-American Samoa, 2016. PLoS Negl Trop Dis 12: e0006583. [ Ref ]
Witt C, Ottesen EA (2001) Lymphatic filariasis: an infection of childhood. Trop Med Int Heal 6: 582-606. [ Ref ]
Michael E, Grenfell BT, Bundy DAP (1994) The association between microfilaraemia and disease in lymphatic filariasis. Proc R Soc London Ser B Biol Sci 256: 33-40. [ Ref ]
Chan MS, Srividya A, Norman RA, Pani SP, Ramaiah KD, et al. (1998) Epifil: a dynamic model of infection and disease in lymphatic filariasis. Am J Trop Med Hyg 59: 606-614. [ Ref ]
Meyrowitsch DW, Simonsen PE, Makunde WH (1995) A 16-year followup study on bancroftian filariasis in three communities of north-eastern Tanzania. Ann Trop Med Parasitol 89: 665-675. [ Ref ]
Mahoney LE, Kessel JF (1971) Treatment failure in filariasis mass treatment programmes. Bull World Health Organ 45: 35-42. [ Ref ]