Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 31 May, 2021
Accepted date: 22 June, 2021
Published date: 29 June, 2021
Citation: Joshi J, Paudel P, Bhatt P, Regmi D, Bhattarai T Sreerama L (2021) Characterization of Ethanol Producing Yeasts for their Efficiency in Ethanol Production, Salt Tolerance, and Utilization of Glucose and Xylose. J Appl Microb Res. Vol: 4 Issu: 1 (42-49).
Copyright: © 2021 Sreerama L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Yeast is the mainstay in ethanol production industry. Search for efficient salt tolerant as well as hexose and pentose utilizing yeast strains is important in fermentation industry. In this regard, 12 yeast strains, viz., CDBT1-12, were isolated from various sources and characterized. Molecular characterization of the yeast strains was done by sequencing their D1D2 region of 26S rRNA gene. Out of 12, 10 were found to be Saccharomyces cerevisiae, 1 was Wikerhamomyces anomalous (CDBT7), and the other was Cyberlindnera fabianii (CDBT8). All of the strains were found to be good ethanol producers. CDBT2 was found to have tolerance for high salt (up to 15%) and ethanol (up to 16%) concentrations. CDBT7 was both salt tolerant (up to 15%) as well as utilizes glucose and xylose without compromising on ethanol production efficiency. CDBT2’s ethanol production efficiency was further enhanced by application of low voltage. Under such conditions alcohol dehydrogenase (ADH1) and pyruvate decarboxylase (PDC1) mRNA levels were increased by 2.78 ± 0.80 and 1.12 ± 0.37 fold, respectively, in CDBT2. This observation is novel, it has not been reported previously.
Keywords
Yeast, Molecular Characterization, Alcohol Dehydrogenase, Pyruvate Decarboxylase, External Voltage.
Abstract
Yeast is the mainstay in ethanol production industry. Search for efficient salt tolerant as well as hexose and pentose utilizing yeast strains is important in fermentation industry. In this regard, 12 yeast strains, viz., CDBT1-12, were isolated from various sources and characterized. Molecular characterization of the yeast strains was done by sequencing their D1D2 region of 26S rRNA gene. Out of 12, 10 were found to be Saccharomyces cerevisiae, 1 was Wikerhamomyces anomalous (CDBT7), and the other was Cyberlindnera fabianii (CDBT8). All of the strains were found to be good ethanol producers. CDBT2 was found to have tolerance for high salt (up to 15%) and ethanol (up to 16%) concentrations. CDBT7 was both salt tolerant (up to 15%) as well as utilizes glucose and xylose without compromising on ethanol production efficiency. CDBT2’s ethanol production efficiency was further enhanced by application of low voltage. Under such conditions alcohol dehydrogenase (ADH1) and pyruvate decarboxylase (PDC1) mRNA levels were increased by 2.78 ± 0.80 and 1.12 ± 0.37 fold, respectively, in CDBT2. This observation is novel, it has not been reported previously.
Keywords
Yeast, Molecular Characterization, Alcohol Dehydrogenase, Pyruvate Decarboxylase, External Voltage.
Introduction
Yeast strains are the common dwellers of most of nutrient rich media/sources such as fruits, tree bark, soils etc. [1]. They form one of the important classes of microorganisms that are more complex than bacteria. Yeasts are ovoid single cells that are about 8 μm long and 5 μm in diameter. Their doubling times are 1-3h under optimal growing conditions [Morris et al, 1992]. According to published news reports the global market for yeast and their products has reached nearly $7.6 billion in 2017and it is increasing rapidly and expected to grow to nearly $10.7 billion by 2022 [2]. The most commonly used yeast in baking and brewing industry is Saccharomyces cerevisiae. Besides, many varieties of yeasts, including S. cerevisiae are used in manufacturing of shoyu, miso and production of various fermentation products, e.g., enzymes, vitamins, capsular polysaccharides, carotenoids, polyhydric alcohols, lipids, glycolipids, citric acid etc. [3]. Given the importance of the yeasts and yeast by-products described above, extensive research has been undertaken to identify, catalog and preserve yeast strains worldwide [4].
The process of identification of yeasts involves sequence analysis of conserved ribosomal RNA genes. The ribosomal RNA genes coding for both 18s and 26s RNA have been extensively analyzed and the analysis has proven that it is not only important in establishing them as useful molecular markers for studying evolutionary relationships between organisms but also useful tools for molecular characterization of yeasts [5]. Early studies related to characterization of yeasts and their classifications have shown a widespread pattern of disparity between phenotypes and genotypes. For the purpose of clarity and to systematically classify yeasts, analysis of genes coding for 18S rRNA, internal transcribed spacer of 18s rRNA and the DNA sequences for domains 1 and 2 (D1/D2) of 26s rRNA gene have proven to be optimal [4-6].
