Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 17 February, 2021
Accepted date: 05 May, 2021
Published date: 12 May, 2021
Citation: Li B, Liu v, Xing Y, Guo S (2021) Cell wall Strengthening and Remodeling Provide New Insights on Sclerotia development of Medicinal Fungus Polyporus umbellatus. J Appl Microb Res. Vol: 4 Issu: 1 (34-41).
Copyright: © 2021 Guo S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Sclerotium is a special form of many species of fungi and cell wall thickening is a common phenomenon in sclerotium. Rational manipulation of sclerotium would be an innovative approach for pathogenic control as well as for medicinal fungal resource revival. Sclerotia of Polyporus umbellatus were used to treat multiple human diseases. However, the mechanism of thickened cell wall (TCW) is still unclear.
Methodology:In this study, Sequential Windowed Acquisition of all Theoretical fragment ions spectra-MS (SWATH-MS) and parallel reaction monitoring (PRM) technology were introduced to demonstrate biomarkers at protein level associated with TCW as sclerotia development from initial sclerotia (IS) to developmental and mature sclerotia (DS and MS).
Results:72 differentially expressed proteins (DEPs) were associated with TCW, and evidence supported presence and accumulation of chitin, glycan, xylcan and hydrophobins in the cell wall of P. umbellatus sclerotia. Pump122, a new hydrophobin in P. umbellatus expressed up sharply with DS/IS 119.85 and MS/IS 128.17, and located at cell wall and septum hypha in of sclerotia by immune colloidal gold technique. In addition, sclerotial cell wall could be remodeled via O-mannosylation, O-fucosylation and deacetylation to strengthen cell wall. Conclusion: This study provides new insights on fungal cell wall especially to sclerotia-formed fungus.
Keywords
Polyporus umbellatus,S, cell wall, Cell wall strengthening, Cell wall remodeling.
Abstract
Background: Sclerotium is a special form of many species of fungi and cell wall thickening is a common phenomenon in sclerotium. Rational manipulation of sclerotium would be an innovative approach for pathogenic control as well as for medicinal fungal resource revival. Sclerotia of Polyporus umbellatus were used to treat multiple human diseases. However, the mechanism of thickened cell wall (TCW) is still unclear.
Methodology:In this study, Sequential Windowed Acquisition of all Theoretical fragment ions spectra-MS (SWATH-MS) and parallel reaction monitoring (PRM) technology were introduced to demonstrate biomarkers at protein level associated with TCW as sclerotia development from initial sclerotia (IS) to developmental and mature sclerotia (DS and MS).
Results:72 differentially expressed proteins (DEPs) were associated with TCW, and evidence supported presence and accumulation of chitin, glycan, xylcan and hydrophobins in the cell wall of P. umbellatus sclerotia. Pump122, a new hydrophobin in P. umbellatus expressed up sharply with DS/IS 119.85 and MS/IS 128.17, and located at cell wall and septum hypha in of sclerotia by immune colloidal gold technique. In addition, sclerotial cell wall could be remodeled via O-mannosylation, O-fucosylation and deacetylation to strengthen cell wall. Conclusion: This study provides new insights on fungal cell wall especially to sclerotia-formed fungus.
Keywords
Polyporus umbellatus,S, cell wall, Cell wall strengthening, Cell wall remodeling.
Introduction
Polyporus umbellatus (Zhuling in Chinese) is a traditional Chinese medicinal fungus and performs a good diuretic effect with history of more than 2000 years. Modern pharmacology shows that P. umbellatus or its metabolites have nephro-protective activity, hepato-protective effect, immuno-enhancing activity, antioxidative activity and anti-inflammation [1]. Ergosta-4, 6, 8 (14), 22-tetraen-3-one (ergone) isolated from P. umbellatus has been proven to prevent the progression of renal injury and the subsequent renal fibrosis by inhibiting the increase of creatinine, urea nitrogen, urine protein and N-acetyl-b-D-glucosaminidase levels [2]. The Polysaccharide InjectionTM of P. umbellatus (Zhuling Duotang Zhusheye) is used for adjuvant therapy of antitumor [3]. P. umbellatus and its products are still wildly used in Asia.
In fact, the medicinal parts of P. umbellatus displayed these bioactivities are a specialized structure named sclerotia which differentiated from hypha [4]. The sclerotium is a structure that supports long-term survival and is intricately linked with structural changes to the thick-walled cell induced adverse environmental conditions [5-8]. However, the detail mechanism for thickened cell wall (TCW) is still unknown.
In wild condition, sclerotia can generate and develop after co-cultured with symbolized fungus Armillaria mellea. Once A. mellea invade P. umbellatus, sclerotia can build a belt like Great Wall to stop the further invasion of A. mellea, which resembled epidermal tissue of sclerotia [5]. These suggest that the cell wall of sclerotia may be closely with some proteins and can be strengthen and remodeled as sclerotia development. Previously, Chen et al. found that cell wall proteins (covalent and non-covalent cell wall proteins) were major contribution to the cell wall of sclerotium, though the total number of proteins is greater in the mycelium and fruiting body [9]. Therefore, there might be some significant expressed proteins contributed to the thickened cell wall of P. umbellatus sclerotia.
