Journal Name: Journal of Applied Microbiological Research
Article Type: Clinical Trial
Received date: 27 October, 2022
Accepted date: 25 November, 2022
Published date: 02 December, 2022
Citation: Belà B, Pignataro G, Di Prinzio R, Simone DD, Crisi PE, Gramenzi A (2022) Effects of Lactobacillus reuteri NBF 1 DSM 32203 Supplementation on Healthy Bouledogue Francais Performance. J Appl Microb Res. Vol: 5 Issu: 2 (19-29).
Copyright: © 2022 Belà B et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The present study wants to evaluate the effectiveness of a potential probiotic strain, L. reuteri NBF 1 DSM 32203, on the intestinal health of healthy Bouledogue Francais adult dogs by analyzing their bodyweight (BW), body condition score (BCS), fecal quality and fecal moisture. Furthermore, the composition of the intestinal microbiota was also evaluated to quantify the total lactobacilli and coliforms. The dogs included in the study were divided into two groups: the control group, fed the standard commercial diet with the addition of a placebo and the treated group to which the probiotic was administered. The study lasted 35 days in line with the time needed to assess any effects. While the body weight showed no differences between the two groups of dogs, the fecal moisture was significantly lower at the end of the trial in the treated group respect to the control group; the beneficial effect of Lactobacillus reuteri NBF 1 DSM 32203 was also confirmed by the values of the fecal score recorded among the two groups of dogs. At the end of the study period was also registered a significant increase of Lactobacilli in the treated group respect to the control group (P= <0.001). This study shows the ability of the bacterial strain L. reuteri NBF 1 DSM 32203 to improve fecal quality parameters in healthy Bouledogue Francais increasing the amount of lattobacilli accompanied by a little reduction of total coliforms.
Keywords
Bouledogue Francais Dog, Fecal Quality Parameters, Body Condition Score, Intestinal Microbiota, Probiotic.
Abstract
The present study wants to evaluate the effectiveness of a potential probiotic strain, L. reuteri NBF 1 DSM 32203, on the intestinal health of healthy Bouledogue Francais adult dogs by analyzing their bodyweight (BW), body condition score (BCS), fecal quality and fecal moisture. Furthermore, the composition of the intestinal microbiota was also evaluated to quantify the total lactobacilli and coliforms. The dogs included in the study were divided into two groups: the control group, fed the standard commercial diet with the addition of a placebo and the treated group to which the probiotic was administered. The study lasted 35 days in line with the time needed to assess any effects. While the body weight showed no differences between the two groups of dogs, the fecal moisture was significantly lower at the end of the trial in the treated group respect to the control group; the beneficial effect of Lactobacillus reuteri NBF 1 DSM 32203 was also confirmed by the values of the fecal score recorded among the two groups of dogs. At the end of the study period was also registered a significant increase of Lactobacilli in the treated group respect to the control group (P= <0.001). This study shows the ability of the bacterial strain L. reuteri NBF 1 DSM 32203 to improve fecal quality parameters in healthy Bouledogue Francais increasing the amount of lattobacilli accompanied by a little reduction of total coliforms.
Keywords
Bouledogue Francais Dog, Fecal Quality Parameters, Body Condition Score, Intestinal Microbiota, Probiotic.
Background
It’s well known how important the intestinal microbiota is for the general health of the whole organism. Its importance is closely correlated with the main functions it performs like:
• Protection against pathogenic bacteria: the intestinal microbiota acts as a barrier against harmful bacteria competing for nutrition and intestinal site colonization;
• Improve the digestive process: intestinal bacteria are able to complete the digestion of substances that arrive undigested in the colon fermenting the dietary fiber;
• Metabolic function: the intestinal flora is able to produce vitamins like Vitamin K and synthesize aminoacids;
• Release of metabolites like Short Chain Fatty Acids (SCFAs) decreasing the intestinal pH making the environment more hostile to putrefactive bacterial species, the SCFAs production is also an important energy source for colonocytes;
• Development of the immune system participating in the production of molecules that regulate immune responses [1].
The term intestinal microbiota defines the microbial community of the gastrointestinal tract consisting mainly of bacteria, as well as yeasts, parasites and viruses; among the bacterial population there are not only beneficial but also harmful species. When these communities live in equilibrium there is a condition called eubiosis and this is very important because it allows the various components of the intestinal microbiota to be functionally effective and above all to be synchronized both with each other and with the other components of the intestinal ecosystem [1]. Eubiosis condition is essential to maintain a health organism. However, multiple factors can influence the composition of the intestinal microbiota causing an imbalance between beneficial and pathogenic species, among which we remember:
• Diet: eating habits affect well-being and intestinal balance so, a healthy and complete diet is essential not only to avoid nutritional deficiencies but also to maintain a healthy intestinal ecosystem;
• Use of antibiotic drugs and antimicrobial exposure: some antibiotics are able to destroy some common bacterial phyla; although antibiotics can improve many health condition, they are also the cause of a reduction in the number of commensal bacteria crucial for a healthy gut. Furthermore, an excessive use of antimicrobials promotes the colonization of Clostridium difficile, an opportunistic pathogen that causes diarrhea associated with consequent reduction of intestinal microbial diversity;
• Age: as the time goes on, the intestinal microbiota appears less rich and varied losing beneficial bacterial populations with probiotic function like Lactobacillus and Bifidobacterium; less variability also means greater vulnerability to the negative effects of dysbiosis, it is therefore not uncommon to find accumulation of opportunistic bacteria such as Enterobacteria able to damage the intestinal ecosystem in stressful situations.
