Journal Name: Journal of Applied Microbiological Research
Article Type: Review
Received date: 12 October, 2017
Accepted date: 17 October, 2017
Published date: 24 November, 2017
Citation: Koiri RK, Naik RA, Rawat D, Chhonker SK, Ahi JD (2017) Bioecological Perspective of Entomopathogenic Fungi with Respect to Biological Control. J Appl Microb Res Vol: 1, Issu: 1 (07-14).
Copyright: © 2017 Koiri RK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Entomopathogenic fungi are anticipating alternatives to chemical insecticides. Indeed, fungal entomopathogens have been widely looked into as biological control agents of insect’s pest in efforts to improve the sustainability of crop protection. Fungal entomopathogens have evolved some elaborate relations with arthropods, plants and other microorganisms. Critical parameters for choosing a fungal pathogen for its role in biocontrol include the cost-effective fabrication of a stable, infective propagule that is appropriate for use in the environment where the insect must be controlled. Efficiency and cost are the two valuable parameters that need to be looked at while comparing the entomopathogens (biopesticides) with the conventional chemical pesticides. In addition to effectiveness, there are advantages in employing microbial control agents, such as human safety and other non-target organisms; pesticide residues are downplayed in food and biodiversity would be increased in managed ecosystems. Production of enzymes at large scale and some other metabolites in grade of increasing the entomopathogenic fungi virulence, in the control of insects and potentially in some diseases affecting plants, opens new potentialities in order to enhance the entomopathogenic fungi in use. This approach of using biocontrol agents instead of chemical pesticides seems to be very promising in the coming years as it heads towards sustainable agricultural practices and defending surroundings, which is the need of the hour.
Keywords
Entomopathogenic fungi; Biological control.
Abbreviations and Acronyms:IPM (Integrated Pest Management); EST (Expressed Sequence Tag); ETPs (Epipoly-thiodioxo-piperazines); HA (Hazianum A); JA (jasmonate); SA (Salicyclic Acid); ZrMEP (zincdependent metalloprotease)
Abstract
Entomopathogenic fungi are anticipating alternatives to chemical insecticides. Indeed, fungal entomopathogens have been widely looked into as biological control agents of insect’s pest in efforts to improve the sustainability of crop protection. Fungal entomopathogens have evolved some elaborate relations with arthropods, plants and other microorganisms. Critical parameters for choosing a fungal pathogen for its role in biocontrol include the cost-effective fabrication of a stable, infective propagule that is appropriate for use in the environment where the insect must be controlled. Efficiency and cost are the two valuable parameters that need to be looked at while comparing the entomopathogens (biopesticides) with the conventional chemical pesticides. In addition to effectiveness, there are advantages in employing microbial control agents, such as human safety and other non-target organisms; pesticide residues are downplayed in food and biodiversity would be increased in managed ecosystems. Production of enzymes at large scale and some other metabolites in grade of increasing the entomopathogenic fungi virulence, in the control of insects and potentially in some diseases affecting plants, opens new potentialities in order to enhance the entomopathogenic fungi in use. This approach of using biocontrol agents instead of chemical pesticides seems to be very promising in the coming years as it heads towards sustainable agricultural practices and defending surroundings, which is the need of the hour.
Keywords
Entomopathogenic fungi; Biological control.
Abbreviations and Acronyms:IPM (Integrated Pest Management); EST (Expressed Sequence Tag); ETPs (Epipoly-thiodioxo-piperazines); HA (Hazianum A); JA (jasmonate); SA (Salicyclic Acid); ZrMEP (zincdependent metalloprotease)
Introduction
The Kingdom Fungi is one of the foremost groups of eukaryotic microorganisms in terrestrial ecosystems [1]. There are just about 100,000 depicted species of Fungi [2], which only constitutes a fraction of its variety, estimated to be between 1.5 and 5 million species [3,4]. Significantly, one of the trademarks of fungi is their tendency to form intimate interactions and associations with other groups of life on Earth [5]. According to Hawksworth [6], 21% of all species of reported fungi are linked with algae as lichens and 8% form intimate affiliation with plants as mycorrhiza, being this an outstanding example of such close relationship, which occur in rhizosphere (around roots), where arbuscular mycorrhizal and ectomycorrhizal fungi form relations with plants. Few if any organisms in terrestrial ecosystems subsist in nature in the complete nonexistence of fungi and for this reason they are vital players in the maintenance of ecosystem health. Another group of magnitude are the Oomycetes. These are so-called water molds and fit into a very distant Kingdom (Stramenopila), more closely associated to brown algae [7]. However, it is suitable to confer them with fungi as they were long regarded to be fungi and are ecologically very similar. Entomopathogenic fungi are found out in the divisions of Zygomycota and Ascomycota (Table 1). [8], as well as the Chytridiomycota (fossil fungi) and Oomycota, which were earlier classified within the fungi. Many of the taxa of entomopathogenic fungi currently under research either fit into the class Entomophthorales in the Zygomycota or the class Hyphomycetes in the Deuteromycota.