Proteomics has served at the forefront as a tool for filamentous fungi and other pathogens of human and plants [10]. Label-free quantitative proteomics based on Sequential Window Acquisition of all Theoretical fragment ion spectra (SWATH)-MS on data independent acquisition (DIA) model can provide differentially expressed proteins (DEPs) [11]. For targeted protein (s), parallel reaction monitoring (PRM)-MS technology performs a full scan of each transition by a precursor ion to achieve quantitative observations, and is widely applied to the validation of DEPs un-relying on specific antibodies [12,13]. SWATH-MS combined with PRM will be powerful technology to obtain biomarkers.
Initial sclerotia (IS) differentiated from hypha and then developed to development sclerotia (DS) and mature sclerotia (MS) [4]. In the current study we obtained biomarkers at the protein level contributing to thickened cell wall in sclerotia and how they aide regulation of cell wall strengthening and remodeling of P. umbellatus. Understanding the mechanisms of cell wall thickening helps to manipulate sclerotia development rationally, which would be beneficial for the revival of medicinal fungi resources, as well as for fungal pathogen control.
Methods
P. umbellatus culture
P. umbellatus were derived from wild triennial sclerotia, and were cultured as described previously [8]. In the current study, P. umbellatus were cultured on plates containing fructose complete medium at room temperature in the dark to obtain sclerotia. P. umbellatus sclerotia collected on day 30, 40 or 90 after inoculation were defined as initial sclerotia (IS), developmental sclerotia (DS) and mature sclerotia (MS), respectively for PRM validation, ELISA analysis and enzyme activity assays.
PRM validation
For PRM analysis, proteins and peptides were prepared as previously reported [4]. Briefly, proteins were extracted from sclerotia, and then purified with ice cold acetone twice and dissolved with 0.2% RapiGestSFTM (Waters) in 50 mM ammonium bicarbonate. Peptides were digested using sequencing grade modified trypsin digestion (enzyme to protein ratio about 1:40) with final protein concentrations of approximately 1μg/μL. Candidate DEPs in this work were confirmed using parallel reaction monitoring (PRM) method. Nano LC-MS/MS analysis was carried out using an Orbitrap Fusion (Thermo-Fisher Scientific, San Jose, CA) mass spectrometer equipped with a nanospray Flex Ion Source and the UltiMate3000 RSLC nano (Dionex, Sunnyvale, CA). Peptide samples were injected onto a PepMap C-18 RP nano trap column (3μm, 100 μm×20 mm, thermo-Fisher Scientific) at a 6 uL/min flow rate for on-line desalting, and separated on a PepMap C-18 RP nano column (2 μm, 75 μm×25 cm). Peptides were eluted at a flow rate of 300 nL/ min in 0.1% formic acid at 55°Cwith a linear, segmented gradient of 3-8-28-95% of solvent B (0.1% FA, 80% ACN) over 120 min prior electro spray ionization.
The Orbitrap Fusion was operated in positive ion mode with nano spray voltage set at 2.1 kV and source temperature at 275°C. The instrument was operated in PRM mode. MS survey scanned at a resolving power of 60,000 FWHM (full width half maximum) at m/z 200, for the mass range of m/z 350~2000, and MS/MS scanned at 30,000 with Q isolation window (m/z) at 1.2 for the mass range m/z 105~2000. All data were acquired under Xcalibur 4.3 operation software.
The PRM data were processed using Skyline (v.3.6). Peptide settings: the enzyme was set as trypsin [KR/P], and the maximum number of missed cleavages was set as 2. The peptide length was set as 8-25; the variable modifications were set as carbamidomethyl on Cys and oxidationon Met; and the maximum number of variable modifications was set as 3.
Transition settings: the precursor charges were set as 2+, 3 and 4+; the ion charges were set as 1 and 2; and the ion types were set as b and y. The product ions were set as ranging from ion 3 to the last ion, and the ion match tolerance was set as 0.02 Da.
Enzyme activity assay
Laccase were extracted from fresh sclerotia and enzyme activity assays were carried out according to the laccase kit guideline (Solarbio Life Science Co. Ltd.). 40 μL of the sample protein solution were used as test sample whereas boiled sample protein solutions with the same volumes were set as control. Each sample was incubated at 60°C in a water bath for 3min after added 240μL of the working solution. Next, 200 μL solution were placed into one well of a 96-well plate to measure absorbance at 420 nm on an EnSpire® multimode reader (PerkinElmer Co. Ltd.). Three biological samples were taken, and each biological sample was measured twice. The optical density (OD) value of the test minus the OD value of the control tube acted as the actual OD value (δA). Enzyme activity (U/g) were calculated according to 65*δA /w in which W were the weight of sample.
ELISA tests of β-mannosidase and α-L fucose
Concentrations of β-mannosidase (β-manase) or α-fucosidase (α-L fucose) were measured using ELISA according to kit guidelines (MSKBIO Co. Ltd.) by standard curve method with a gradient of β-mannosidase standard solutions 5 pg/mL, 10 pg/mL, 20 pg/mL, 40 pg/mL or 60 pg/ mL and a gradient of α-L fucose enzyme standard solutions 20 ng/L, 40 ng/L, 80 ng/L, 160 ng/L or 320 ng/L.
The major procedure as follow: 10μL of sample solution and 40μL of sample dilution solution were added to one well of ELISA test plate, and incubated at 37°C for 30 minutes. Discarded the solution and washed each well with 200μL washing solution. And then, added 50μL of enzyme-labeled reagent to every well and incubated at 37°C for 30min. After that, added 50μL developer A and B solution, mixed gently, and developed at 37°C in the dark for 15min. Next, Added 50μL stop solution to stop reactions. Finally, the OD values of all samples were measured at 450 nmon an EnSpire® multimode reader. Wells without samples and enzymelabeled reagents acted as blank wells.