All the factors above mentioned lead to an imbalance in the composition of the intestinal microbiota known as dysbiosis [2]. Dysbiosis is the opposite of eubiosis, in fact, while an eubiosis condition guarantees a healthy intestine, dysbiosis is often associated with various gastrointestinal disorders and pathologies such as inflammatory bowel disease (IBD). The term dysbiosis identifies an alteration of the physiological bacterial flora generally associated with an increase in potentially harmful bacterial species compared to beneficial ones. Fortunately, dysbiosis is not always an irreversible condition, although it requires important attention, the bacterial imbalance can be resolved through various strategies. Amon these strategies, recent studies focus the attention on the administration of specific microorganisms with beneficial functions for intestinal health; these specific microorganisms are called probiotics. “Probiotic” is a word that derives from “pro” (in favor of) and “bios” (life) and is used to indicate those microorganisms that are shown to be able, once ingested in adequate quantities, to exercise beneficial functions for the body (Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), 2001) [3]. Probiotics can be added to foods, improving their functional properties, or can be used as food supplements; to exert their beneficial functions, a probiotic microorganism must:
• fall within the species traditionally used to integrate intestinal microbiota;
• be considered safe (not capable of causing disease);
• arrive alive and active in the intestine and be able to multiply within it.
Currently, the microorganisms most used as probiotics are bacteria belonging to the genera Lactobacillus and Bifidobacterium (both belonging to the group of so-called “lactic ferments”), Bacillus, Enterococcus, Streptococcus and Escherichia. Furthermore, probiotic microorganisms are often associated with prebiotics that are non-vital substances that confer a benefit to health by modulating the microbiota; a combination of pro and prebiotics seems to have synergistic effect enhancing one the effect of the other. The probiotic use has been proposed, often with encouraging results, in the treatment of various conditions, pathological and not. The positive effect on health depends, in the first instance, on the inhibitory effect on the proliferation of pathogens in the intestine. Although most of the probiotic studies have focused more on humans, in recent years, some interesting research has also been conducted on the effect of these microorganisms in companion animals (dogs and cats). The probiotics’ effects on these animals have shown very similar results to those found in humans, in fact, these microorganisms seem to perform the same beneficial functions at the level of the canine/feline intestinal microbiota. As for canine gut microbiota, 16S rRNA sequencing showed that the main bacterial genera present are: Firmicutes, Fusobacteria, Bacteroidetes and Proteobacteria with a predominance of Clostridia in the duodenum and jejunum, while Fusobacteria and Bacteroides are highly abundant in the ileum and colon [4]. As for lactobacilli, they are distributed in any part of the canine intestine ranging from 104 to 108 CFU/ml; among them, Lactobacillus acidophilus is dominant. However, it was seen that the presence of Lactobacillus fermentum, Lactobacillus rhamnosus and Lactobacillus salivarius is associated with a health canine intestine. Lactobacillus murinus and Lactobacillus reuteri are other two abundant species found in the canine gut [4]. Several studies analyzed different species of canine intestinal lactobacilli in order to assess their potential probiotic functions; the bacterial species most analyzed belong to the genera Lactobacillus and they are: L. plantarum, L. fermentum, L. acidophilus and also L. reuteri. These studies reported very good results as these species are able to colonize the canine intestinal mucosa and proliferate at the expense of harmful bacteria. Of course, the research has also analyzed other bacterial species and genera that can exert a probiotic action but lactobacilli seem to be among the best candidates as they are able to colonize and proliferate in different sites of the canine gastrointestinal tract. Finding new bacterial species with probiotic action represents a recent important goal given their ability to collaborate in the resolution or improvement of different diseases aiming a rebalancing of the intestinal ecosystem often correlated to pathological states.
Aim of the Study
The main objective of this study was to assess the impact of a potential probiotic strain L. reuteri NBF1 (DSM 32203) on the body weight and fecal quality of healthy Bouledogue Francais adult dogs also evaluating its ability to change the intestinal microflora composition by increasing the number of beneficial bacterial species such as lactobacilli and decreasing the pathogenic ones such as E. coli.
Material and Methods
Feed additive under test
The zootechnical feed additive Lactobacillus reuteri NBF 1 DSM 32203 was produced by Centro Sperimentale del latte (CSL); it’s a freeze-dried microbial preparation of Lactobacillus reuteri DSM 32203.
Ethical statement
The research was conducted according to the directive 2010/63/EU; the study did not imply any form of animal suffering or health risk, since it focused on the administration of a natural substance.