| Division | Class | Order | Family | Genus |
|---|---|---|---|---|
| Zygomycota | Zygomycetes | Entomophthorales | Entomophthoraceae | Entomophaga Entomophthora Erynia Eryniopsis Furia Massospora Strongwellsea Pandora Tarichium Zoophthora |
| Ascomycota | Sordariomycetes | Hypocreales | Clavicipitaceae | BeauveriaCordyceps Cordycepioideus Metarhizium Nomurae Lecanicillium |
Table1: Current classification of the genera of entomopathogenic fungi.
The Insects with over 900,000 reported species symbolize the most species-richness groups of eukaryotes [9]. They are known to form close relationships with many fungal groups: mutualistic endosymbionts that support in nutrition [10], fungi as food sources that insects form [11], sexuallytransmitted parasites and commensals [12], and pathogens proposed to have prominent effects on host populations [13,14]. However, nevertheless we know many diverse fungal-insect associations exist and this area remains one of the most understudied areas of fungal diversity and likely entertains one of the largest reservoirs of undocumented fungal species [5]. A prominent characteristic of insects is a chitinous body covering, which the great preponderance of entomopathogenic fungi and oomycetes need to infiltrate and prevail over the cellular and humoral defences in the hemocoel [15]. Asexually produced fungal spores or conidia are normally responsible for infection and are disseminated throughout the surroundings in which the insect hosts are present. When conidia land on the cuticle of a suitable host, they fasten and germinate; initiating showers of recognition and enzyme activation reactions both by the host and the fungal parasite [8]. Incursion of the insect body and circulatory system (haemolymph) happens once the fungus has run through the cuticle of the external insect skeleton. Structures and actions for the invasion of insect tissues are similar to plant pathogens, including the formation of germ tubes, appresoria and penetration pegs [8]. After the colonization of the insect’s body, the fungi and water moulds require developing structures to produce and disseminate their spores. Most of the entomopathogenic fungi execute their hosts before spore production starts, but few of them, particularly some Zygomycota species, sporulate from the living body of their hosts [16]. Having provided a short introduction, the aim of this paper is to ask the role of entomopathogenic fungi in evolving the ability to exploit the insect body. For this purposes, we will ponder on examples of work with entomopathogenic fungi, which exemplify the principles or strategies which can be used to diminish losses by insect pests and the role played by these fungi in biological control with special reference to metabolites, ecological perspective and genetics level.
Entomopathogenic Fungi in Biological Control of Pests
Indeterminate and unstructured use of formal chemical insecticides has led to improvement in developing resistance towards various chemicals present in the plant protection products, in the insects. More than 500 species of the arthropods have become immune to more than one type of synthetic pesticides [17]. Incursive and highly persistent species that are brought in accidently to a new continent or country and escape their natural microorganisms and predators, pose another serious problem. Thus, there is a need to seek new, safer options of reducing the outbreaks of pests [18]. Biological control for agricultural systems is not a new initiative. During the last century greater than 2,000 non-native (exotic) control agents have been employed in at least 200 countries or islands with few predictable problems to flora, fauna or environment. Biological control of insect pests is steadily gaining momentum. Biological control is a module of an integrated pest management (IPM) strategy [19]. It is really viewed as a “systems approach” to IPM [20]. Biological control is delineated as the decrease of pest populations by natural enemies and generally involves an active human role. It admits the control of animals, weeds and disease. A biological control platform dilutes, but does not eliminate pests and it is used to suppress populations of pest organisms below levels that would have pessimistic economic impact [21]. Natural enemies used in biocontrol measures include parasitoids, predators, microbes and beneficial nematodes [22].
There are three universal approaches to biocontrol: (A) Classical biocontrol which is the rehearsal of diluting the populations of bizarre pests for long periods by the release of imported (exotic) natural enemies of the pest. Successful biological control is nearly permanent because the agent is permanently established [23]. (B) Augmentation biocontrol is the repeated release of natural enemies in periodic applications. Treatments (inoculations) may be small numbers during periods when pest populations are low, or large numbers of control agents may be released (inundative) as a remedial procedure for immediate results [24]. Control is usually attained by released individuals, not the off-spring. Inundative releases of biocontrol species that are not able to set up permanently are securer than classical releases. With augmented control repeated applications or additional methods may be used to maintain control. (C) Conservation control is the use of indigenous natural enemies.