Localization of target protein Pump122
The localization of hydrophobin Pump122 (hydrophobin B-like protein) in initial sclerotia of P. umbellatus was tested by immunocolloidal gold localization technology. The experimental process refer to the previous article and the general steps were as follows: Sclerotia was cut into small pieces with a diameter of about 0.3 cm, and fixed with 4% glutaraldehyde solution (4°C) and then dehydrated with gradient ethanol solution (4°C) [8]. The dehydrated samples were treated with ethanol-LR White epoxy resin mixture (ethanol: LR White=3:1, 1:1 and 1:3) successively, and then imbedded in the LR white resin. The pellets were cut into 50~80 nm ultra-thin sections and washed three times with distilled water, PBS-glycine solution and PBS-T in proper order. The slices were incubated with diluted anti-Pump122 antibody solution (6000 times) overnight at 4ºC following goat anti-mouse secondary antibody solution incubating for 90 min at room temperature. Finally labeled proteins in samples were visualized by using JEM-1230 transmission electron microscope (Tokyo, Japan).
Data processing and biological information analysis
Quantified proteins of sclerotia by SWATH were selected, normalized and compared via t-test to identify differentially expressed proteins (DEPs) of MS vs. IS, MS vs. DS and DS vs. IS. Union of the DEPs was done via Venn diagram, and then GO annotation and KEGG pathway enrichment analysis were performed by omicsbeanTM (Shanghai Gene for health Co.). Those DEPs involved in defensive structures of sclerotia were selected for further analyses to investigate morphological changes in sclerotia. Calculated results are presented as M±SD (n=3) followed Grubbs test to remove suspicious data.
Results
Differentially expressed proteins contribute to thickened cell wall of sclerotia
Proteins in initial sclerotia (IS), development sclerotia (DS) and mature sclerotia (MS) were identified by our previous work, so did DEPs of DS vs. IS, MS vs. DS and MS vs. IS [4]. 611 merged DEPs were obtained among the DS vs. IS, MS vs. DS and MS vs. IS groups (as shown in Supplementary material 1 (S1)) including 85 unknown proteins (comp11158_c0, comp13970_c0, comp142821_ c0, etc) and 526 identified proteins (Q49W60, O14241 and Q1K9C2 etc.) based on results of the t-tests and Venn diagram comparison. Gene Ontology (GO) enrichment and KEGG metabolic pathway enrichment of these merged DEPs were shown in Supplementary material 2-3 (S2-S3).
Bioinformatics results of merged DEPs demonstrated that the development of sclerotia of P. umbellatus was characterized by synthesis and hydrolysis of polysaccharides, the main components of P. umbellatus cell wall. Therefore, changes in polysaccharides can directly affect the sclerotia. It has been reported that monosaccharide of P. umbellatus include xylose, glucose, mannose, galactose, and fructose [3]. Therefore, 72 DEPs related to components of the cell wall from merged DEPs listed as shown in Supplementary material 4 (S4).
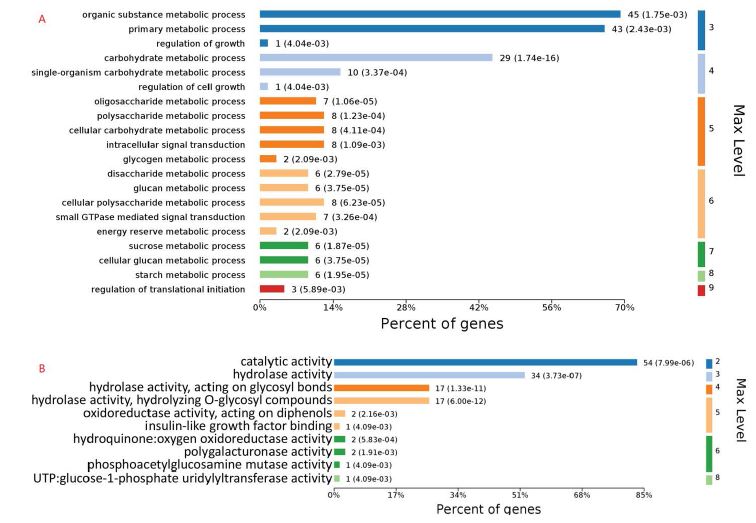
The 72 DEPs could be classified into functional categories including carbohydrate metabolism, small GTPase mediated signal transduction, intracellular signal transduction, organic substance metabolism, metabolic processes, cell wall biogenesis, and other biological processes. Carbohydrate metabolism (29) and singleorganism carbohydrate metabolism (10) were significantly enriched (Figure 1A). Some DEPs’ functional categories were associated with disaccharide, sucrose, oligosaccharide, glucan, polysaccharide metabolic processes, and these were also found to enrich in the KEGG pathways of carbohydrate metabolism (AA) and glycan biosynthesis and metabolism (AG) (Supplementary material 5 (S5)). The patterns in differentially expressed pathways are known to be related to morphogenesis of the fungal cell wall which is composed of complex polysaccharides (such as glucan, chitin, etc) and proteins [14]. Additionally, P. umbellatus have been demonstrated to biosynthesize polysaccharides composed of monosaccharide such as mannose, galactose, glucose and fructose [3]. Furthermore, the biological processes of cell wall organization or biogenesis were enriched (Figure 1A), indicating that the cell wall of sclerotia were synthesized simultaneously as P. umbellatus developed and matured. In the molecular function subcategory, 34 DEPs were related to hydrolase activity, and 17 DEPs were associated with hydrolase activity, acting on (O-) glycosyl compounds (Figure 1B). Therefore, we suggest that the process of cell wall organization is a continuous process, and that hydrolysis and modification of cell wall architecture occur throughout sclerotium development and maturation. The DEPs’ biological functions and expression levels were list as Supplementary material 4 (S4).
