Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 09 June, 2020
Accepted date: 01 July, 2020
Published date: 08 July, 2020
Citation: Garima S, Kumud T, Singh SK (2020) Curing of Mammalian Cell Lines from Severe Bacterial Contamination. J Appl Microb Res. Vol: 3 Issu: 2 (01-06).
Copyright: © 2020 Singh SK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Mammalian cell culture has proven to be crucial in studying human cellular physiological processes. However, microbial contamination continues to be a common and serious problem faced by researchers worldwide, which unfortunately persists even after different aseptic measures are meticulously followed. This can have serious consequences like spurious, non-reproducible data leading to compromised results and loss of important biological samples. In this article, we report identification and eradication of bacterial contamination encountered during maintenance of mammalian cell line culture. The contamination was so severe that we were on verge of losing the cell lines. 16S rDNA sequencing identified the contaminant as gram-positive bacteria from genus Bacillus which was already resistant to penicillin and streptomycin. In order to contain and finally get rid of the bacterial contaminants, the cultures were treated for two weeks with a cocktail mixture of antibiotics ciprofloxacin and gentamicin in different doses, to estimate the effective curing dose with least cytotoxicity. Eventually, the cells got completely cured of bacterial contamination. Henceforth, cell lines maintained even without additional antibiotic pressure remained healthy and normal for long term (under the microscope). Finally, by fluorescence-activated cell sorting (FACS) it was confirmed that these cells have retained their normal cell cycle properties.
Keywords
Mammalian cell culture, Bacterial Contamination, Curing.
Abstract
Mammalian cell culture has proven to be crucial in studying human cellular physiological processes. However, microbial contamination continues to be a common and serious problem faced by researchers worldwide, which unfortunately persists even after different aseptic measures are meticulously followed. This can have serious consequences like spurious, non-reproducible data leading to compromised results and loss of important biological samples. In this article, we report identification and eradication of bacterial contamination encountered during maintenance of mammalian cell line culture. The contamination was so severe that we were on verge of losing the cell lines. 16S rDNA sequencing identified the contaminant as gram-positive bacteria from genus Bacillus which was already resistant to penicillin and streptomycin. In order to contain and finally get rid of the bacterial contaminants, the cultures were treated for two weeks with a cocktail mixture of antibiotics ciprofloxacin and gentamicin in different doses, to estimate the effective curing dose with least cytotoxicity. Eventually, the cells got completely cured of bacterial contamination. Henceforth, cell lines maintained even without additional antibiotic pressure remained healthy and normal for long term (under the microscope). Finally, by fluorescence-activated cell sorting (FACS) it was confirmed that these cells have retained their normal cell cycle properties.
Keywords
Mammalian cell culture, Bacterial Contamination, Curing.
Introduction
Over the last half-century, cell culture has become a common model to study and investigate human cellular processes which has proved to be a breakthrough in understanding how the various mechanisms inside the cell work. The limitless availability, inexpensiveness and relatively easy handling of mammalian cells have made cell culture technology an important biomedical tool. Amongst all problems, the most common problem affecting this technology worldwide is unexpected contamination either from cross-contamination or from micro-organisms. The major microbial contaminants of mammalian cell cultures are bacteria, fungi, yeast and viruses, which may be sourced from the cells itself, media, inefficient aseptic practices or airborne (Table 1) [1]. The most common causative agent contaminating mammalian cell lines are mycoplasma (5-35%) [2]. Mycoplasma contamination could be detected by various kits available in the market and cured using antimycotic agents like plasmocin etc. [3]. Bacterial contamination can trigger series of mammalian signaling pathway via Toll-like Receptors (TLRs) [4-6], which can influence the cell milieu and can affect various cellular characteristics and parameters that can sabotage the results. Hence, contamination of cell lines could have severe consequences resulting in unreliable data, loss of money, loss of time and in extreme cases loss of valuable cell lines. Therefore, identification of these contamination(s) in time is very important. Thus, in such cases, all possible attempts need to be made towards removal of the contamination from the cell lines without harming or changing their inherent properties. Several practices like filtration, supplementation of antibiotic/antimycotic into the medium have been adopted by scientists to keep the contamination in check. Streptomycin in combination with penicillin is the most commonly used antibiotic supplement in the medium which shows an effective response against most of the bacterial species.