Biocontrol models: group of fungi infecting major pests
Metarhizium anisopliae:First documented as a biocontrol agent in 1880’s, found in soil; used as a biocontrol agent against different insects and pests including beetles, spittle bugs and locusts [25]Zimmerman, 1993]. Different spores or conidial formulations of M. anisopliae are prepared and applied. After achieving the initiation of the fungal epizootic control, new spores and the vegetative cells are created in the infected insect. These spores rapidly extend to the healthy insect population and encourage persistent control.
Beauveria bassiana:Beauveria bassiana belongs to the class deuteromycete (fungi imperfecta). These are thread like fungi; different strains of Beauveria are highly introduced to a particular host insect. A broad range of medically or agriculturally noteworthy strains of Beauveria bassiana have been derived from various insects worldwide. B. bassiana possesses no well-known sexual cycle. Insects are infected by conidia (asexual propagules) which adhere to the host cuticle. Conidia grow in an environment with high humidity. The germ tubes arising from the conidia infiltrate the host cuticle and invade the haemocoel. A successful infection by B. bassiana is dependent mainly on various enzymatic activities for degradation of proteins, chitin and lipids in the insect integument [26,27].
Trichoderma:Various species belonging to Trichoderma genus are well recognized for their capability to generate industrially valid enzymes. Trichoderma species also have potential role in the biological control of plant pathogens. Mycoparasitism (kill/parasitize fungal pathogens) against the fungal microorganisms of crop plants is one of the major strategies of biocontrol, used by Trichoderma species. A number of signalling cascades are triggered against fungal pathogen during the mycoparisitic activity of Trichoderma. The Trichoderma species are detected in almost every region, throughout the world and are secluded simply from different soil forms, sporocarps and decomposing woods. Trichoderma species have been established as effective biocontrol agents against different pathogens, mostly soil borne which are causative agents of innumerable plant diseases. Strains of Trichoderma are widely used as an alternative in place of chemical pesticides to undertake many plant pathogens. This use is credited to their association and mycolytic activity and to the sensitivity to physiological changes mediated by host [28,29]. They generate various antimicrobial secondary metabolites like gliovirin, peptaibols and gliotoxin, which are known to inhibit numerous plant pathogens. Trichoderma virens and Trichoderma atroviride are the examples of two proven biocontrol species, these contain diverse reservoir of secondary metabolite biosynthetic genes [30,31]. Products of these genes aid for the secondary metabolite production and are linked with mycoparasitism by Trichoderma against other microbes. Steroids, terpenoids, pyrones and polyketides are some of the highly characterized secondary metabolites, these are non-polar in nature and possess low molecular mass.
Trichoderma spp. are well-known for the production of non-ribosomal peptides such as epipoly-thiodioxopiperazines (ETPs) and siderophores that are mainly antimicrobial in nature, these improve the cell wall lysis by acting in a synergistic manner with hydrolytic enzymes that are involved in cell wall dissolution [32]. Malmierca et al., [33] elucidated that the trichothecenes such as trichodermin and hazianum A (HA) are formed by Trichoderma species and disruption of the gene (tri gene) that impedes the amalgamation of trichothecenes is responsible for lowering the biocontrol efficiency against Botrytis cinerea and Rhizoctonia solani pathogens. Silencing of the tri4 gene contributes to down regulation of some defence genes of jasmonate (JA) and salicyclic acid (SA) pathways against B. cinerea in tomato plant whereas, the expression of these genes in the wild type strain is much higher. The results suggested that the pretreated plants were senisitized by the HA produced by Trichoderma, an increase in the expression of defence genes was also observed when they were challenged against B. cineria. Thus, Trichoderma species not only inhibit the proliferation of fungal pathogen but also improves the growth of treated plant and induce the expression of the defence genes. The widespread and special mechanisms prevailed in most of the Trichoderma spp. Include antagonism, parasitism, or even killing other fungi. The biocontrol efficient strains of Trichoderma spp. are found to successfully establish in the rhizosphere of the treated plants and encourage growth of plants and motivated defence responses when encountered by pathogens [34].
Pros and Cons associated with the use of entomopathogenic fungi as bio-control agent
Fungi exhibit higher degree of host specificity. They can be used for controlling the virulent insect pests without inducing any harm to beneficial insects. Advantages of using fungi as an insecticide are (1) less hazards found in contrast to chemical insecticide application, such as environmental pollution and the absence of effects on mammals, (2) Prolonged pest control and lack of insect resistance related problems, (3) Fungi show high degree of perseverance and hence present prolong pest control, (4) Further expansion in this field by biotechnological research can help in producing better options that can replace the chemical pesticides and insecticides.