PRM validation results
Appropriate peptides of all the 72 DEPs were selected as targets of PRM tests. The same amounts of indexed Retention Time (iRT) peptides were added into each sclerotium sample and retention times were calibrated. Finally, 27 proteins and 54 peptides could be quantified by PRM, including 11 onepeptide proteins, 8 two-peptide proteins, 6 three-peptide proteins, 1 four-peptide protein, and 1 five-peptide protein.
Figure 1: The biological processes (A) and molecular function (B) annotation enrichment of 72 DEPs involved in cell wall of P. umbellatus.
The precursor ion m/z, changes, start time, end time and fragment ions for each proteins were pooled and presented in Supplementary material 6 (S6A). Quantified peptides were processed using Skyline (v.3.6) and the area, median area and standard deviation of quantified proteins were calculated and are presented in Supplementary material 6 (S6B). The relative expression levels of quantified proteins were calculated among IS, DS and MS were shown in table 1.
Results of ELISA tests
Concentrations of α-fucosidase or β-manase in IS, DS and MS of P. umbellatus were calculated by standard curve method with standard curve y=0.0071x+0.0088 (R2=0.998) for α-fucosidase and y=0.0353x+0.1738 (R2=0.964) for β-manase in which x was concentration for sample and y was δabsorbance (δA). The concentrations and the relative ratio among the sclerotia at different phases were presented in table 2. Expressions of α-fucosidase were upregulated throughout growth (MS/DS 2.45, MS/IS 2.63) and had the same trend as identified with SWATH and PRM quantification. β-manase was down-regulated as sclerotia matured with DS/IS 0.242 and MS/DS 0.806.
Enzyme activity analysis
Laccase activities of P. umbellatus sclerotia at the initial, developmental or maturation phase were calculated using the formula 65*δA/w (w was the weight of fresh material and δA was absorbance of the sample after blanking). In general, laccase activity increased as sclerotia developed and matured, and a maximum was reached during the maturation phase with MS/DS 3.11 (Table 2).
Hydrophobin Pump122 located at cell wall and septum of hypha
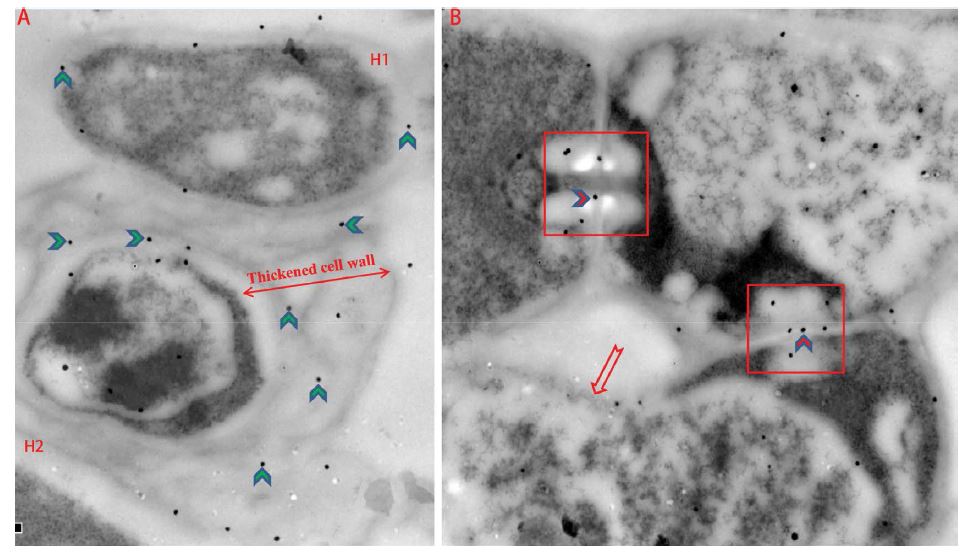
Pump122 was one type of hydrophobins identified from P. umbellatus and was homologous with hydrophobin B [15], named hydrophobin B-like protein. By transmission electron microscope, the hypha in sclerotia of P. umbellatus were intertwined and adhered, and the cytoplasm concentrated as sclerotia development. For some hypha, cell walls had been significantly thickened, and some hypha had merged into a pellucida among hypha. Pump122 was located at the cell wall and septum of hyphae and clamp connection (Figure 2A and B) by immunocolloidal gold localization technology. This indicated Pump122 was not only the cell wall component, but also contributed to sclerotia development.
Discussion
Sclerotium was initiated from hypha, and transformed from white clusters at initial phase to a rigid and resting structure accompanying morphogenesis change of hypha in sclerotia [6,8]. The significant morphogenesis were the thickened cell wall of hypha in sclerotia with little cytoplasm shown in figure 2. These results indicated that a number of proteins could involve in cell wall metabolism. Using proteomic analysis, proteins associated with cell wall architecture were identified, and cell wall was strengthen and remolded as sclerotia developed and matured.