Table 1: Mammalian Cell Culture Contaminants and Their Detection.
| Micro-organismic Contaminants | General Indication | Microscopic Appearance | Common Source | Detection Methods | Reference |
|---|---|---|---|---|---|
| Mycoplasma | Changes in cell growth characteristics (poor adherence, reduced growth rate) | Cannot be detected | Cross-contamination and airborne microscopic aerosols | PCR (16S rDNA sequencing), microbiological culture, fluorescent staining (e.g., Hoechst 33258), ELISA, immunostaining, autoradiography and nucleic acid hybridization | [7-10] |
| Bacteria | Increased turbidity, shift in media pH (change in medium color) and cell destruction | Fine black dots/granules in the space between cells | Water bath, FBS, during cell culture handling | Microscopy, 16S rDNA sequencing bacterial culture | [7,11,12] |
| Fungus | Furry growth leading to cloudiness in medium and basic pH | Budding Chain/Oval. Organism can sometimes be multi-branched. Whitish, yellowish, or black mycelium in culture Later stages-- appear large fuzzy patches in the dish | Cross-contamination and airborne aerosols, spores from heating and air-conditioning systems | Microscopy, fungal culture, PCR | [7,13] |
| Virus | Cell destruction | Cannot be detected | Serum based medium, FBS | Hemadsorption, electron microscopy, immunofluorescence and PCR | [14] |
Recently, while propagating Human Embryonic Kidney 293T (HEK293T) and C33A cervical cancer (HPV negative) cell lines, which we had just acquired from a cell line repository, it was discovered that the cells have got severe bacterial contamination, so much so that we were on verge of losing the cell lines. Under low-resolution microscope, the contaminants looked like swimming black dots in the medium [15]. Advice and help were sought from researchers at several research blogs and other online discussion forums, but received no certain response. In this process, interestingly, we discovered that there are lots of researchers/labs who had or continues to face this kind of problem and have lost important cell lines. Hence, it was decided to solve this problem systematically. This problem has been processed through the identification of bacterial contaminant and successful curing method of cell lines. Also, results showed that upon curing, the cells were normal in their morphology and cell cycle behavior. That is why the idea of the study emerged to solve the problems of workers in this field.
Materials and Methods
Mammalian cell lines and culture condition
HEK293T is an immortalized cell line containing SV-40 large T antigen and C33A is an epithelial cervical cancer cell line. Both of these mammalian cells were purchased from NCCS (National Centre for Cell Science, Pune) and were grown in DMEM medium (Cell Clone, India) supplemented with 10%-20% (v/v) fetal bovine serum (FBS) (Gibco Life Technologies, USA), 1% (v/v) routine antibiotics (penicillin and streptomycin, Cell Clone, India) at 37ºC incubator with 5% CO2 and 95% humidity. The cell growth was monitored by counting the cells using hemocytometer. Morphology of the cells was observed under a phase-contrast microscope (Leica, Germany) at 10X, 20X and 40X magnifications routinely.
Mycoplasma detection by PCR
A 500 μl volume of cell suspension was aseptically removed from 70-80% confluent cells and used for mycoplasma detection using EZdetectTM PCR Kit (Himedia, India) according to the manufacturer’s instruction [2]. The PCR condition was 95ºC for 5 min, 35 cycles of 30 sec at 95ºC, 30 sec at 55ºC, and 1 min at 72ºC, with final elongation step of 10 min at 72ºC and hold the reaction at 4ºC. The amplified products were then run on 2% agarose gel electrophoresis and visualized by ethidium bromide staining [16].
Anti-mycoplasma treatment
Anti-mycoplasma treatment was given routinely to the cells using a mycoplasma elimination kit (Himedia, India) as per the protocol mentioned in the manual.