Animals and study design
Healthy adult male and female non pregnant (age > 1year) dogs (n = 30, Bouledogue Francais; 10 females, 20 males; 10 + 20 = 30) were selected for the study and were randomly assigned to the control group (CTR; n=15; male: female = 2:1) and to the treated group (LACTO; n=15; male: female = 2:1); table 1 reports age and body weight of each dog included in the study. The design of the practice is a blind trial: the operator on the farm is aware of the animals belonging to the two experimental groups while the operator of the analysis laboratory is not aware of the origin of the sample; the analyzed dogs were assigned randomly (Survey Monkey Excel) between the control and the treated group minimizing selection bias errors. In this way two similar groups were obtained, allowing to better identify the effect of the treatment. The control group diet was supplemented by maltodextrin (used as placebo) while, the treated group diet was supplemented with L. reuteri NBF 1 DSM 32203. Cleaning and disinfecting procedures of the single fences were carried out and the animals have been individually stabulated. Before starting the trial, an antiparasitic treatment (ecto and endo) was carried out using commercial molecule drugs with no antibacterial effect. The dogs were evaluated daily by a veterinarian for any health and welfare concerns throughout the experimental period (two-week acclimation and 35-day study). Table 1 refers to the age and body weight of each dog included in the study.
Feed supplement and diet
A dry extruded commercial petfood for adult dogs (table 2) was fed to both the experimental groups, CTR and LACTO, specifically, the commercial diet used in the experiment was the Royal Canin mini adult. A freeze-dried microbial preparation of L. reuteri NBF 1 DSM 32203 (5 x 109 colony-forming units (CFU)/kg feed) was added to the LACTO group diet. Dogs were fed a commercial dry pet food once a day based on their maintenance energy requirements (adult dogs: 100 kcal x BW0.67 kg) and they had free access to potable water [5]. Consumption for each dog was measured by weighing the residue before the next day’s meal was administered. The dogs were fed once a day and the residue was zero. Table 3 (A-F) reports sex, body weight and amount of feed given to each dog at the beginning of the study (T0), after 1 week (T1), after 2 weeks (T2), after 3 weeks (T3), after 4 weeks (T4) and at the end of the experiment (T5); the amount of feed to be administered was calculated each time on the basis of the live body weight of the animal monitored weekly.
Table 1:Age (month), body weight (Kg) and sex of Bouledogue Francais dogs included in the study: A. Control group, B. Lacto group.
| Dogs (CTR group) | Age | Body weight | Sex |
|---|---|---|---|
| 2 | 22.4 | 11.2 | M |
| 4 | 22.4 | 11.6 | M |
| 5 | 22.4 | 11.7 | M |
| 7 | 32.2 | 11.5 | M |
| 8 | 28.4 | 11.8 | M |
| 9 | 32.2 | 11.9 | M |
| 10 | 22.4 | 11.6 | M |
| 13 | 28.4 | 11.7 | M |
| 19 | 28.4 | 11.8 | M |
| 22 | 28.4 | 11.6 | M |
| 23 | 32.2 | 9.5 | F |
| 26 | 32.2 | 9.6 | F |
| 27 | 32.2 | 9.7 | F |
| 29 | 22.4 | 9.8 | F |
| 30 | 28.4 | 9.4 | F |
| Average ± SD | 1.7 ± 4.2 | 11.0±1.0 |
B. Control group
| Dogs (Lacto group) | Age | Body weight | Sex |
|---|---|---|---|
| 1 | 28.4 | 11.4 | M |
| 3 | 32.2 | 11.9 | M |
| 6 | 28.4 | 11.7 | M |
| 11 | 28.4 | 11.6 | M |
| 12 | 28.4 | 11.7 | M |
| 14 | 32.2 | 11.8 | M |
| 15 | 22.4 | 11.5 | M |
| 16 | 32.2 | 11.9 | M |
| 17 | 32.2 | 11.8 | M |
| 18 | 22.4 | 11.7 | M |
| 20 | 32.2 | 9.8 | F |
| 21 | 28.4 | 9.7 | F |
| 24 | 22.4 | 9.6 | F |
| 25 | 22.4 | 9.6 | F |
| 28 | 22.4 | 9.5 | F |
| Average ± SD | 27.7 ± 4.2 | 11.0 ± 1.0 |
B. Lacto group
Table 2:Diet chemical composition (label).
| Analytical components | Percentage (%) |
|---|---|
| Moisture (%) | 9.00 |
| Crude protein (%) | 27.00 |
| Fat (%) | 16.00 |
| Fibre (crude) (%) | 2.75 |
| ME* (kcal/kg) | 3818.90 |
| *ME= Metabolizable energy | |
Table 3:Sex, body weight and amount of feed given to each dog at the beginning of the study (T0; A), after 1 week (T1; B), after 2 weeks (T2; C), after 3 weeks (T3; D), after 4 weeks (T4; E) and at the end of the experiment (T5; F).