Table 1: Purification Table for Pectinase from Bacillus subtilis.
| Protein | Name | Ratio by SWATH | Ratio by PRM | ||||
|---|---|---|---|---|---|---|---|
| DS/IS | MS/DS | MS/IS | DS/IS | MS/DS | MS/IS | ||
| A2R797 | Probable alpha-fucosidase A | - | 15.8 | 22.5 | 6.98 | - | 9.73 |
| P82476 | Chitin deacetylase | 1.70 | - | - | 0.42 | - | 0.54 |
| Q70J59 | Tripeptidyl-peptidase sed2 or Sedolisin-B | - | - | - | 0.19 | 6.18 | 1.15 |
| Q9VR81 | N-acetylglucosamine-6-phosphate deacetylase | 0.66 | 2.16 | - | 0.13 | 0.17 | 0.02 |
| A1CA51 | Probable beta-glucosidase I | 0.59 | 4.20 | 2.49 | 0.45 | 0.53 | 0.24 |
| O80327 | Thaumatin-like protein 1 | 8.27 | 3.28 | 27.2 | 1.85 | 3.70 | 6.83 |
| O93869 | Glycogen [starch] synthase | - | - | - | 1.32 | 0.23 | 0.30 |
| P13860 | Exoglucanase 1 | - | 28.5 | 27.6 | 0.63 | - | 0.50 |
| P49426 | Glucan 1,3-beta-glucosidase | - | 24.2 | 29.3 | 3.08 | - | 2.85 |
| Q06625 | Glycogen debranching enzyme | 0.55 | 0.52 | 0.28 | - | 0.63 | 0.64 |
| Q0CI96 | Probable glucan endo-1,3-beta-glucosidase btg C | - | 3.03 | 1.84 | - | 3.18 | 3.73 |
| Q96VA4 | 1,4-alpha-glucan-branching enzyme | 0.56 | 0.48 | 0.27 | 0.52 | - | - |
| Q9Y8H3 | 1,4-alpha-glucan-branching enzyme | 0.48 | 0.60 | 0.29 | 0.04 | - | 0.04 |
| P78811 | Probable UTP--glucose-1-phosphate uridylyltransferase | 0.52 | - | 0.60 | 0.51 | 0.84 | 0.43 |
| Q04336 | Uncharacterized protein YMR196W | 0.22 | - | 0.17 | 3.05 | 0.38 | - |
| Q5B7W2 | Beta-mannosidase B | - | - | - | 35.23 | 0.19 | 6.60 |
| O14224 | Rho GDP-dissociation inhibitor (Rho GDI) | 0.61 | 0.65 | 0.40 | 5.41 | 0.12 | 0.65 |
| O59810 | Vigilin 1 | 0.74 | 1.25 | 0.58 | 2.58 | 0.37 | - |
| P0CM16 | ADP-ribosylation factor (Arf) | - | 0.35 | 0.35 | 8.89 | 0.47 | 4.16 |
| P33723 | GTP-binding protein ypt1 | - | 0.24 | 0.22 | - | - | 0.65 |
| P49605 | cAMP-dependent protein kinase regulatory subunit | 0.56 | - | - | 0.82 | - | 0.65 |
| P55039 | Developmentally-regulated GTP-binding protein 2 | 5.29 | 0.13 | - | 0.55 | 0.20 | 0.11 |
| Q00078 | Protein kinase C-like | - | 0.33 | 0.32 | 0.04 | - | 0.03 |
| Q5RBG1 | Ras-related protein Rab-5B | 0.28 | - | 0.28 | 2.77 | 1.66 | 4.60 |
| Pump122 | hydrophobin B-like protein | 3.99 | - | 4.11 | 119.85 | - | 128.17 |
| P04158 | Hydrophobin SC1 | 4.86 | 2.12 | 10.3 | 0.41 | 4.68 | 1.89 |
| Note: “-“ there is no significant difference (fold change≥1.50 or ≤0.67). | |||||||
Table 2: ELISA and enzyme activity test results.
| Enzyme | IS | DS | MS | DS/IS | MS/DS | MS/IS |
|---|---|---|---|---|---|---|
| β-manase | 0.041±0.009 | 0.010±0.010 | 0.008±0.053 | 0.242 | 0.806 | 0.195 |
| α-fucosidase | 0.0389±0.0204 | 0.0417±0.0236 | 0.102±0.0409 | 1.07 | 2.45 | 2.63 |
| Laccase | 114.6±66.3 | 314.2±10.5 | 356.2±209.7 | 2.74 | 3.11 | 1.13 |
Figure 2: Immunolocalization of hydrophobin B-like protein Pump122 on hypha in sclerotia of P. umbellatus. Pump122 (black spot pointed by chevron arrow) located at cell wall (A and B) and septum (B) of hyphae. The thickened cell wall was obviously in hyphae H2 compared with hyphae H1 (A). Pump122 located at cell wall, and much more the protein expressed as cell wall thickening. Pump122 (black spot pointed by red chevron arrow) also located at septum including hphae and clavicular union (red arrow) (B).