16S rDNA sequencing and identification
One ml of the confluent cell suspension (90 % contaminated based on cell count) was removed and pelleted down at 10000 rpm for 5 mins. Bacterial genomic DNA was isolated using Qiagen kit following their protocol. The DNA was then amplified using 16S rDNA universal primers; 8F-5’ AGATTTGATCCTGGCTCAG 3’ and 1492R- 5’ GGTTACCTTACGACTT 3’ as described by Sambrook et al [16]. For sequencing, the amplified product, along with forward (8F) and reverse (1492R) primers were submitted to GeNei (India). The sequences obtained were then blasted to NCBI’s BlastN to find the corresponding match. The blast results showed 99 % identity with various species of genus Bacillus.
Antibiotic treatment
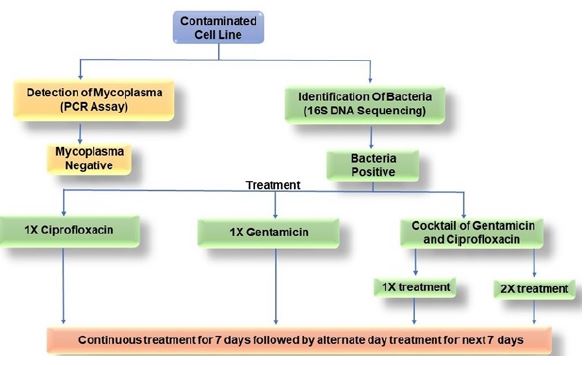
After referring numerous literatures [17,18] and going through initial screening, two antibiotics, ciprofloxacin hydrochloride and gentamicin sulphate (SRL; India), were used in two different concentrations and four different combinations as mentioned in Table 2 and Figure 6. Antibiotic doses were supplemented in the DMEM medium every time the medium was changed.
Cell cycle analysis by flow cytometry
Approximately two million cells were harvested either by pipetting (loosely adhered) or by trypsinization (Trypsin, Cell Clone, India.) followed by 2 washes of phosphate buffered saline (PBS). The washed cells were then fixed with freshly prepared 70% ethanol (in PBS) and incubated at -20oC overnight for proper permeabilization. Fixed cells were washed once with PBS and then stained with propidium iodide (PI) staining solution containing 100 μg/ ml PI (Himedia, India), 100 μg/ul RNase A (Himedia, India) and 0.1% Triton X-100 (SRL, India) [19]. The stained sample was then run on Beckman Coulter fluorescence-activated cell sorter (USA) and the results were analyzed by the Navios software provided with the machine.
Results and Discussion
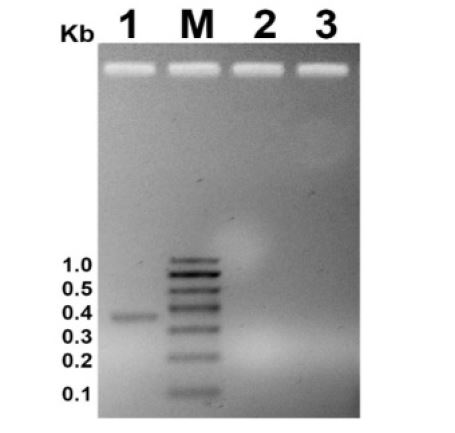
Immediately, after acquiring the cells from repository they were seeded back to propagate further and maintain the stock. After seeding HEK293T and C33A cells, change in cell behavior, such as loss of adherence followed by a high rate of cell death was observed via microscopic examination. Since 5-35% of mammalian cell contaminations showing this kind of symptoms are due to mycoplasma [2,8], the first suspect was mycoplasma infection. A PCR based assay was used to detect the mycoplasma contamination (if any). The PCR assay detected mycoplasma specifically on the basis of highly conserved spacer region present between 16S and 23S rRNA genomic DNA sequence, among various mycoplasma species. In case of positive contamination, the amplified product should be of 370-500 bp long. Using specific primers for mycoplasma detection, no bands could be observed of the specified length (Figure 1 Lane 1 vs 2 and 3). Hence, absence of any amplified product in the PCR assay, rules out the presence of mycoplasma contamination in the mammalian cell cultures.