Many efforts have been made to isolate and characterize yeasts from various climates of Nepal with applications in baking and brewing, however their molecular characterization and systematic evaluation of their application, especially, in brewing is lacking [7]. An important parameter in selecting brewing yeast is its tolerance to salt and ethanol because the polysaccharide hydrolysates used for fermentation normally contain high salt concentrations and ethanol produced in the process along with salt will destabilize the cultures via the damage they cause to lipid layers [8,9]. Our laboratory has long been interested not only isolating and characterizing various yeast strains from Nepal for various industrial applications, but also assesses their ability to enhance ethanol production under an externally applied electric voltage [10]. Further, we are also interested in isolating yeasts that utilize both glucose and xylose for alcohol fermentation from xylose containing substrates such as lignocellulosic biomass [11]. Described in this study is the isolation and characterization of 12 yeast strains by (i) nucleotide sequencing of D1/D2 domains of 26s rRNA genes, (ii) their ability to tolerate salt and ethanol as well as utilize glucose and xylose as substrates for efficient ethanol production, and (iii) effect of voltage supply on the expression of two important enzymes, viz., alcohol dehydrogenase (ADH1) and pyruvate decarboxylase (PDC1) that are important in ethanol fermentation.
Materials and Methods
Sample collection
Various samples with confirmed yeast sources were collected from different parts of Kathmandu valley, Nepal (Table 1). All of the samples were collected during the months of September and October, and the samples were placed in sterile zip lock bags and stored at 4oC until further analysis.
Table 1: Purification Table for Pectinase from Bacillus subtilis.
| S. No. | Sample | Sampling location | Substrate | Purpose of use/Source | |
|---|---|---|---|---|---|
| 1 | Murcha* | Lubhu, Lalitpur, Nepal | Steamed rice | Brewing | |
| 2 | Manna* | Lubhu, Lalitpur, Nepal | Steamed wheat | Brewing | |
| 3 | Murcha* | Bhaktapur, Nepal | Steamed rice | Brewing | |
| 4 | Manna* | Bhaktapur, Nepal | Steamed wheat | Brewing | |
| 5 | Freshblack grape | Balkhu, Nepal | Grape pulp | Fruit Pulp | |
| 6 | Oak tree bark | Tribhuvan University premises, Kirtipur, Nepal | Oak bark | Wood source | |
| 7 | Guava fruit | Tribhuvan University premises, Kirtipur, Nepal | Guava fruit | Fruit | |
| 8 | Oak Wood | Tribhuvan University premises, Kirtipur, Nepal | Oak tree stem | Wood source | |
| *Manna (yeast starter culture in rice substrate) and Murcha (yeast starter culture in wheat grain). | |||||
Isolation, characterization and selection of yeasts for efficient ethanol production
Isolation of yeasts: Yeast were isolated from various samples (Table 1) by either making an impression on Yeast Maltose Agar (YMA) media (Yeast extract: 3 gm·L-1, malt extract: 3 gm·L-1, peptone: 5 gm·L-1, glucose: 10 gm·L-1 agar: 1.5 gm·L-1 and pH 4.5) or by overtaxing the sample in Yeast Maltose Broth (YMB), followed by serial dilution and spreading them on YMA media [12,13]. The isolated yeast colonies were sub-cultured and stored in YMA slants and/or as 15% glycerol stocks.
Biochemical characterization of yeast isolates: Isolated yeasts were studied for their efficiency of budding, utilization of D-glucose and D-xylose, ethanol production from glucose and xylose, ethanol and salt tolerance. Yeasts were allowed to grow in Peptone Yeast extract Nutrient (PYN) media (Peptone: 3.5 gm·L-1, yeast extract: 3 gm·L-1, KH2PO4: 2 gm·L-1, MgSO4: 1 gm·L-1 and (NH4)2SO4:1 gm·L-1) and observed microscopically to see budding. PYN media supplemented with 2% glucose or xylose was used to determine the growth and ethanol production efficiency. PYN media supplemented with 1-22% salt (sodium chloride) or ethanol was used for the salt and ethanol tolerance test [14].
Studies on glucose and xylose utilization and ethanol production: All of the isolated yeasts were cultured separately in PYN media supplemented with glucose or xylose as a carbohydrate source. The growth of yeast was observed by measuring absorbance at 600 nm (turbidity changes) as described by Sherman [15]. Successively, ethanol production was also measured using the protocol of Seo and associates [16]. The culture broth was centrifuged at 10000 x g for 15 min. One mL of the supernatant was added to1 mL tri-n-butyl phosphate (TBP). The mixture was vortexed for 15 min. Finally, the vortexed mixture was centrifuged at 10000 x g for 15 min to separate layers. About 750 μL of upper layer was transferred to another tube and mixed with equal volume of acidified 5% potassium dichromate reagent. The process of vortexing and centrifuging was repeated. The lower layer was then pooled and absorbance was measured at 595 nm using spectrophotometer.