Strengthening of sclerotial cell wall
The cell wall of sclerotia is composed of chitin (chitosan), glucan, GPI anchors and cell wall proteins etc. Chitin forms part of the mesh network of the cell wall and provides mechanical stability [16]. In addition, chitin can be used to produce a second layered structure along the spore thicken-wall, next to the outer dityrosine layer in response to environmental stress [17,18]. Increased expression of chitinase 2 and sedolisin-B displayed functions in chitin metabolism (Table 1) and might contributed to cell wall thickening in sclerotia of P. umbellatus during sclerotia maturation.
Glycan is a major component in fungal cell walls. The glycan chain is elongated by adding glucose in beta- 1, 3-glucan linkages, and it branches in α-1, 4-glucan linkages. The cell wall is not a static structure and keeps the balance between hydrolysis and biosynthesis. Elongation of glucan chains was a major response when sclerotia differentiations were initiated as expression levels of β-1, 3-glucanosyltransferases gel 4 were elevated [19]. However, when sclerotia matured, hydrolases could be activated, especially glucan 1, 3-beta-glucosidase, while β-1, 3-glucanosyltransferases gel 4 were up-regulated, which might result in the assembly and arrangement of fungal cells, allowing them to maintain their shape and protecting them from osmotic lysis [19]. Glycogen (starch glycan) and branching of glucan play important roles in fungi. Glycogen is a multi-branched polysaccharide of glucose that serves as a form of energy storage in fungi. Both the formation and degradation of glycogen occur from the non-reducing ends of its a-1, 4-linked chains. Branching increases the number of available ends and therefore the rate at which glycogen can be broken down or re-synthesized. Glycogen levels are elevated, and polysaccharides are less branched when exposed to nutrient limitation or other severe conditions [20,21]. In the sclerotia of P. umbellatus, branching enzymes were down-regulated (as verified by PRM in Table 1), though glycogen (starch) synthase were up-regulated, indicating that glycogen were stable in the sclerotia and that the less branched glucan can promote adaptation of sclerotia to environmental stress. In addition, hydrolyzed glycogen can release glucose as a nutrient for phase growth or cell wall synthesis and serve as a remodeling basic material. However, we do not know this occurred in P. umbellatus.
Cell wall proteins also contributed to cell wall strengthening including covalent cell wall proteins and non-covalent cell wall proteins. Covalent cell wall proteins combine with other components of the cell wall, while noncovalent cell wall proteins are associated with cell wall biosynthesis or metabolism (Supplemental material 1 (S1)). In this study, three covalent cell wall proteins were notable: hydrophobin SC1, SC3 and Pump122 (homologous protein of hydrophobin B) by PRM. Based on GO annotation results, we speculate that hydrophobins acted as adhesion agents to aide intertwining of hyphae. Pump122 was immunodetected and located at cell wall and septum of hypha in sclerotia (Figure 2) indicating it was the component of cell wall and associated with sclerotial development. All of the three proteins expressed differentially in sclerotia; especially Pump122 which had DS/IS 119.85 and MS/IS 128.17 using PRM for validation. The thicker the cell wall, the more Pump122 expressed (Figure 2A). These results indicated that increased amounts of hydrophobins not only strengthen hypha intertwining, but also strengthen cell walls of sclerotia.
Modifications of the cell wall components
In addition to the proteins involved in sclerotia cell wall architecture, some proteins which contribute to modification of cell wall components or protein post translational modifications as P. umbellatus sclerotia matured. These proteins were responsible for O-mannosylation, O-fucosylation and deacetylation, and for strengthening cell wall stability and integrity.
O-mannosylation is a crucial and conserved modification process in eukaryotes and exerts a variety of different functions, such as impacting cell wall integrity, cell polarity and morphogenesis [22]. O-mannosylation is catalyzed by dolichol-phosphate mannosyltransferase (P54856) and is an essential enzyme that synthesizes mannosylate covalent bonds in the GPI anchor [23]. Dolichol-phosphate mannosyltransferase transfers mannose from GDP-mannose to dolichol monophosphate to form dolichol phosphate mannose (Dol-P-Man), which is the mannosyl donor in pathways and results in protein O-linked mannosylation. We observed down-regulation of O-mannosylation in sclerotia during the maturation phase, indicating that O-mannosylation in the GPI anchor was elevated, which could help strengthen the stability and integrity of the cell wall [24]. On the other side, down-regulation of O-mannosylation related proteins in P. umbellatus sclerotia during the maturation phase indicated that hydrolysis of mannan might be inhibited. In addition, the uncharacterized protein YMR196W (Q04336) and beta-mannosidase B involved in protein N-linked glycosylation, were highly expressed (validation by PRM in table 1) during the developmental phase and might accelerate sclerotia thickening when comparing IS and DS [25]. Therefore, mannosylation was a key function in strengthening cell wall of P. umbellatus.
O-fucosylation is an essential post-translational modification. In P. umbellatus, alpha-xylosidase (P31434) and probable alpha-fucosidase A (A2R797) were synergistically involved in complete deconstruction of the xyloglucan backbone. Alpha-xylosidase catalyzes hydrolysis of xyloglucan side chains to remove unsubstituted D-xylose residues attached to glucose, while probable alphafucosidase A is involved in the degradation of fucosylated xyloglucans. A size reduction of xyloglucan chains in growing tissues could promote cell wall loosening, and lessfucosylated xyloglucans are also less tightly associated with other cell wall components, resulting in reduced extensibility [26,27]. No significant difference in protein trends related to O-fucosylation were observed between IS and DS (Table 2), whereas fold change increased sharply during maturation (Table 1 and 2). Increased expression during maturation might be due to reduce extensibility of cell wall because matured sclerotia got hard.