New Insights into Ecological Role of Entomopathogenic Fungi
Insect pathogenic fungi in recent times have been shown to provide protection against insects, plant parasitic nematodes, and plant pathogens [35]. Beauveria bassiana and other entomopathogenic species have been reported endophytic in corn, tomato, cocoa, pine, opium poppy, date palm, bananas, and coffee. The mode of accomplishment largely remains anonymous although the entomopathogens are known to produce fungal metabolites that cause feeding deterrence or association. Some endophytic strains have been shown to infect insects in bioassay but field reports of infection after contact with endophytic plants are presently wanting. In addition to their endophytic abilities, there have been substantial and far attainment advances made in the soil and plant associated bionomics of entomopathogenic fungi. Not only have recent phylogenies emphasized the host jumping of plant and animal/invertebrate abilities among fungi [36], but new studies have confirmed that entomopathogenic fungi inhabit the rhizosphere and act as plant pathogen antagonists [35,37,38]. Building on the study of [39] where advanced endurance of a green-fluorescent protein expressing strain of Metarhizium anisopliae in the cabbage rhizosphere was shown, an expanded field trial is underway with Metarhizium anisopliae strains expressing two fluorescent protein genes, a deletion mutant of a gene highly expressed in the haemolymph of the host and mandatory for immune evasion [40] and a mutant of MAD2, a plant adhesive protein [41]. These field trials are planned to scrutinize the adaptations of Metarhizium anisopliae to survive in the soil and rhizosphere.
Role of Metabolites
A number of studies have sophisticated familiarity on the genetics and function of secondary metabolites and toxins from entomopathogens, especially Beauveria and Metarhizium, which can be useful in realizing infection procedures and developing biocontrol. Large EST (Expressed Sequence Tag) or genome studies have confirmed regulation of known enzyme or toxin genes during contact to the cuticle or other circumstances and several studies established the involvement of well known metabolites in virulence. Bassianolide, a cyclo oligomer depsipeptide secondary metabolite from Beauveria bassiana, was shown to be a highly noteworthy virulence factor through targeted deactivation studies. Interruption of bassianolide did not affect another metabolite, beauvericin [42], another cyclodepsipeptide, which was recognized as a nonessential virulence factor during transmission of Galleria mellonella, Spodoptera exigua, and Helicoverpa zea. Beauvericin was also highly toxic in vitro to cells of the fall armyworm, Spodoptera exigua [43]. Although, [44] established that another metabolite of Beauveria bassiana, tenellin, had no role in virulence. The cyclic depsipeptides destruxins, produced by Metarhizium anisopliae, have insecticidal, antiviral, and phytotoxic abilities and are also considered for their toxicity to cancer cells. Gene expression studies on Drosophila melanogaster following injection of destruxin showed a novel role for destruxin A in specific suppression of the humoral immune response in insects [45]. Subtilisins (Pr1) are known to be involved in virulence of some entomopathogenic fungi. Metarhizium strains with broad host ranges expressed up to 11 subtilisins during growth on insect cuticle [46] and up to 8 in Beauveria [47]. Pr1 was also shown to be upregulated during mycelial surfacing in the host [48], depicting that, as the nutrition within the host is depleted; Pr1 is upregulated to assist breaching the host cuticle again. A zinc-dependent metalloprotease, ZrMEP1, was derived from Zoophthora radicans, the first report of this type of metalloprotease from an entomopathogenic fungus. It appears to have a role in the infection process [49].
The Fungal Infection Cycle and Host Specificity
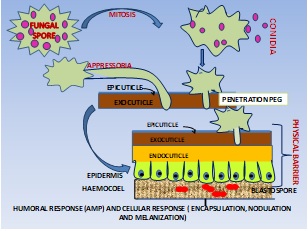
Entomopathogenic fungi recognize and infect insects through the spore adhesion and formation of appressoria that penetrate the cuticle (Figure 1). After reaching the hemocoel (body cavity) of an insect, fungal filaments will shift into yeast-like cells that experience budding for rapid proliferation and neutralize the immune response of the hosts. For the transmission cycle to complete, dead insects must be either mycosed to produce asexual conidial spores or colonized to form a fruiting body to yield sexual spores for the next infection. Diverse species of parasitic fungi have different insect host scopes and it has been observed that species, such as Beauveria bassiana and Metarhizium robertsii, can infect hundreds of insect species of various orders, whereas species such as Metarhizium acridum (specific to locusts and grasshoppers) [50,51], Cordyceps militaris (specific to caterpillars) [52], and O. unilateralis sensulato (specific to formicine ants), only infect a small number of insects [53,54]. While studying the fungal hostspecificity evolution, the study of Metarhizium species with different host ranges have shown a directional trajectory of speciation from being specialists to becoming generalists, and the process has been fixed with protein family expansions [50]. Specifically, the number of divergent G-protein coupled receptors is extensively correlated with host specificity [50,51].