Study of salt and ethanol tolerance by yeast isolates: All the isolated yeast strains were cultured separately in PYN media supplemented with 0-22% sodium chloride or ethanol respectively and allowed to grow at pH 4.5 and temperature 28oC for 96 h [14]. Microbial growth pattern was observed spectrophotometrically for changes in turbidity (Thermo- Scientific, USA) at 600 nm against medium blank [15].
Molecular characterization of yeasts
Extraction of DNA from yeasts:Total DNA was extracted from broth culture using DNA isolation kit (Promega, Madison, WI, USA). DNA pellet were dried for 15 min in air and finally re-suspended in 40 μL Tris-HCl buffer (10 mM, pH 8). The genomic DNA was verified by running DNA in 0.8% agarose gel electrophoresis. Remaining DNA was stored for PCR analysis.
Amplification of D1D2 region: The 26S rRNA gene D1/ D2 region was amplified by PCR using forward and reverse primers for D1D2 amplification [NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG- 3′) and NL-4 (5′-GGTCCGTGTTTCAAGACGG- 3′) respectively]. The expected amplified PCR fragment was 680 bp [17]. PCR was performed in 25 μL reaction volume containing: 1 μL (45 ng) genomic DNA, 1μL (25 mM) MgCl2, 12.5 μL (2x) master mix (premixed Taq DNA polymerase and mixture of NTPs), 1.5 μL (10 pM) of each primer pair and 7.5 μL nuclease free water. All the reagents for PCR amplification were purchased from New England Biolabs (Boston, Ma, USA). Thermo-cycling conditions were 96oC for 2 min for initial denaturation, followed by 35 cycles of 96oC for 45 sec, 52oC for 45 sec, 72oC for 2 min. Final elongation was done at 72oC for 10 min and, storage temperature was kept at 4oC. PCR was performed in Bio-Rad Thermocycler (Bio-Rad Laboratories, Irvine CA, USA).
Phylogenetic analysis: The PCR products of CDBT1- 8 were sequenced at Yeast Genomics Laboratory, Nova University, Lisbon, Portugal and of CDBT9-12 were sequenced in Excelris Laboratory, Ahmadabad, India. The sequences obtained were edited, compiled, and aligned using Bio-Edit software. Sequence similarity searches were performed using GenBank Blastn protocol. A phylogenetic tree was generated using the neighbor-joining algorithm in MEGA6 (Molecular Evolutionary Genetics Analysis) software.
Effect of applied-electrical current on expression of ADH1 and PDC1: The yeast strains were cultured in an electrochemical cell (ECC) under an applied electrical current as described previously [10,11]. The yeast strains were cultured as described above in PYN media in an ECC. The fermentation cultures in ECC without an external source of electrical current served as controls.
RT PCR (qPCR) was performed to determine the expression of ADH1 and PDC1 with or without supply of electric field by analyzing the mRNA level.
Isolation of RNA from yeasts:Quick-RNATM MiniPrep kit (Zymo Research, Irvine, Ca, USA) was used to isolate RNA from yeast isolated. Yeast samples (200 μl) were suspended and lysed using 600 μl RNA lysis buffer and centrifuged to remove cell debris. The clear supernatant was transferred into spin-away filter fitted with a collection tube, and centrifuged. The filtrate recovered was mixed with equal volume of ethanol (95-100%) and vortexed. The mixture was then transferred to Zymo-spin IIICG column in a collection tube and centrifuged. The flow through was discarded. The column was first washed with 400 μl RNA prep buffer and centrifuged, and flow through was discarded. Again washed two times with 700 μl and 400 μl of RNA wash buffer and centrifuged for 2 min to completely ensure removal of wash buffer. RNA was eluted with 100 μl nuclease free water by centrifugation. The flow through consisted of RNA, which was immediately used to prepare cDNA for further study.
Synthesis of cDNA:Bio-Rad iScriptTM cDNA synthesis kit was used for preparation of cDNA. Reaction parameters used for cDNA synthesis was as per the protocol provided by the manufacturer. The total reaction mixture was 20 μL and it consisted of 5x iScript reaction mix (4 μL), iScript reverse transcriptase (1 μL), nuclease free water (7 μL) and RNA template (8 μL).The components were mixed by pipetting up and down. PCR cycling conditions were as follows: priming (25oC for 5 min), reverse transcription (46oC for 20 min), reverse transcription inactivation (95oC for 1 min) and holding step (4oC). The synthesis of cDNA was tested using 0.8% agarose gel and stored at -20oC for further use.