| Dogs (CTR group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
|---|---|---|---|---|---|---|
| 2 | M | 11.2 | 6.12 | 673.45 | 3823 | 176 |
| 4 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 5 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 7 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 8 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 9 | M | 11.9 | 6.41 | 704.78 | 3823 | 184 |
| 10 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 13 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 19 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 22 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 23 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 26 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 27 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 29 | F | 9.8 | 5.54 | 609.27 | 3823 | 159 |
| 30 | F | 9.4 | 5.37 | 590.53 | 3823 | 154 |
| Dogs (LACTO group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
| 1 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 3 | M | 11.9 | 6.41 | 704.78 | 3823 | 184 |
| 6 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 11 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 12 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 14 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 15 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 16 | M | 11.9 | 6.41 | 704.78 | 3823 | 184 |
| 17 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 18 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 20 | F | 9.8 | 5.54 | 609.27 | 3823 | 159 |
| 21 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 24 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 25 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 28 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| *ME = Metabolizable energy | ||||||
B. Sex, body weight (Kg) and amount of feed given to each dog after 1 week of study (T1).
| Dogs (CTR group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
|---|---|---|---|---|---|---|
| 2 | M | 11.1 | 6.08 | 668.94 3823 175 | 3823 | 175 |
| 4 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 5 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 7 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 8 | M | 11.7 | 6.33 | 695.88 3823 182 | 3823 | 182 |
| 9 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 10 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 13 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 19 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 22 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 23 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 26 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 27 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 29 | F | 9.8 | 5.54 | 609.27 | 3823 | 159 |
| 30 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| Dogs (LACTO group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
| 1 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 3 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 6 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 11 | M | 11.6 | 6.29 | 691.41 | 3823 | 181< |
| 12 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 14 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 15 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 16 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 17 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 18 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 20 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 21 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 24 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 25 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 28 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| *ME = Metabolizable energy | ||||||
C. Sex, body weight (Kg) and amount of feed given to each dog after 2 weeks of study (T2).
| Dogs (CTR group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
|---|---|---|---|---|---|---|
| 2 | M | 11.2 | 6.12 | 673.45 | 3823 | 176 |
| 4 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 5 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 7 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 8 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 9 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 10 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 13 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 19 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 22 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 23 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 26 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 27 | F | 9.8 | 5.54 | 609.27 | 3823 | 159 |
| 29 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 30 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| Dogs (LACTO group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
| 1 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 3 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 6 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 11 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 12 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 14 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 15 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 16 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 17 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 18 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 20 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 21 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 24 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 25 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 28 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| *ME = Metabolizable energy | ||||||
D. Sex, body weight (Kg) and amount of feed given to each dog after 3 weeks of study (T3).
| Dogs (CTR group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
|---|---|---|---|---|---|---|
| 2 | M | 11.1 | 6.08 | 668.94 | 3823 | 175 |
| 4 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 5 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 7 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 8 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 9 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 10 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 13 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 19 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 22 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 23 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 26 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 27 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 29 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 30 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| Dogs (LACTO group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
| 1 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 3 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 6 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 11 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 12 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 14 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 15 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 16 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 17 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 18 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 20 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 21 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 24 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 25 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 28 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| *ME = Metabolizable energy | ||||||
E. Sex, body weight (Kg) and amount of feed given to each dog after 4 weeks of study (T4).
| Dogs (CTR group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
|---|---|---|---|---|---|---|
| 2 | M | 11.2 | 6.12 | 673.45 | 3823 | 176 |
| 4 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 5 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 7 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 8 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 9 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 10 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 13 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 19 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 22 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 23 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 26 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 27 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 29 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 30 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| Dogs (LACTO group) | Sex | Body weight | Kg 0.67 | ME* Kcal/d | ME* Kcal/Kg feed | g/d |
| 1 | M | 11.4 | 6.20 | 682.45 | 3823 | 179 |
| 3 | M | 11.9 | 6.41 | 704.78 | 3823 | 184 |
| 6 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 11 | M | 11.5 | 6.24 | 686.94 | 3823 | 180 |
| 12 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 14 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 15 | M | 11.6 | 6.29 | 691.41 | 3823 | 181 |
| 16 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 17 | M | 11.7 | 6.33 | 695.88 | 3823 | 182 |
| 18 | M | 11.8 | 6.37 | 700.33 | 3823 | 183 |
| 20 | F | 9.7 | 5.50 | 604.60 | 3823 | 158 |
| 21 | F | 9.8 | 5.54 | 609.27 | 3823 | 159 |
| 24 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| 25 | F | 9.6 | 5.45 | 599.92 | 3823 | 157 |
| 28 | F | 9.5 | 5.41 | 595.23 | 3823 | 156 |
| *ME = Metabolizable energy | ||||||
F. Sex, body weight (Kg) and amount of feed given to each dog at the end of the study (T5).
Diet composition: Fresh chicken 20%, corn, dehydrated poultry 15% (of which chicken 6%), rice, poultry fat, wheat, barley, hydrolysed poultry liver 2.5%, beet pulp 2.5%, dehydrated fish 1%, whole dehydrated eggs 0.5%, mineral substances, brewer’s yeast, chicory 0.5%, Yucca crush 170 mg/kg, mannanoligosaccharides 60 mg/kg.