Figure 1: Structure of insect cuticle and mode of penetration, thereby eliciting the humoral and immune response in the hemocoel
Field Application of Entomopathogenic Fungi
Laboratory tests always pave the way for the practical application of the entomopathogenic fungi in the classical or inundation biological control strategies. These tests are carried on for the selection of highly virulent strains, determining inoculum dosage, to observe the impacts of both biotic and the abiotic factors on the fungus used as biocontrol agent and to test different mode in which the fungi can be brought into the fields [55-59]. Although, sometimes these laboratory tests do not orchestrate later with the practical use of the entomopathogenic fungi, but they provide beneficial and relevant data about the activity and the possible role of the fungus in biocontrol of many dangerous pests [60]. The practical use of microorganisms is not easy; it is associated with numerous problems and the biggest problem being the difficulty to anticipate the effects of these microbes used as biocontrol agents before actually releasing them into the environment. Various factors on which the achievement of the field trials depends need to be taken into consideration. The event of lesser effectiveness of the biocontrol agent, utilized in the field in contrast to that observed in the laboratory tests is observed quite often. Many features of the entomopathogenic fungi like higher degree of virulence against the target species; no infestation in the nontarget organisms including animals and humans; resistance towards abiotic and biotic factors of the environment are determinative in attaining satisfactory results in the field trials [61,62]. The impact of entomopathogenic strains on the non-targets is always considered into account as side effects while the field application of the organism. It has been elucidated in various researches that entomopathogenic fungi exhibit very less impact on the non-target insects [63- 66]. Large-scale use of entomopathogenic fungi depends on economic and cheaper mass production of the synthetic media required. However, most of the fungal biopesticides are compiled up of the hypocrealen fungi, majority of them belonging to polyphagous species, representing broad host spectrum. Among different species, the entomophthoralen fungi are highly focused or monophagous and are not of great interest from the mycoinsecticides production point of view due to the problems in their proliferation and development on artificial medium and mass scale propagation of the infective material [67]. Barley kernels colonized by Beauveria brongniarti based product was tried for field applicability under BIPESCO-EU (Biological Pest Control) funded project, recently. The objective of the project was analysis and development of entomopathogenic fungi to control subterranean insect pests like weevils and scarabs [18]. Introduction of barley kernels colonized by fungus into the soil is the most commonly used method in case of soil dwelling pests. This approach has been utilized in order to control the populations of larvae of Melolontha melolontha in different crop varieties [68]. Using fungal bands that are infused with entomopathogenic fungi, is another common method used for biocontrol. The bands are placed near the trunk or around the branches of the tree and it provide protection against the invading pests. The method was first used to control Monochamus alternatus which is the major carrier of wilting in pines caused by Bursaphelenchus xylophylus [69]. Presently, the fiber band approach gives acceptable outcomes in biocontrol of Anoplophora glabripennis and Agrilusplani pennis invasive species [18].
Future Perspectives
Insects are exceptional models for studying host fungal interactions and immune responses. Recognition of host immune reactions or immune related molecules in Drosophila has already greatly benefited human health by aiding schemes for controlling human pathogens. Early studies on fungal diseases concentrated on molecules implicated in sensing and signalling, whereas more recent studies have started to scrutinize immunity using a more holistic access to provide a more complete perceptive of disease. These studies can disclose how immunity and its dysregulation can modulate whole body pathophysiology. They have also revealed that innate immune genes are under firm positive selection, which depicts that parasites inflict firm evolutionary pressures on their hosts. Certainly, the power of Drosophila genetics, counting purposeful genomics analysis, in combination with genetic and RNAi screens will maintain to offer a powerful approach for elucidating the regulation of insect immune genes accountable for antifungal reactions. This will in turn help us to examine and realize the evolutionary preservation of antifungal immune responses in vertebrate animals. It is likely that receptiveness to disease will involve alleles with various levels of penetrance and in which many ordinary or rare variants are caught up, each having an unpretentious effect. Association studies based on whole-genome sequencing of hundreds of wild Drosophila strains have recognised the genetic underpinnings of considerable heterogeneity in Drosophila populations regarding their responses to various stresses, including starvation [70-72]. Similar approaches incorporating whole-genome sequencing data, proteomic profiles, RNA-seq, mass spectrometry, epigenetic markers e.g., genome-wide methylation profiles), and evolutionary and population genetics data should assist in the recognition of functionally important genes and variants that lead to heterogeneity between individuals and populations of insects in terms of vulnerability to infection and disease progression. These studies will provide new insights into the physiological effects of microbial colonization and how immunity interacts with behaviour, metabolism, physiology, and hormonal regulation. They will persist to further our understanding of the genetic basis for any tolerance mechanisms and the means by which a host adjusts to infection and associated damage. They will also open up new routes for translational research into human and animal diseases and new schemes for insect pest control.