Quantification of ADH1, PDC1 and TFC1 gene expression:The levels of expression of ADH1 (Alcohol dehydrogenase 1), PDC1 (Pyruvate decarboxylase 1) with reference to TFC1 (transcription factor 1) genes was quantified by RT PCR [18]. The relative quantification technique was used for the comparison of gene expression relative to the reference gene. TFC1 (a housekeeping gene) was used as the reference gene. Advanced Universal SYBER green super-mix dye was used as detector. All the components were thawed to room temperature before use. These reagents and components were centrifuged to collect solutions at bottom of the tube and then stored on ice and protected from light. The reaction mixture contained 15 μL with SYBER green super mix (7.5 μL), forward primer (0.35 μL), reverse primer (0.35 μL), nuclease free H2O (5.8 μL) and cDNA template (1.0 μL). All experiments were done in triplicate to optimize the result. Primers used for RT PCR are shown in Table 2
The PCR cycling conditions included denaturation at 95oC for 2 min, followed by 34 cycles of denaturation at 95oC for 30 sec, annealing at 64oC for 30 sec and extension at 72oC for 30 sec. Melting curves were monitored and when the PCR run was completed, the data obtained was saved. Further calculations were done manually as described by Yuan and associates [19].
ΔCt = Ct (Test sample) – Ct (Reference)
ΔΔCt = Ct (Test sample) – Ct (Control)
Statistical Analysis
All graphs and statistical analysis were performed using Graph Pad Prism 8.0.1. Values reported herein are mean ± standard deviation of three independent experiments.
Results
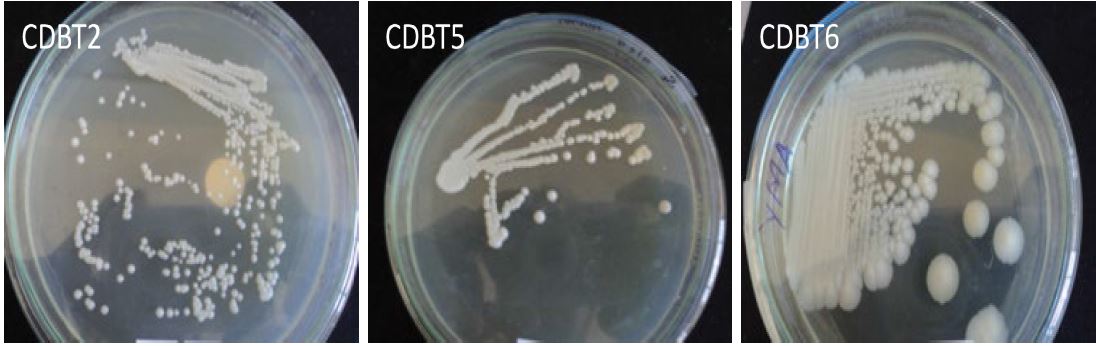
Isolation of yeasts and molecular characterization Morphological study: From the eight different substrates (Table 1) tested, 12 different yeast colonies (CDBT1 to CDBT12) were isolated (Table 3). The isolated yeasts werewhite or creamy colonies with variability in consistency and texture as described by Cletus and associates [20]. All isolates have cottony or rubbery like appearance (Figure 1, Table 3).
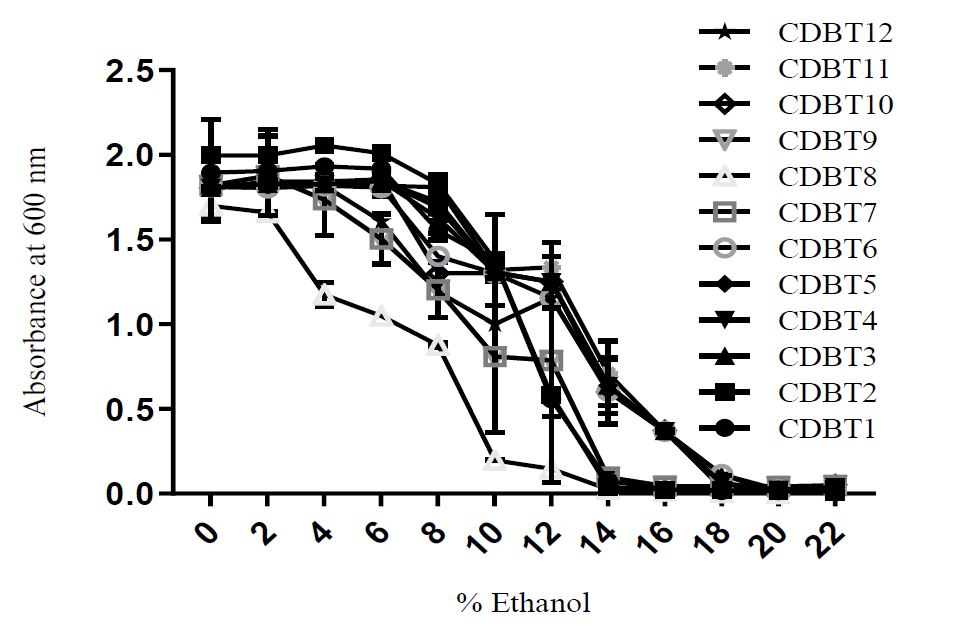
Biochemical characterization of yeast: All the yeast was multiplied by budding and were good ethanol producers (Table 4). CDBT7 and CDBT8, in addition to glucose, were also found to utilize xylose. CDBT2, CDBT3, CDBT7 and CDBT11 were found to tolerate high salt concentrations (15%). All the yeast strains showed normal growth in the presence of ethanol up to 4%, except CDBT8 that can only tolerate 2% ethanol (Figure 2). CDBT2 was found to grow normally in the presence of 6% ethanol. Almost all yeast strains were found to grow in media with 14% ethanol, with the exception of CDBT2, which can resist up to 16% ethanol in the medium. Overall, from biochemical characterization, CDBT2 and CDBT7 were found to be potent strains for ethanol production as they are high salt and ethanol tolerant, and can produce ethanol from glucose as well as xylose.