Nutritional additives per Kg:Vitamin A 15,000 IU/kg, Vitamin D3 2,000 IU/kg, Vitamin E 150 mg/kg, iron (iron sulfate (II) monohydrate) 75 mg/kg, iodine (potassium iodide) 3.5mg/kg, copper (copper sulfate(II) pentahydrate) 10 mg/kg, manganese (manganose sulphate, monohydrate) 7.5 mg/kg, zinc (zinc oxide) 120 mg/kg, selenium (sodium selenite) 0.12mg/kg, L-carnitine 40 mg/kg.
Data collection
Bodyweight (BW) and body condition score (BCS) were recorded at days 0 (T0), 7 (T1), 14 (T2), 21 (T3), 28 (T4) and 35 (T5), according to the American Animal Hospital Association (AAHA) Nutritional Assessment Guidelines for Dogs and Cats [6]. Each morning, before feed administration, an operator measured the body weight of each animal. At the same time, BCS assessment was carried out by visual examination and palpation of the animal on a scale between 1 and 9 (Figure 1) where a score of 4 or 5 is reflecting the ideal body condition depending on the breed. The Fecal Score (FS) and Fecal Moisture (FM) were evaluated to analyze the effect of the probiotic on fecal quality; fecal score was evaluated using a 7-point scoring chart according to Bybee and colleagues, as described in table 4, at all six sampling times (T0–T5). In the laboratory, collected fecal samples were analyzed to determine the fecal moisture.
Fecal sampling was carried out at T0, T1, T2, T3, T4 and T5 and the collected samples were stored at +4°C until they are brought to the laboratory, where they are stored at −20°C. An aliquot of 5–10 g of stool was weighed and dried in an oven at a temperature of 105°C–110°C for 20–24 hours, cooled down in a desiccator for another 20–24 hours, after which the FM content was calculated as lost weight after desiccation. Microbiological analysis was performed at T0, T1, T3 and T5. One gram of fresh stool was diluted in sterile saline solution with a ratio of 1:10. Diluted feces were vortexed for two minutes to obtain a homogeneous suspension, which was then streaked on different culture media for total bacterial count and identification [7]. Specifically, for Escherichia coli and total coliforms (E. coli), eosin methylene blue agar (Oxoid, Italy) was used. After an incubation time of 24 hours at 37°C, E. coli colonies show growth with a green metallic reflex, while coliforms show growth with blue, red or uncolored colonies. For Lactobacilli, Man Rogosa and Sharpe agar (Oxoid) was used and plates were incubated under anaerobic condition at 37 °C for 48 hours. All the analysis was performed in duplicate.
Figure 1:Body Condition Scoring by WSAVA guidelines.
Statistical analysis
For the statistical analysis a Mixed Model with repeated measurements has been used, which allows to estimate the parameters considering both random effect and fixed effect. Following, the model has been estimated as the following [7]:
yijk = μ + Si + Gj + Tk+ Gj * Tk + ei,j,k
where y = dependent variable (FM, FS, BW, BCS, LB, COLI); μ = overall mean; Si = fixed effect of the ith sex (I = 1,2); Gj = fixed effect of the jth group (j = 1,2); Tk = fixed effect of the kth time (k = 0.5) and eijk = error. The software used was: R Core Team, R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria) and for the different analysis was used the Mixed Model; nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-141) and the Least Squares, Least- Squares Means [8]: The R Package lsmeans. Time was used as repeated measurement and therefore each subject has been analyzed in every different temporal instant. The autoregressive covariance structure was used. Least Square Means were estimated and they were being statistically tested using Student’s t test (with Tukey p-value adjustment). In order to be able to describe the goodness of the fit of the mixed model, we used the R squared described by Nakagawa and Schielzeth, [9]. No outliers and missing data were found.