Change in the host behaviour during fungus-insect interactions are diverse, complicated, and of great scientific interest. Passive or active behavioural changes in insects are significant of evolutionary adaptations that either encourages cross-kingdom control by fungi or philanthropic behaviour by the hosts. There are still significant gaps in our understanding of the molecular mechanisms implicit in behavioural alterations in insects during their interactions with fungi. Due to the taxonomic diversity of both insects and fungi, the molecular machinery involved in insect behaviour changes could vary among the acting species pairs or function on a case-by-case foundation.
Conclusion
Insects have developed an alarming array of defences against microbes that are omnipresent in the environments that they inhabit. These expand from the cuticle that can be armoured with antimicrobial compounds and secretions to behavioural adjustments including induced fever, grooming, and burrowing to developmental curricula of moulting which successfully results in ablution of the outer surface of the insect to the enrolment of antibiotic or other defence compound giving rise to (symbiotic) bacteria. Furthermore, the potential role of plants in supporting either the (fungal) pathogen or the insect host cannot be ignored. In response, entomopathogenic fungi must breach the cuticle, detoxify host and/or endogenous microbial defences, equivocate grooming and other behavioural responses, and potentially restrain other pathogens and parasites. Regarding virulence, two hostile pressures are likely to be exerted on the fungus: (a) to specialize on specific (abundant) target hosts; and/or (b) to maintain broad host range. Fungal adaptive reactions may be arbitrated by epigenetic mechanisms that would allow for short-term interest while maintaining the broad host range potential. Current evidence is growing suggesting that a major factor driving the co-evolutionary arms race between the pathogen and the host occurs on the cuticular surface, and although noteworthy progress has been made in recent years, much regarding the molecular epitopes that reconcile these interactions in both the pathogen and the host remains to be uncovered. Further research examining genetic variation regarding cuticular degradative processes amongst hypervirulent fungal strains on the one hand, and cuticular defence responses in resistant insect species on the other, is warranted. One of the best utilization of entomopathogenic fungi is when complete wipe out of the pest is not needed instead; the pest populations are managed to a nominal level below which they could not be able to cause any effect on production or wealth of crop production. However, more work needs to be done in this field to develop stable, easy to produce, cost effective and easy to apply formulations of the same.
It is clear that realizing the genetics, biology, and ecology of entomopathogenic fungi is entering a new era. New insights into the ecological roles these fungi occupy, have been strengthened by advances in the ‘‘omics,’’ contributing awareness on the genetics underpinning substrate exploitation and infective determinants. The phylogenetic associations between the fungi are also becoming clearer, showing new and interesting links to other fungal groups. We may yet see entomopathogenic fungi fully live up to their potential as extensive and resourceful control agents of invertebrate pests.
Acknowledgement
RAN, DR, SKC thanks Dr. Harisingh Gour Central University, Sagar for fellowship. This work was financially supported by a project SERB (SERB/LS-329/2013), Govt. of India, sanctioned to RKK. The authors are thankful to Department of Zoology, Dr. Harisingh Gour Central University, Sagar, for providing infrastructural and financial support.
1. Mueller GM and Schmit JP (2007) Fungal biodiversity: what do we know? What can we predict? Biodivers Conserv16: 1-5.
2. Kirk PM, Canon PF, Minter DW, Staplers JA (2008) Dictionary of the Fungi 10th Edition CABI.
3.Hawksworth DL, Rossman AY (1997) Where are all the undescribed Fungi? Phytopathol 87: 888-891.
4. Blackwell M (2011) The Fungi: 1,2,3... 5.1 million species? American J Botany 98: 426-438.
5. Vega FE, Blackwell M (2005) Insect-Fungal Associations: Ecology and Evolution. Oxford University Press, Oxford.
6. Hawksworth DL (1998) The consequences of plant extinctions for their dependent biotas: An overlooked aspect of conservation science. Academia Sinica, Taipei, pp 1–15
7. Kamoun S (2003) Molecular genetics of pathogenic oomycetes. Eukaryotic Cell, 2: 191-199.
8. Samson RA, Evans HC, Latge JP (1988) Atlas of Entomopathogenic Fungi. Springer.
9. Grimaldi D, Engel MS (2005) Evolution of the insects. Cambridge University Press.
10. Suh SO, McHugh JV, Pollock DD, Blackwell M (2005) The beetle gut: a hyperdiverse source of novel yeasts. Mycol Res 109: 261-265.
11. Currie CR, Wong B, Stuart AE, Schultz TR, et al. (2003) Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299: 386-388.
12. De-Kesel A (1996) Host specificity and habitat preference of Laboulbenia slackensis. Mycologia 88: 565-573.
13. Evans HC, Samson RA (1982) Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. I. The Cephalotes (Myrmicinae) complex. T Brit Mycol Soc 79: 431–453.