Table 2: List of primers used in RT PCR of ADH1, PDC1 and TFC1.
| S.No. | Primer Name | Primer Sequence(5’-3’) |
|---|---|---|
| 1. | ALD1F | CGTTTCCGAAGCCGCTATTG |
| ALD1R | GCATACCGACCAAAACGGTG | |
| 2. | PDC1F | GCCAAACGATGCTGAATCCG |
| PDC1R | CCTTGACGTCGTGTCTGGAA | |
| 3. | TFC1F | GCTGGCACTCATATCTTATCGTTTCACAATGG |
| TFC1R | GAACCTGCTGTCAATACCGCCTGGAG |
Table 3: Morphological characterization of yeast strains.
| S.No. | Isolate Designation | Colony Morphology | Isolate identified as |
|---|---|---|---|
| 1 | CDBT1 | Ovoid, smooth | Saccharomyces cerevisiae |
| 2 | CDBT2 | Ovoid, smooth | S. cerevisiae |
| 3 | CDBT3 | Ovoid smooth | S. cerevisiae |
| 4 | CDBT4 | Ovoid, smooth | S. cerevisiae |
| 5 | CDBT5 | Ovoid, smooth | S. cerevisiae |
| 6 | CDBT6 | Diffused | S. cerevisiae |
| 7 | CDBT7 | Diffused | Wickerhamomyces anomalous |
| 8 | CDBT8 | Ovoid, smooth | Cyberlindnera fabianii |
| 9 | CDBT9 | Diffused | S. cerevisiae |
| 10 | CDBT10 | Ovoid, smooth | S. cerevisiae |
| 11 | CDBT11 | Ovoid, smooth | S. cerevisiae |
| 12 | CDBT12 | Diffused | S. cerevisiae |
Figure 1: Yeast isolates grown on YMA media - colony morphologies of representative yeast isolates.
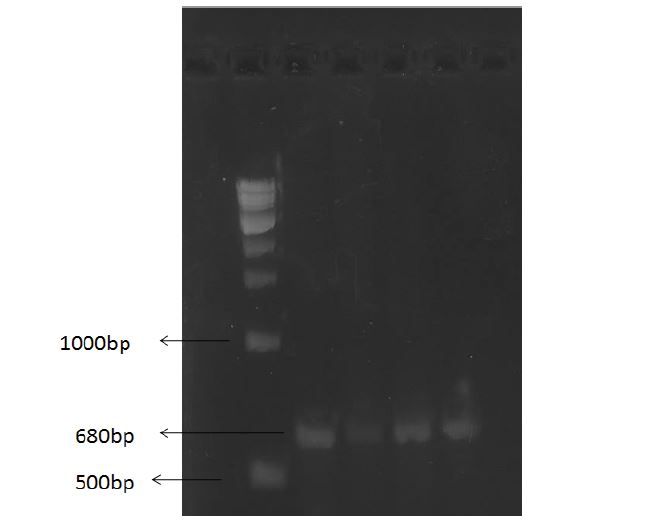
Molecular characterization of yeast:26S ribosomal D1/D2 segment analysis: The 680 bp amplified 26S rDNA products were confirmed by electrophoresis in 1.0% agarose gel (Figure 3). The sequences were edited by BioEdit software and analyzed by NCBI blast [21]. Out of twelve yeasts, ten of them were Saccharomyces cerevisiae and CDBT7 and CDBT8 were Wikerhamomyces anomalous and Cyberlindnera fabianii respectively (Table 3). A phylogenetic tree was developed to see the relatedness between the yeasts (Figure 4) using MEGA6 software. The 26S rDNA fragments of potent yeast strains CDBT2 and CDBT7 were given the gene bank accession numbers MK910215 and MK910216 respectively [10].
Figure 2: Effect of ethanol concentration on yeast growth. Ethanol concentrations tested ranged of 0 - 22%.
Figure 3: Amplification of 680bp fragment of D1D2 of 26S rDNA from representative yeast isolates. L1: NEB 100bp ladder, L2:CDBT1, L3:CDBT2, L4:CDBT3 and L5:CDBT4.
Figure 4:Phyl ogenetic tree based on sequences of the D1/D2 region of the rDNA 26S gene. The tree shows the position of CDBT isolates to be closely related yeast species. The tree was constructed based on the genetic distances obtained according to MEGA6 using the neighbor-joining method.