Results
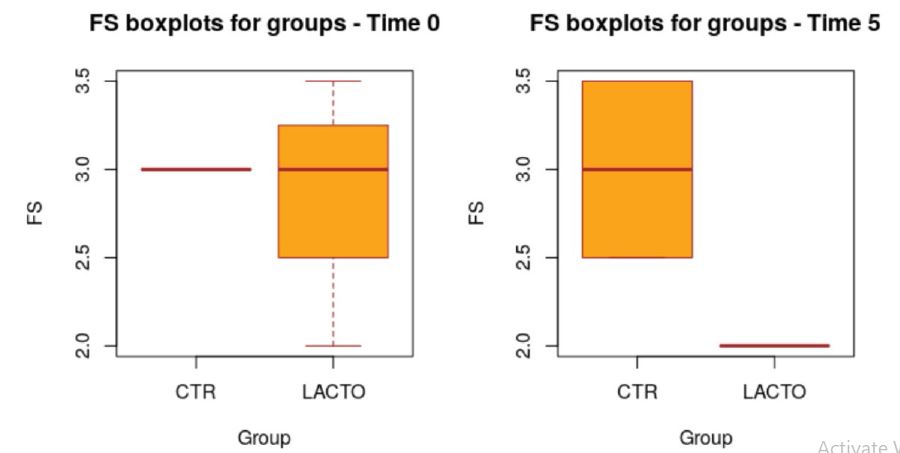
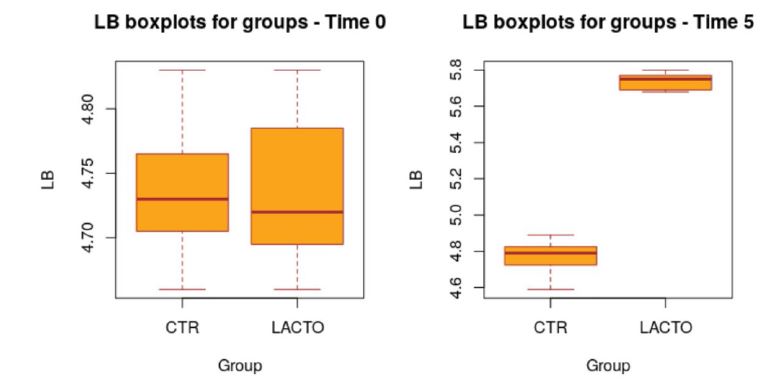
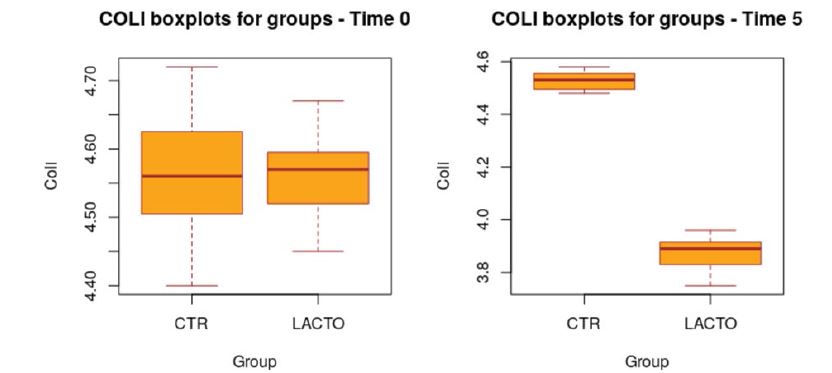
All dogs were healthy during the trial, no side effects and no case of death were recorded as evidenced by the certificate issued by the veterinarian. No residual pet food was found after consumption throughout the experimental period. BW and BCS did not change during the trial in either group showing no significant values (Table 5; A-B) and the animals maintained an ideal body condition. BW data show no differences between the two groups. FM was significantly lower at the end of the trial in the LACTO group compared with the CTR group (P<0.001; table 6); in fact a lower humidity content was found especially in the last three weeks of the experimental period (T3-T5) in the fecal samples of the group treated with L. reuteri NBF 1 DSM 32203 compared with the values recorded in the CTR group (P<0.001) where the fecal humidity remains almost the same; the beneficial effect of Lactobacillus reuteri NBF 1 DSM 32203 was also confirmed by the values of fecal score (FS) recorded among the two groups of dogs (Table 7; Figure 2). Specifically, FS values fluctuate between 2.97±0.29 at the beginning of the study and 3.00±0.29 at the end in the control group, so, they don’t show any variation; while, the LACTO group shows much lower values between the 2.93±0.29 at the beginning of the trial and the 2.00±0.29 at the end of the experiment with an overall value of 2.92±0.15 (P<0.001) for the control group and 2.32±0.16 (P<0.001) for the LACTO group. The decrease of about 1 point (registered in the LACTO group) on a scale of 1 to 7 can certainly have implications for the intestinal health of dogs with an important biological relevance [10]. With regard to the microbiological analysis, at the end of the experimental period (T5), was observed a significant increase of Lactobacilli in the treated group (LACTO) respect to the control group moving from a concentration of 4.75±0.06 log CFU/g (P=0.990) at the beginning of the experiment to a concentration of 5.73±0.06 log CFU/g (P<0.001) at the end of the trial (Table 8; Figure 3) followed by a decrease in the total coliforms’ amount (P<0.001) (Table 9; Figure 4).
Figure 2:Box plot showing the effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to the diet on the fecal score (FS) of Bouledogue Francais dogs in the overall period (P<0.001; t-test). CTR, control group; LACTO, treated group.
Discussion
This study evaluated the effect of a potential probiotic strain: L. reuteri NBF 1 DSM 32203 on body weight, fecal consistency and microbiological analysis of fecal samples of healthy Bouledogue Francais adult dogs. L. reuteri NBF 1 DSM 32203 promoted a significant reduction in fecal humidity (FM) and fecal score (FS) in the group of dogs treated with the probiotic (LACTO) with a mean value closer to the ideal one compared with the control group (CTR). The potential probiotic strain showed a positive effect at the intestinal level of healthy dogs decreasing fecal humidity giving more consistency to the stool. During the last three weeks of the study (T3-T5) were recorded the lowest fecal humidity values in the LACTO group and the fecal score displayed values closer to those of the ‘ideal’ condition respect to the control group (CTR). The effects of the administration of L. reuteri NBF 1 DSM 32203 on the intestinal microbial ecosystem showed the ability of this potential probiotic to increase, especially in the last two weeks, the amounts of lactobacilli, followed by a decrease of total coliforms. The increase in lactobacilli is certainly positive: they promote the integrity of the intestinal barrier by preventing the adhesion of pathogenic bacteria and their proliferation [11].