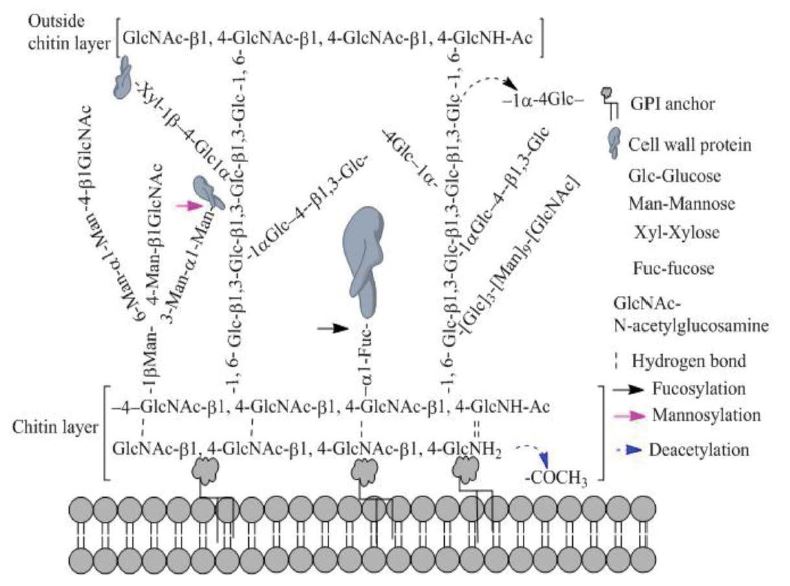
Figure 3:Hypothesis of cell wall architectures of P. umbellatus sclerotia. The supposed cell wall architecture was sketched as a reference in yeast including chitin, GPI anchor, and glucan. Not only enriched biosynthesis of cell wall, but also remodeling including O-fucosylation, O-mannosylation and deacetylation etc.
It has been reported that deacetylation plays an important role in biological attack and defense systems, especially in chitin and chitosan of the fungal cell wall [28]. In P. umbellatus, two types of enzymes are associated with chitin and chitosan metabolism. Chitin synthase takes part in chitin synthesis to form a linear homopolymer composed of β-1-4 linked N-acetylglucosamine (GlcNAc) residues [29]. The conversion of chitin to chitosan is catalyzed by chitin deacetylase and N-acetylglucosamine-6-phosphate deacetylase, which can hydrolyze the N-acetamido groups of N-acetyl-Dglucosamine residues in chitin [30]. The expression of the two deacetylases decreased significantly as sclerotia developed and maturated (validated by PRM as shown in table 1) suggesting that chitin was a basic component of the cell wall in sclerotium and its deacetylation was completed during the initial phase. Deacetylation could expose the amino group in GlcNAc residues, leading to strengthening of hydrogen bonds among chitin homopolymers which are distributed in layers (Figure 3). Moreover, exposed chitin is much easier to conjugate covalently with other cell wall components, such as glucan, proteins and the GPI anchor, and contributes to strengthening cell wall stability.
Conclusion
Cell wall thickening is a common phenomenon in fungi, especially in sclerotia-formed species, such as medicinal fungus Polyporus umbellatus. Understanding the mechanisms of cell wall thickening would be beneficial for the revival of medicinal fungi resources, as well as for fungal pathogen control rationally. 72 biomarkers at protein level were obtained associated with thickened cell wall (TCW) by quantitative proteome analysis and parallel reaction monitoring (PRM) validation among initial sclerotia (IS), development sclerotia (DS) and matured sclerotia (MS). Evidence supported presence and accumulation of chitin, glycan, xylcan and hydrophobins in the cell wall of P. umbellatus sclerotia. Hydrophobin B-like protein Pump122, a new hydrophobin in P. umbellatus expressed up sharply with DS/IS 119.85 and MS/IS 128.17, and located at cell wall and septum of hypha in sclerotia contributing to cell wall thickening and sclerotia development. In addition, sclerotial cell wall could be remodeled via O-mannosylation, O-fucosylation and deacetylation to strengthen cell wall. These findings reveal a novel mechanism for sclerotial cell wall thickening in P. umbellatus, which may also be applicable for other fungus.
Authors’ Contribution
BL and SXG conceived and designed the experiments. BL performed the proteomics studies. BL and LL carried out bioinformatic analysis for this work. YMX and LL assisted BL in PRM quantification. SXG gave the help to carry out targeted protein cellular location tests with immune colloidal gold technique. All author approved the final manuscript.
Conflict of Interests
The authors declare no competing financial interests.
Funding
This research was financially supported by grants from the National Natural Sciences Foundation of China (No. 81773843), Youth Program of National Natural Sciences Foundation of China (No. 81803666) and Chinese Academy of Medical Science Innovation Fund for Medical Sciences (CIFMS, No.2017-I2M-3-013).