Table 4: Study of different features and characteristics of yeasts: Summary
| Yeast (CDBT)/ Characters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Budding | + | + | + | + | + | + | + | + | + | + | + | + |
| Growth/D-Glucose | + | + | + | + | + | + | + | + | + | + | + | + |
| Growth/D-Xylose | - | - | - | - | - | - | + | + | - | - | - | - |
| Ethanol production from glucose | + | + | + | + | + | + | + | + | + | + | + | + |
| Ethanol production from xylose | - | - | - | - | - | - | + | + | - | - | - | - |
| Salt tolerance (% w) | 9 | 15 | 15 | 6 | 6 | 6 | 15 | 9 | 9 | 10 | 15 | 8 |
Table 5: Average Ct values of ADH1, PDC1 and TFC1 genes obtained from RT PCR.
| S. No. | Culture Types | Ct values | ||
|---|---|---|---|---|
| ADH1 | PDC1 | TFC1 | ||
| 1 | CDBT2 (normal growth) | 3.35 ± 0.51 | 3.45 ± 0.10 | 2.49 ± 0.21 |
| 2 | CDBT2 (electrochemically enhanced) | 1.95 ± 0.26 | 3.36 ± 0.01 | 2.57 ± 0.29 |
ADH1 and PDC1 Expression Analysis
Previously, we have demonstrated enhancement of ethanol production in CDBT2 cultures supplied with low levels of applied electrical current [10]. The rationale for the increased levels of ethanol could be overexpression of key alcohol fermentation genes, viz., ADH1and PDC1. Accordingly, total RNA was isolated from CDBT2 strain cultured under normal conditions and in an electrochemical cell in the presence of 2V, applied electric current [Joshi et al 2019]. The cDNA was prepared from isolated RNA. Both the RNA and cDNA preparations were confirmed by running in 1% agarose gel. Real time qPCR was used to quantify the relative expression of “PDC1 and ADH1” in normal and electrochemically enhanced yeasts.
Gene expression was analyzed taking same amount of template for both reference/ housekeeping gene TFC1 and test genes PDC1 and ADH1. Relative expression of PDC1 and ADH1 was then calculated comparing the expression of TFC1 gene as reference gene. Gene expression in CDBT2 cultured under normal growth conditions was used as control and CDBT2 cultured in an electrochemical cell under applied electric current (2V) was used as test sample. The Ct data obtained in Table 5 clearly revealed high expression of both the genes than in normal condition. When the obtained Ct data were used to calculate the relative expression of ADH1 and PDC1 genes using the protocol given by Yuan et al. (2006), ADH1 and PDC1 genes were found to express 2.78 ± 0.80 and 1.12 ± 0.37 fold more than normal fermentation indicating that external voltage supply during growth of yeast enhanced enzyme expression. While the overexpression of PDC1 was, although lower, it was always consistently higher than the control in all of our experiments. On the other hand, ADH1 is always consistently and significantly overexpressed (Table 5).
Discussion and Conclusion
Among the 12 yeast isolates, most were found to be S. cerevisiae strains. It is long been known that many strains of S. cerevisiae are involved in alcohol fermentation, accordingly exhibit polymorphism [22]. The polymorphism in S. cerevisiae has no correlation between phenotypic and genotypic characteristics [23]. All isolates utilize glucose as substrate for fermentation. Additionally, CDBT7 (W. anomalous) and CDBT8 (C. fabianii) also utilize xylose as substrate for fermentation. W. anomalous strain isolated from sugar beet thick juice was found to have a comparable ethanol yield, but needed longer fermentation time and can utilize xylose [24]. CDBT2 strain, on the other hand, is found to be a potent yeast strain for ethanol production using glucose as substrate, with tolerance to high salt and ethanol concentrations. Yeast strain CDBT7 is not only tolerant to high salt and ethanol concentrations; but also utilize both glucose and xylose to produce ethanol from xylose rich source like lignocellulosic biomass hydrolysate which are most common and widely available substrates. [11]. Selection of salt and ethanol tolerance strain is a must when a yeast strain is used for industrial production of ethanol using neutralized media in optimized condition [25]. Among the various conditions that cause stress to yeast cells during ethanol fermentation, include ethanol toxicity, adverse environmental factors, osmotic shock and salt pressure [26]. The inability of yeast to adapt to these stressful conditions results in slow or incomplete alcohol fermentation [27]. According to Sutticha and associates, a strain with ethanol tolerance of up to 5% is considered as good isolate for ethanol production [28]. Most of the isolates of S. cerevisiae reported herein could retain viability up to 46% in the presence of 5% ethanol up to 48h. This observation is consistent with literature reports [14]. In this regard, CDBT2 can be a good strain for industrial ethanol production as it grows normally up to 6% ethanol. The other stains described herein show significant decrease in growth after 4%. These results were similar to those reported by Chiranjeevi and associates [29]. On the other hand, according to Gonzalez and associates, the ethanol tolerance is found to vary slightly with media composition and culture condition [30].