Figure 3:Box plot showing the effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet on total Lactobacilli count (LB) in the overall period (P<0.001; t-test). CTR, control group; LACTO, treated group.
Figure 4:Box plot showing the effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet on total coliform (Coli) in the overall period (P<0.001; t-test). CTR, control group; LACTO, treated group.
Table 4:Fecal scoring chart by Nestle Purina fecal score system (modified).
| Score | Characteristics |
|---|---|
| 1 | Very hard and dry; Often expelled as individual pellets; Requires much effort to expel from the body; Leaves no residue on ground when picked up. |
| 2 | Firm, but not hard, pliable; Segmented in appearance; Little or no residue on ground when picked up. |
| 3 | Log-shaped, moist surface; Little or no visible segmentation; Leaves residue on ground, but holds form when picked up. |
| 4 | Very moist and soggy; Log-shaped; Leaves residue on ground and loses form when picked up. |
| 5 | Very moist but has a distinct shape; Present in piles rather than logs; Leaves residue on ground and loses form when picked up. |
| 6 | Has texture, but no defined shape; Present as piles or spots; Leaves residue on ground when picked up. |
| 7 | Watery; No texture; Present in flat puddles. |
Table 5:Effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet on body weight (BW) (A) and body condition score (BCS) (B) of Bouledogue Francais dogs.
| Groups | |||
|---|---|---|---|
| Time | CTR | LACTO | P- value |
| Overall | 10.6 ± 0.1 | 10.6 ± 0.1 | 0.322 |
| T0 | 10.6 ± 0.1 | 10.7 ± 0.1 | 0.990 |
| T1 | 10.6 ± 0.1 | 10.6 ± 0.1 | 0.990 |
| T2 | 10.6 ± 0.1 | 10.6 ± 0.1 | 0.999 |
| T3 | 10.6 ± 0.1 | 10.6 ± 0.1 | 1.000 |
| T4 | 10.5 ± 0.2 | 10.6 ± 0.1 | 0.880 |
| T5 | 10.6 ± 0.1 | 10.7 ± 0.1 | 0.976 |
A. Effect of Lactobacillus reuteri addition to diet on body weight (BW) of Bouledogue Francais dogs (LS Mean ± SE).
| Groups | |||
|---|---|---|---|
| Time | CTR | LACTO | P- value |
| Overall | 4.71 ± 0.11 | 4.67 ± 0.11 | 0.494 |
| T0 | 4.70 ± 0.25 | 4.63 ± 0.25 | 1.000 |
| T1 | 4.73 ± 0.25 | 4.70 ± 0.25 | 1.000 |
| T2 | 4.70 ± 0.25 | 4.70 ± 0.25 | 1.000 |
| T3 | 4.76 ± 0.25 | 4.66 ± 0.25 | 1.000 |
| T4 | 4.70 ± 0.25 | 4.66 ± 0.25 | 1.000 |
| T5 | 4.70 ± 0.25 | 4.66 ± 0.25 | 1.000 |
B. Effect of Lactobacillus reuteri addition to diet on body condition score (BCS) of Bouledogue Francais dogs (LS Mean ± SE)
Table 6:Effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet on fecal moisture in dogs: results of mixed models showing least square means ± SE in CTR (control group) and LACTO (treated group) dogs for the six individual sampling times and overall throughout the study.
| Time | CTR | LACTO | P- value |
|---|---|---|---|
| Overall | 0.67 ± 0.01 | 0.56 ± 0.01 | <0.001 |
| T0 | 0.67 ± 0.02 | 0.70 ± 0.01 | 0.001 |
| T1 | 0.68 ± 0.02 | 0.64 ± 0.02 | <0.001 |
| T2 | 0.67 ± 0.01 | 0.59 ± 0.02 | <0.001 |
| T3 | 0.66 ± 0.02 | 0.54 ± 0.02 | <0.001 |
| T4 | 0.65 ± 0.02 | 0.48 ± 0.02 | <0.001 |
| T5 | 0.67 ± 0.02 | 0.40 ± 0.01 | <0.001 |
Table 7:Effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet on the fecal score of Bouledogue Francais adult healthy dogs: results of mixed models showing least square means ± SE in CTR (control group) and LACTO (treated group) dogs for the six individual sampling times and overall throughout the study.