Zhao YY (2013) Traditional uses phytochemistry pharmacology pharmacokinetics and quality control of Polyporus umbellatus (Pers) Fries: A review. J Ethnopharmaco 149: 35-48. [ Ref ]
Zhao Y, Park RD, Muzzarelli RA (2012) Chitin Deacetylases: Properties and Applications. Mar Drugs 8: 24-46. [ Ref ]
Bandara AR, Rapior S, Bhat DJ, Kakumyan P, Chamyuang S, et al. (2015) Polyporus umbellatus an edible-medicinal cultivated mushroom with multiple developed health-care products as food medicine and cosmetics: a review. Cryptogamie Mycol 36: 3-42. [ Ref ]
Li B, Tian XF, Wang CL, Zeng X, Xing YM, et al. (2017) SWATH labelfree proteomics analyses revealed the roles of oxidative stress and antioxidant defensing system in sclerotia formation of Polyporus umbellatus. Sci Rep 7: 41283. [ Ref ]
Guo S X, Xu JT (1993) Cytological studies on the process of Armillaria Mellea infection through the sclerotia of Grifola Umbellata. Acta Bot Sin 35: 44-50. [ Ref ]
Xing XK, Guo SX (2005) Morphological characteristics of scletotia formed from hyphae of Grifola umbellate under artiticial conditions. Mycopathologia 159: 583-590. [ Ref ]
Xia HY, Guo SX (2000) Generation and variety of active oxygen species in the hyphae of Grifola umbellate. Mycosystema 19: 576-579. [ Ref ]
Xing YM, Zhang LC, Liang HQ, Lv J, Song C, et al. (2013) Sclerotial formation of Polyporus umbellatus by low temperature treatment under artificial conditions. PLOS One 8: 1-14. [ Ref ]
Chen L, Zhang BB, Cheung PCK (2012) Comparative proteomic analysis of mushroom cell wall proteins among the different developmental stages of Pleurotus tuberregium. J Agric Food Chem 60: 6173-6182. [ Ref ]
Kim Y, Nandakumar MP, Marten MR (2007) Proteomics of filamentous fungi. TRENDS Biotechnol 25: 395-400. [ Ref ]
Liu YS, Borel C, Li, L. Muller T, Williams GE, et al. (2017) Systematic proteome and proteostasis profiling in human Trisomy 21 fibroblast cells. Nat Commun 8: 1212. [ Ref ]
Aebersold R, Burlingame AL, Bradshaw RA (2013) Western blots versus selected reaction monitoring assays: time to turn the tables? Mol Cell Proteomics 12: 2381-2382. [ Ref ]
Chen IH, Xue L, Hsu CC, Paez JS, Pan L, et al. (2017) Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. P Natl Acad USA 114: 3175-3180. [ Ref ]
Wessels JGH (1994) Developmental regulation of fungal cell wall formation. Annu Rev Phytopathol 32: 413-437. [ Ref ]
Linder MB, Szilvay GR, Nakari-Setälä T, Penttilä ME (2005) Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiology Reviews 29: 877-896. [ Ref ]
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. Bio Essays 28: 799-808. [ Ref ]
Christodoulidou A, Briza P, Ellinger A, Bouriotis V (1999) Yeast ascospore wall assembly requires two chitin deacetylase isozymes. FEBS Lett 460: 275-279. [ Ref ]
Matsuo Y, Tanaka K, Matsuda H, Kawamukai M (2005) cda1+ encoding chitin deacetylase is required for proper spore formation in Schizosaccharomyces pombe. FEBS Lett 579: 2737-2743. [ Ref ]
Gastebois A, Fontaine T, Latge JP, Mouyna I (2010) β- (1-3) Glucanosyltransferase Gel4p is essential for Aspergillus fumigates. Eukaryotic cell 9: 1294-1298. [ Ref ]
Lillie SH, Pringle JR (1980) Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol 143: 1384-1394. [ Ref ]
Wilson WA, Hughes WE, Tomamichel W, Roach PJ (2004) Increased glycogen storage in yeast results in less branched glycogen. Biochem Bioph Res Co 320: 416-423. [ Ref ]
Lehle L, Strahl S, Tanner W (2006) Protein glycosylation conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl 45: 6802-6818. [ Ref ]
Lommel M, Strahl S (2009) Protein O-mannosylation: conserved from bacteria to humans. Glycobiology 19: 816-828. [ Ref ]
Arroyo J, Hutzler J, Bermejo C, Ragni E, Garcia-Cantalejo J, et al. (2011) Functional and genomic analyses of blocked protein O-mannosylation in baker’s yeast. Mol Microbiol 79: 1529-1546. [ Ref ]
Goggin DE, Powles SB, Toorop PE, Steadman KJ (2011) Dark-mediated dormancy release in stratified Lolium rigidum seeds is associated with higher activities of cell wall-modifying enzymes and an apparent increase in gibberellin sensitivity. J Plant Physiol 168: 1-533. [ Ref ]
Shigeyama T, Watanabe A, Tokuchi K, Toh S, Sakurai N, et al. (2016) α-Xylosidase plays essential roles in xyloglucan remodelling maintenance of cell wall integrity and seed germination in Arabidopsis thaliana. J Exp Bot 67: 5615-5629. [ Ref ]
Günl M, Pauly M (2011) AXY3 encodes a α-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 233: 707-719. [ Ref ]
Zhao Y, Park RD, Muzzarelli RA (2010) Chitin Deacetylases: Properties and Applications. Mar Drugs 8: 24-46. [ Ref ]
Ruiz-Herrera J (1992) Chemical differentiation of the cell wall In Fungal cell wall: structure synthesis and assembl. CRC Press, Boca Raton. [ Ref ]
Davis LL, Bartnicki-Garcia S (1984) Chitosan synthesis by the tandem action of chitin synthetase and chitin deacetylase from Mucor rouxii. Biochemistry 23: 1065-1073. [ Ref ]