14. Evans HC, Samson RA (1984) Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. T Brit Mycol Soc 82: 127–150.
15. Evans HC (1988) Coevolution of entomogenous Fungi and their insect hosts. Academic Press, London.
16. Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic Fungi and their arthropod hosts. Annu Rev Entomol 51: 331–357.
17. Mota-Sanchez D, Bills PS, Halon ME, Wheeler WB (2002) Pesticides in agriculture and the environment.
18. Augustyniuk-Kram A, Kram KJ, Blanco JA, Lo YH (2012) Entomopathogenic Fungi as an Important Natural Regulator of Insect Outbreaks in Forests. InTech Croatia 265.
19. Hoffmann MP, Frodsham AC (1993) Natural Enemies of Vegetable Insect Pests. Cooperative Extension. Cornell University, NY 63.
20. Jean-Christophe C, Damien J, Guy T, Banpot N (1999) A systems approach to understanding obstacles to effective implementation of IPM in Thailand: key issues for the cotton industry.Agriculture. Ecos Environ 72: 17-34.
21. Bale JS, Lenteren JC, Bigler F (2008) Biological control and sustainable food production. Phil Trans R Soc B 363: 761–776.
22. Mahr D, Whitaker, P, Rigdway N (2008) Biological control of insects and mites: An introduction to beneficial natural enemies and their use in pest management. Cooperative extension publishing, Madison.
23. Tu M, Hurd C, Randall J (2001) Biological control. The Nature Conservancy. U.S. government documents.
24. Sloderbeck P, Nechols J, Greene G (1996) Biological control of insect pests on field crops in Kansas. Kansas State University, Manhattan.
25. Zimmerman G (1993)The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. Pestic Sci 37: 375-379.
26. Ferron P, Fargues J, Riba, G (1991) Fungi as microbial insecticides against pests, in Handbook of Applied Mycology. New York 2: 665-706.
27. Khachatourians GG (1991) Physiology and genetics of entomopathogenic fungi, in Handbook of Applied Mycology. New York 2: 613-663.
28. Howell CR (2003)Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis 87: 4-10.
29. Singh A, Jain A, Sarma BK, Upadhyay RS, et al. (2013)Rhizosphere microbes facilitate redox homeostasis in Cicer arietinum against biotic stress. Ann Appl Biol 163: 33–46.
30. Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, et al. (2011)Trichoderma: The genomics of opportunistic success. Nat Rev Microbiol 9: 749-759.
31. Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, et al. (2011)Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol 12: 40.
32. Lorito M, Peterbauer C, Hayes CK, Harman GE (1994)Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiol 140: 623-629.
33. Malmierca MG, Cardoza RE, Alexander NJ, McCormick SP, et al. (2012)Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl Environ Microbiol 78: 4856-4868.
34. Benitez T, Rincón AM, Limón MC, Codón AC (2004)Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7: 249-260.
35. Ownley BH, Gwinn KD, Vega FE (2010)Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. Biol Cont 55: 113–128.
36. Humber RA (2008) Evolution of entomopathogenicity in fungi. J Invertebr Pathol 98: 262–266.
37. Jaronski ST (2007) Soil ecology of the entomopathogenic ascomycetes: a critical examination of what we (think) we know. Research Sign Posts, India.
38. Bruck DJ (2009)Fungal entomopathogens in the rhizosphere. Biol Cont 55: 103–112.
39. Hu G, St Leger RJ (2002)Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveals that it is rhizosphere competent. Appl Environ Microbiol 68: 6383–6387.
40. Wang C, St. Leger RJ (2006)A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc Natl Acad Sci USA 103: 6647–6652.
41. Wang C, St. Leger RJ (2007)The MAD1 Adhesin of Metarhizium anisopliae Links Adhesion with Blastospore Production and Virulence to Insects, and the MAD2 Adhesin Enables Attachment to Plants. Eukaryot cell 6: 808–816.
42. Xu Y, Orozco R, Wijeratne EMK, Artiles PE, Gunatilaka AAL, et al. (2009)Biosynthesis of the cyclo oligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genetics Biol 46: 353–364.
43. Fornelli F, Minervini F, Logrieco A (2004)Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J Invertebr Pathol 85: 74–79.
44. Eley KL, Halo LM, Song Z, Powles H, Cox RJ, et al. (2007)Biosynthesis of the 2-pyridone tenellin in the insect pathogenic fungus Beauveria bassiana. Chem Bio Chem 8: 289–297.
45. Pal S, St-Leger RJ, Wu LP (2007)Fungal peptide Destruxin A plays a specific role in suppressing the innate immune response in Drosophila melanogaster. J Biol Chem 282: 8969–8977.
46. Bagga S, Hu G, Screen SE, St-Leger RJ (2004)Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene 324: 159–169.
47. Cho EM, Boucias D, Keyhani NO (2006)EST analysis of cDNA libraries from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. II. Fungal cells sporulating on chitin and producing oosporein. Microbiol 152: 2855–2864.