As mentioned earlier and in the coming results and their explanations, it would be worthy to mention that, the microscopic observation at 10X revealed the presence of black shimmering dots in the culture, which at 20X and 40X seemed to be rod-shaped and wiggling (Figure 2: a,b,c,d and Figure 3: a, d). Also, the change in media color from dark pink to orange indicated the change in pH of the medium and thus a possible bacterial contamination [12]. In order to verify and identify the bacterial contamination, 16S rDNA gene sequencing of the samples was performed using universal primers (as mentioned in materials and methods). The blast result showed more than 99 % identity with genus Bacillus. The highest identity (100%) was with Bacillus cereus. Bacillus cereus is a gram-positive, sporeforming opportunist pathogen. Earlier reports had shown that this pathogen is highly toxic to mammalian cell cultures. It produces cytotoxic compounds which permeabilize and kill mammalian cells. Moreover, the effect becomes more potent if higher amount of serum is present in the medium [20]. Hence, we speculate that the mammalian culture cells were also getting killed by the same mechanism.
Figure 1:PCR profile for mycoplasma detection. M is molecular marker. Size of the bands are indicated on left side in kb. Lane 1 represents positive control (mycoplasma DNA provide with the kit). Lane 2 and 3 represents HEK293T and C33A sample respectively.
Table 2: Antibiotic Doses Used in the Study.
| Treatment doses | Ciprofloxacin Hydrochloride | Gentamicin Sulphate |
|---|---|---|
| CPFX | 10 μg/ml | ------- |
| GM | -------- | 50 μg/ml |
| 1X Cocktail | 10 μg/ml | 50 μg/ml |
| 2X Cocktail | 20 μg/ml | 100 μg/ml |
The chronically contaminated mammalian cells were then treated with ciprofloxacin hydrochloride and gentamycin sulphate in four different predefined conditions (as described in Table 2) since it had already shown to be resistant to the penicillin-streptomycin cocktail (which were used in the medium as a default practice). The abovementioned antibiotic is used either individually or in cocktail because ciprofloxacin (CPFX) is a broad-spectrum antibiotic belonging to the fluoroquinolone class. It acts as a cell division inhibitor by blocking DNA gyrase activity and hence bacterial DNA synthesis [21]. Whereas, gentamycin (GM) is an aminoglycoside and acts as a protein inhibitor by binding to the 30s subunit of the bacterial ribosome [22]. Hence, combination of these two antibiotics was of good chance to address the treatment of severe Bacillus contamination. There could be three probable outcomes of the treatment: (i) cured: the cells became all free from bacterial contamination; (ii) resistant: there would be no effect on contaminants and eventually resulting in the loss of culture due to cytotoxic effect of bacterial contamination; (iii) stress: the cells became free from bacterial contamination, but due to treatment the cell culture would be affected in such a way that cells lose their inherent property or get arrested in certain phase of the cell cycle.
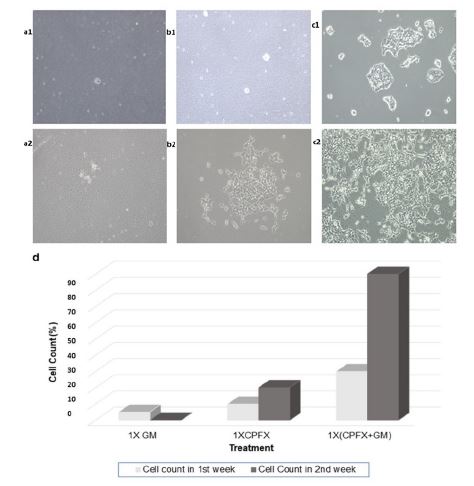
Figure 2:Effect of antibiotic/s on HEK293T cells. a1 and a2 shows GM treatment effect after first and second week of the treatment respectively. b1 and b2 shows CPFX treatment effect after first and second week of the treatment respectively. c1 and c2 shows 1X cocktail treatment effect after first and second week of the treatment respectively. d Curing efficiency of different doses of the treatment.