Induced expression of ADH1 and PDC1 in CDBT2 in an electrochemical cell under low applied electrical field (2 V) is a novel observation and this observation is being further evaluated to enhance ethanol production by various strains of yeasts, fungi and bacteria in our laboratory. TFC1, a housekeeping gene, was used as reference gene to compare induced expression of ADH1 and PDC1 genes. TFC1 is one of the six subunits of RNA polymerase III-transcription factor complex TFIIIC. It is an essential factor to regulate expression of housekeeping genes. TFC1 gene is located in chromosome II (484742--- 486691). PDC1 is the major isozyme among the three pyruvate decarboxylases present in yeast. PDC1 is a key enzyme in alcoholic fermentation that decarboxylates pyruvate to acetaldehyde. PDC1 is also involved in amino acid catabolism. In the yeast genome, PDC1 is located on chromosome XII (232390 --- 234081) [Kellermann et al., 1986]. Given the fact that, PDC1 is involved in decarboxylation of both amino acids as well as alpha-keto acids, it may serve as a housekeeping gene and its expression levels are always higher. This may be reason for low levels of its overexpression under applied electrical current in this study. It is important to note that, in all of the experiments we have conducted so far, although low, PDC1 levels are consistently higher compared to the controls. ADH1 is the main isozyme required for the reduction of acetaldehyde to ethanol (a rate determining step in ethanol fermentation) in yeast out of four isozymes (ADH 1, 2, 3 and 5). ADH1 gene is located on yeast chromosome XV (159548 --- 160594). ADH5 is a paralog of ADH1, which arose from the whole genome duplication. The significant overexpression of ADH1, make sense as the enzyme not only catalyzes a rate determining step but also involved in oxidative detoxification reaction. It is well established that aldehydes cause oxidative stress and their reduction to alcohol is normally considered a detoxification process. In the present study, we have clearly shown the over expression of both PDC1 (although to a lower extent) and ADH1 mRNA in S. cerevisiae CDBT2 strain cultured in an electrochemical cell under low levels of applied electrical current (2V). Previously, we have also reported increased levels of ethanol production by CDBT2 yeast strain under applied electrical field [10]. Induced expression of PDC1 and ADH1 under applied electrical current reported herein is a novel observation. Consistent with this observation is the fact that ADH1 and PDC1 over expression leads to increased production of ethanol in yeast and bacteria [31,32].The combination of the two strains can be good candidates for industrial ethanol production from lignocellulosic biomass hydrolysates.
Acknowledgement
The authors are thank full to Central Department of Biotechnology, Tribhuvan University for providing, laboratory space and instrumental facilities. Our sincere thanks to Dr. Paula Gunclaves, yeast Genomics Laboratory, Nova University, Lisbon, Portugal for sequencing PCR products and valuable suggestions.
Ethical Issues
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
There is no conflict of interest for all authors.
Alfenore S, Molina-Jouve C, Guillouet SE, Uribelarrea JL, Goma G, et al. (2002) Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl Microbiol Biotechnol 60: 67-72. [ Ref ]
GLOBE NEWSWIRE (2017) Yeasts, Yeast Extracts, Autolysates and Related Products: The Global Market. Research and Markets. Dublin. [ Ref ]
Türker M (2014) Advances in Science and Industrial Productions of Baker’s Yeast. Conference: Yeast Biotechnology: Diversity and Applications. Proceedings of 27th VH Yeast Conference, Istanbul. [ Ref ]
James CM, Indge KJ, Oliver SG (1995) DNA sequence analysis of a 35 kb segment from Saccharomyces cerevisiae chromosome VII reveals 19 open reading frames including RAD54, ACE1/CUP2, PMR1, RCK1, AMS1 and CAL1/CDC43. Yeast 11: 1413-1419. [ Ref ]
Ciardo DE, Schär G, Böttger EC, Altwegg M, Bosshard PP (2006) Internal Transcribed Spacer Sequencing versus Biochemical Profiling for Identification of Medically Important Yeasts. J Clin Microbiol 44: 77-84. [ Ref ]
Kurtzman C, Raquel P, Quintilla M, Anna K, Bart T, et al. (2015) Advances in yeast systematics and phylogeny and their use as predictors of biotechnologically important metabolic pathways. FEMS Yeast Research 15: fov050. [ Ref ]