48. Small CLN, Bidochka MJ (2005)Up-regulation of Pr1, a subtilisinlike protease, during conidiation in the insect pathogen Metarhizium anisopliae. Mycol Res 109: 307–313.
49. Xu J, Baldwin D, Kindrachuk C, Hegedus DD (2006)Serine proteases and metalloproteases associated with pathogenesis but not host specificity in the Entomophthoralean fungus Zoophthora radicans. Can J Microbiol 52: 550–559.
50. Hu X, Xiao G, Zheng P, Shang Y, Su Y, et al. (2014)Trajectory and genomic determinants of fungal pathogen speciation and host adaptation. Proc Natl Acad Sci USA 111: 16796–16801.
51. Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, et al. (2011)Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and Metarhizium acridum. PLOS Genet 7: e1001264.
52. Zheng P, Xia Y, Xiao G, Xiong C, Hu X, et al. (2011)Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol 12: R116.
53. De-Bekker C, Quevillon LE, Smith PB, Fleming KR, Ghosh D, et al. (2014)Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evol Biol 14: 166.
54. Evans HC, Elliot SL, Hughes DP (2011)Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLOS One 6: e17024.
55. Lingg AJ, Donaldson MD (1981)Biotic and abiotic factors affecting stability of Beauveria bassiana conidia in soil. J Invert Path 38: 191-200.
56. Kreutz J, Vaupel O, Zimmermann G (2004)Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, Ips typographus L., in the laboratory under various conditions. J Appl Entomol 128: 384-389.
57. Dubois T, Lund J, Bauer LS, Hajek AE (2007)Virulence of entomopathogenic hypocrealean fungi infecting Anoplophora glabripennis. Biol Cont 53: 517-528.
58. Shanley RP, Keena M, Wheeler MM, Leland J, Hajek AE, (2009)Evaluating the virulence and longevity of nonwoven fiber bands impregnated with Metarhizium anisopliae against the Asian long horned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol Cont 50: 94-102.
59. Zhang LW, Liu YJ, Yao J, Wang B, Huang B, et al. (2011)Evaluation of Beauveria bassiana (Hyphomycetes) isolates as potential agents for control of Dendroctonus valens. Insect Sci 18: 209-216.
60. Nielsen C, Keena M, Hajek AE (2005)Virulence and fitness of the fungal pathogen Entomophaga maimaiga in its host, Lymantria dispar, for pathogen and host strains originating from Asia, Europe, and North America. J Invert Path 89: 232-242.
61. Van-Lenteren JC, Babendreier D, Bigler F, Burgio G, Hokkanen HMT, et al. (2003)Environmental risk assessment of exotic natural enemies used in inundative biological control. Biol Cont 48: 3-38.
62. Jackson MA, Dunlop CA, Jaronski ST (2010)Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. Biol Cont 55: 129-145.
63. James RR, Shaffer BT, Croft B, Lighthart B (1995)Field evaluation of Beauveria bassiana: its persistence and effects on the pea aphid and a non-target coccinellid in alfalfa. Biocont Sci Technol 5: 425-438.
64. Parker BL, Skinner M, Gouli V, Brownbridge M (1997)Impact of Soil Applications of Beauveria bassiana and Mariannaea sp. on Non-target Forest Arthropods. Biol Cont 8: 203-206.
65. Traugott M, Weissteiner S, Strasser H (2005)Effects of entomopathogenic fungus Beauveria brongniartii on the non-target predator Poecilus versicolor (Coleoptera: Carabidae). Biol Cont 33: 107-112.
66. Nielsen C, Vestergaard S, Harding S, Eilenberg J (2007)Microbial control of Strophosoma spp. Larvae (Coleoptera: Curculionidae) in Abies procera greenery plantation. J Anhui Agri Uni 34: 184-194.
67. Pell JK, Eilenberg J, Hajek AE, Steinkraus DC, Butt TM, et al. (2001) Fungi as biocontrol agents: Progress, Problems and Potential. CAB International, UK.
68. Keller S, Schweizer C, Keller E, Brenner H, (1997)Control of white grubs (Melolontha melolontha L.) by treating adults with the fungus Beauveria brongniartii. Biocont Sci Technol 7: 105-116.
70. Harbison ST, Carbone MA, Ayroles JF, Stone EA, Lyman RF, et al. (2009)Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nat Genetics 41: 371-375.
71. Ivanov DK, Escott-Price V, Ziehm M, Magwire MM, Mackay TF, et al. (2015)Longevity GWAS using the Drosophila genetic reference panel. J Gerontol 70: 1470-1478.
72. Shorter J, Couch C, Huang W, Carbone MA, Peiffer J, et al. (2015)Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc Natl Acad Sci USA 112: E3555-E3563.