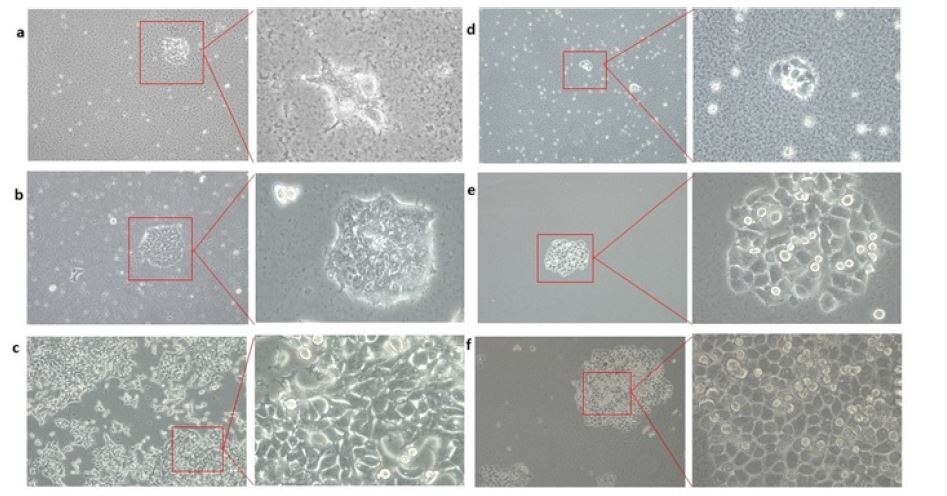
Figure 3:Curing of Bacillus sp. contamination from mammalian cultures. a HEK293T cells before treatment at 10X and 40X magnification. b HEK293T cells after first treatment at 10X and 40X magnification. c HEK 293T cells after curing at 10X and 40X magnification. d C33A cells before treatment at 10X and 40Xmagnification. e C33A cells after first treatment at 10X and 40X magnification. f C33A cells after curing at 10X and 40X magnification.
The infected mammalian cell cultures were divided and treated in parallel as described in table 2. The cell culture treated with 50 μg/ml of GM alone showed no response to the treatment in the 1st week and by the end of the second week all the cells were dead and that batch of the culture was lost (Figure. 2: a1and a2). Hence, one can understand how severe the contamination became eventually in that set of cultures. Whereas, in case of 10 μg/ml CPFX treatment, the culture showed some promising results in the first week and by the end of the second week the cells started proliferating (Figure. 2: b1, b2 and d), but the growth rate was much slower and the bacterial contaminants were still growing in the culture (50% curing). While 1X cocktail treatment consisting of both the antibiotics cured the cells almost more than 90% by the end of the second week of treatment (Figure. 2: c1, c2 and d).
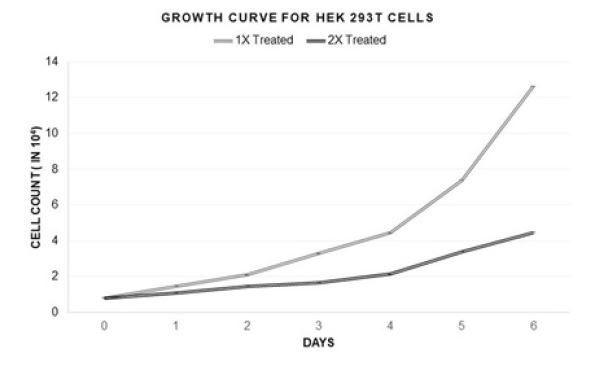
This curing technique has worked for both of the contaminated mammalian cell lines HEK293T and C33A (Figure 3). This treatment method was also found to be successful in rescuing bacterially contaminated A549 epithelial lung carcinoma cell line to normalcy from almost losing them (personal communication with Dutta lab, University of Virginia, USA). Though the cocktail at 1X concentration (as mentioned in Table 2) was working efficiently, and a decision was taken to check the effect of a higher dose (2X concentration) of the same, to see whether that could increase the efficiency and cure the cells faster, which will save valuable time. The results in Figure 4, indicates that the cocktail treatment in 1X concentration was able to cure HEK293T culture with almost no growth suppression, whereas the 2X amount of the same cocktail reduced the growth rate of the cells to more than 60%. Percentage of curing was decided on the basis of the number of live cells and the relative contaminants present in the culture.