Karki TB, Timilsina PM, Yadav A (2017) Selection and Characterization of Potential Baker’s Yeast from Indigenous Resources of Nepal. Biotechnology Research International. [ Ref ]
Kodama S, Nakanishi H, Thalagala TA, Isono N, Hisamatsu M (2013) A wild and tolerant yeast suitable for ethanol fermentation from lignocellulose. J BiosciBioeng 115: 557-61. [ Ref ]
Stanley D, Fraser S, Stanley GA, Chambers PJ (2010) Retrotransposon expression in ethanol-stressed Saccharomyces cerevisiae. Appl Microbiol Biotechnol 87: 1447-1454. [ Ref ]
Joshi J, Dhungana P, Prajapati B, Maharjan R, Poudyal P, et al. (2019) Enhancement of Ethanol Production in Electrochemical Cell by Saccharomyces cerevisiae (CDBT2) and Wickerhamomyces anomalous (CDBT7). Frontiers in Energy Research 7: 61-70. [ Ref ]
Joshi J, Bhattarai T, Sreerama L (2018) Efficient Methods of Pretreatment for the Release of Reducing Sugars from Lignocellulosic Biomass Native to Nepal and Characterization of Pretreated Lignocellulosic Biomass. International Journal of Advanced Biotechnology and Research 9: 9-23. [ Ref ]
Karki T, Shrestha H (1999) Fermentation process of Nepal murcha starters. Proceedings of III National Conference on Science and Technology. [ Ref ]
Middlehoven WJ (2002) Identification of yeast present in sour fermented foods and fodders. Mol Biotechnol 21: 279-292. [ Ref ]
Balakumar S, Arasaratnam V (2012) Osmo, thermo and ethanoltolerances of Saccharomyces cerevisiae. Braz J Microbiol 43: 157-166. [ Ref ]
Sherman F (2002) Getting Started with Yeast. Methods in Enzymology 350: 3-41. [ Ref ]
Seo HV, Kim H, Kim O, Lee H, Jung K (2009) Measurement of ethanol concentration using solvent extraction and dichromate oxidation and its application to bioethanol production process. J Ind Microbiol Biotechnol 36: 285-292. [ Ref ]
Cocolin L, Aggio D, Manzano M, Cantoni C, Comi G (2002) An application of PCR-DGGE analysis to profile the yeast populations in raw milk. Int Dairy J 12: 407-411. [ Ref ]
Smidt OD, James C, Preez D, Albertyn J (2011) Molecular and physiological aspects of alcohol dehydrogenases in the ethanol metabolism of Saccharomyces cerevisiae. FEMS Yeast Research 12: 33-47. [ Ref ]
Yuan J, Reed A, Chen F, Neal SC (2006) Statistical analysis of real-time RT-PCR data. BMC bioinformatics 7: 85-97. [ Ref ]
Cletus P, Kurtzman JWF, Teun B, Vincent R (2011) Methods for isolation, phenotypic characterization and maintenance of Yeasts- Chapter 7. The Yeasts, Taxonomic Study 5: 87-110. [ Ref ]
Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41: 95-98. [ Ref ]
Lene J (2003) Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Research 3: 191-200. [ Ref ]
Granchi L, Ganucci D, Buscioni G, Mangani S, Guerrini S (2019) The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery- Strains. Fermentation 5: 86. [ Ref ]
Ruyters S, Mukherjee V, Verstrepen KJ, Thevelein JM, Lievens B (2015) Assessing the potential of wild yeasts for bioethanol production. J Ind Microbiol Biotechnol 42: 39-48. [ Ref ]
Ekunsanmi TJ, Odunfa SA (1990) Ethanol tolerance, sugar tolerance and invertase activities of some yeast strains isolated from steep water of fermenting cassava tubers. Journal of Applied Bacteriology 69: 672-675. [ Ref ]
Logothetis S, Walker G, Nerantzis E (2007) Effect of salt hyperosmotic stress on yeast cell viability. Proc Nat Sci Matica Srpska Novi Sad 113: 271-284. [ Ref ]
Zhao XQ, Bai FW (2009) Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. Journal of Biotechnology 144: 23-30. [ Ref ]
Sutticha N, Thammasittirong T, Thammasittirong T, Malee S (2013) Improvement of ethanol production by ethanol-tolerant Saccharomyces cerevisiae UVNR56. Springer Plus 2: 583. [ Ref ]
Chiranjeevi T, Osuru H, Navya A, Praveen C, Veera R, et al. (2013) Isolation and characterization of ethanol tolerant yeast strains. Bioinformation 9: 421-425. [ Ref ]
Gonzalez R, Tao H, Shanmugan KT, York SW, Ingram LO (2002) Global gene expression differences associated with changes in glycolyitc flux and growth rate in Escherichia coli during the fermentation of glucose and xylose. BiotechnolProg 18: 6-20. [ Ref ]
Kata I, Marta VS, Justyna R, Kostyantyn VD, Andriy AS (2016) Overexpression of the genes PDC1 and ADH1 activates glycerol conversion to ethanol in the thermotolerant yeast Ogataea (Hansenula polymorpha). Yeast 33: 471-478. [ Ref ]
Tian L, Perot SJ, Hon S, Zhou J, Liang X, et al. (2017) Enhanced ethanol formation by Clostridium thermocellum via pyruvate decarboxylase. Microbial cell factories 16: 171. [ Ref ]
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 25: 402-408. [ Ref ]