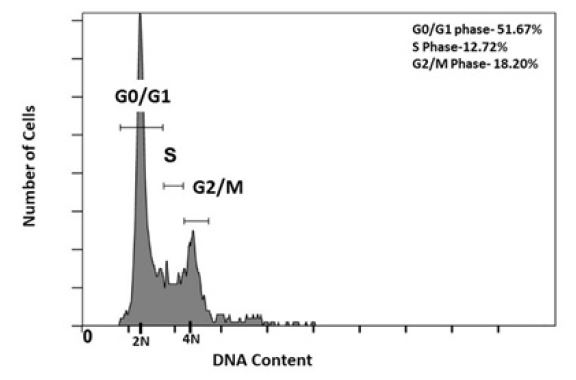
Many drugs interfere and alter the cell cycle behavior of mammalian cells upon treatment. Hence, the best way to know whether the cured cells have healthy physiology or not, is by checking their cell cycle progression behavior. For that, the FACS analysis was carried out after staining cured HEK293T cells with PI. The cell cycle analysis of this cured population shows distribution of cells in 3 major phases; G0/G1, S and G2/M of the cycle (Figure 5). The histogram peaks represent the total population of cells in G0/G1 to be 51.67%, in S phase to be 12.72% and in G2/M Phase to be 18.20% which is highly similar to the normal HEK293T cell behavior [23]. This indicates that the antibiotic treatment did not have any adverse effect on cell cycle physiology. In order to understand in more detail, further proteomics and genomics analysis are recommended, which at this stage is beyond the scope of this article. Therefore, culturing the cured cells was conducted for further several generations. Recurring of contamination in any form and/or loss of normal cellular behavior of the treated cell lines was not observed.
Figure 4:Growth curves for HEK293T cells after 1X and 2X treatments.
Figure 5:Cell cycle analysis of cured HEK293T cells by flow cytometry. The X axis of the histogram represents DNA content of the population. 2N and 4N DNA content represents G0/G1 and G2/M phases of cell cycle respectively.
Figure 6:Workflow of this curing study.
Conclusion
Bacterial contamination can alter mammalian cellular physiology which could lead to undesirable results. If the bacterial contamination is detected in early phases, the chances of getting rid of it by the use of antibiotics are much higher, while heavy infection could become impossible to treat which could result in loss of time, money and unique cell lines. The study performed on contaminated HEK293T and C33A cells revealed that the cultures were contaminated with Bacillus sp., which could be completely cured by antibiotic treatment with regular monitoring. After two weeks of treatment, the cells were completely cured without affecting the morphology and cell cycle behavior. Although, obliteration of bacteria with antibiotic treatment is time consuming, but it is an economical and efficient approach, especially in case of some rare cell lines. This study is the first to describe a detailed curing process of mammalian cell lines severely infected with penicillin and streptomycin resistant Bacillus sp.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgement
We are sincerely thankful to Institute of Medical Sciences, Banaras Hindu University, Director Prof. R. K. Jain and Molecular biology head of the department Prof. Sunit K Singh for all the support. We also want to express our sincere thanks to Director, Institute of Science, BHU, Prof. Anil K Tripathi and Head of School of Biotechnology Prof. S. M. Singh. We also express our thanks to Prof Anindya Dutta and his Laboratory UVA, Virginia, USA for sharing A549 data. We also thank Dr. Arpita Singh, IMS, BHU for suggestions and critical review of the research.
Author Contribution
SKS conceived the idea; SKS, GS and KT performed the experiments. SKS, GS and KT wrote the manuscript.
Funding Statement
The research was funded by Department of Biotechnology (DBT), Govt. of India, RLS grant (BT/RLF/Re-entry/43/2016) to SKS. GS was supported by DBT JRF and KT by Intramural Ph.D. program.
Langdon SP (2004) Cancer Cell Culture: Methods and Protocols. Cell Culture Contamination 9: 309–317. [ Ref ]
Young L, Julia S, Glyn S, John RM (2010) Detection of Mycoplasma in cell cultures. Nat Protoc 5: 929–934. [ Ref ]
Uphoff CC (2012) Treatment of mycoplasma contamination in cell cultures with plasmocin. J Biomed Biotechnol. [ Ref ]
Blazkova H ( 2010) Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J Cell Mol Med 14: 357–367. [ Ref ]
Testro AG, Visvanathan K (2009) Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol 24: 943–954. [ Ref ]
Zenk SF, Jantsch J, Hensel M (2009) Role of Salmonella enterica Lipopolysaccharide in Activation of Dendritic Cell Functions and Bacterial Containment. J Immunol 183: 2697–2707. [ Ref ]
UNC Lineberger comprehenssive Cancer centre (2019) UNC Sch Med. [ Ref ]
Uphoff CC, Drexler HG (2011) Cancer Cell Culture: Methods and Protocols. Detecting Mycoplasma Contamination in Cell Cultures by Polymerase Chain Reaction. [ Ref ]
McGarrity GJ (1980) Mycoplasmal infection of lymphocyte cell cultures: Infection withM. Salivarium. In Vitro 16: 346–356. [ Ref ]
Stanbridge EJ, Leonard H, Frank TP (1971) Modification of Amino-acid Concentrations Induced by Mycoplasmas in Cell Culture Medium. Nat New Biol 232: 242–244. [ Ref ]
Ryan J (2008) Understanding and managing cell culture contamination. Corning Life Sci Tech Lit: 1–24. [ Ref ]
Baumstummler A, Chollet R, Meder H, Rofel C, Venchiarutti A et al. (2010) Detection of microbial contaminants in mammalian cell cultures using a new fluorescence-based staining method. Lett Appl Microbiol 51: 671–677. [ Ref ]
Pincus DH, Orenga S, Chatellier S (2007) Yeast identification — past, present, and future methods. Med Mycol 45: 97–121. [ Ref ]
Kappeler A, Lutz-Wallace C, Sapp T, Sidhu M (1996) Detection of Bovine Polyomavirus Contamination in Fetal Bovine Sera and Modified Live Viral Vaccines Using Polymerase Chain Reaction. Biologicals 24: 131–135. [ Ref ]
Gray JS, Janette MB, Jenifer I (2010) Got black swimming dots in your cell culture? Identification of Achromobacter as a novel cell culture contaminant. Biologicals 38: 273–277. [ Ref ]
Sambrook J, Russell DW (2006) Agarose gel electrophoresis. Cold Spring Harbor Protocols 1: pdb-prot4020. [ Ref ]
Fischer AB (1975) Gentamicin as a bactericidal antibiotic in tissue culture. Med Microbiol Immunol 161: 23–39. [ Ref ]
Walsh C (2003) Antibiotics: actions, origins, resistance. American Society for Microbiology (ASM), Washington 335. [ Ref ]
Kiran S, Ashraf D, Samarendra KS, Kyung Yong L, Anindya D (2018) The Deubiquitinase USP46 Is Essential for Proliferation and Tumor Growth of HPV-Transformed Cancers. Mol Cell 72(5): 823-835. [ Ref ]
Kilcullen K, Allison T, Taissia G, Serguei G (2016) Cytotoxic Potential of Bacillus cereus Strains ATCC 11778 and 14579 Against Human Lung Epithelial Cells Under Microaerobic Growth Conditions. Front Microbiol 7: 69. [ Ref ]
LeBel M (1988) Ciprofloxacin: Chemistry, Mechanism of Action, Resistance, Antimicrobial Spectrum, Pharmacokinetics, Clinical Trials, and Adverse Reactions. Pharmacother. J Hum Pharmacol Drug Ther 8: 3–30. [ Ref ]
Abou-Zeid AA (1978) Mode of Action of Gentamicin Antibiotics Produced by Micromonospora purpurea. Zentralblatt für Bakteriol, Parasitenkunde, Infekt. und Hyg Zweite Naturwissenschaftliche Abteilung: Mikrobiol der Landwirtschaft, der Technol. und des Umweltschutzes 133: 362–368. [ Ref ]
Narayanaswamy N (2014) A Thiazole Coumarin (TC) Turn-On Fluorescence Probe for AT-Base Pair Detection and Multipurpose Applications in Different Biological Systems. Sci Rep 4: 6476. [ Ref ]