| Time | CTR | LACTO | P- value |
|---|---|---|---|
| Overall | 2.92 ± 0.15 | 2.32 ± 0.16 | <0.001 |
| T0 | 2.97 ± 0.29 | 2.93 ± 0.29 | 1.000 |
| T1 | 2.87 ± 0.29 | 2.57 ± 0.29 | 0.470 |
| T2 | 2.90 ± 0.29 | 2.27 ± 0.29 | 0.001 |
| T3 | 2.90 ± 0.29 | 2.10 ± 0.29 | <0.001 |
| T4 | 2.87 ± 0.29 | 2.03 ± 0.29 | <0.001 |
| T5 | 3.00 ± 0.29 | 2.00 ± 0.29 | <0.001 |
Table 8:Effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet, expressed as log CFU/g, on the total amount of Lactobacilli present in the intestinal microflora of Bouledogue Francais adult healthy dogs: results of mixed models showing least square means ± SE in CTR (control group) and LACTO (treated group) dogs for the four individual sampling times and overall throughout the study.
| Time | CTR | LACTO | P- value |
|---|---|---|---|
| Overall | 4.75 ± 0.04 | 5.19 ± 0.04 | <0.001 |
| T0 | 4.76 ± 0.06 | 4.75 ± 0.06 | 0.990 |
| T1 | 4.75 ± 0.06 | 4.98 ± 0.06 | <0.001 |
| T3 | 4.71 ± 0.06 | 5.31 ± 0.06 | <0.001 |
| T5 | 4.78 ± 0.06 | 5.73 ± 0.06 | <0.001 |
Table 9:Effect of Lactobacillus reuteri NBF 1 DSM 32203 addition to diet, expressed as log CFU/g, on the amount of E. coli present in the intestinal microflora of Bouledogue Francais adult healthy dogs: results of mixed models showing least square means ± SE in CTR (control group) and LACTO (treated group) dogs for the four individual sampling times and overall throughout the study.
| Time | CTR | LACTO | P- value |
|---|---|---|---|
| Overall | 4.54 ± 0.05 | 4.25 ± 0.05 | <0.001 |
| T0 | 4.56 ± 0.07 | 4.59 ± 0.07 | 0.960 |
| T1 | 4.53 ± 0.07 | 4.34 ± 0.07 | <0.001 |
| T3 | 4.54 ± 0.07 | 4.18 ± 0.07 | <0.001 |
| T5 | 4.53 ± 0.07 | 3.89 ± 0.07 | <0.001 |
Conclusion
The data collected from the present study report the capacity of the bacterial strain L. reuteri NBF 1 DSM 32203 to ameliorate fecal quality parameters such as FM and FS in healthy adult Bouledogue Francais dogs. Fecal Score is one of the most important parameters of biological relevance and, at the end of the treatment, reported a significant decrease of about 1 point in the group treated with L. reuteri NBF 1 DSM 32203 compared to the control group that showed no decrease respect the beginning. This result was accompanied by a decrease in fecal humidity, causing the feces to be more consistent and well-formed as an indication of good intestinal ecosystem related to a good digestion [12]. In addition, the increase in Lactobacilli, confirms once again the ability of this potential probiotic strain to improve the composition of the intestinal microbiota by promoting an increase in beneficial species capable of promoting the maintenance of the integrity of the intestinal mucosa [13].
Kataoka K (2016) The intestinal microbiota and its role in human health and disease. Review. J Med Invest 63: 27-37. [ Ref ]
Weiss GA, Hennet T (2017) Mechanisms and consequences of intestinal dysbiosis. Review Cell Mol Life Sci 74: 2959-2977. [ Ref ]
Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), 2001. [ Ref ]
Grzeskowiak L, Endo A, Beasley S, Salminen S (2015) Microbiota and probiotics in canine and feline welfare. Anaerobe 34: 14-23. [ Ref ]
FEDIAF (2018) Nutritional guidelines. [ Ref ]
Baldwin K, Bartges J, Buffington T, Freeman LM, Grabow M, et al. (2010) AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 46: 285-296. [ Ref ]
Fusi E, Rizzi R, Polli M, Cannas S, Giardini A, et al. (2019) Effects of Lactobacillus acidophilus D2/CSL (CECT 4529) supplementation on healthy cat performance. Veterinary Record Open 6: e000368. [ Ref ]
Russell V Lent (2016) Least-Squares Means: The R Package lsmeans. Journal of Statistical Software 69: 1-33. [ Ref ]
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods Ecol Evol 4: 133-142. [ Ref ]
Stokes JE, Price JM, Whittemore JC (2017) Randomized, controlled, crossover trial of prevention of clindamycin-induced gastrointestinal signs using a synbiotic in healthy research cats. J Vet Intern Med 31: 1406-1413. [ Ref ]
Cazorla SI, Maldonado-Galdeano C, Weill R, De Paula J, Perdigon GDV (2018) Oral administration of probiotics increases Paneth cells and intestinal antimicrobial activity. Frontiers in microbiology 9: 736. [ Ref ]
Ohno H, Murakami H, Tanisawa K, Konishi K, Miyachi M (2019) Validity of an observational assessment tool for multifaceted evaluation of faecal condition. Scientific Reports 9: 3760. [ Ref ]
Rose C, Parker A, Jefferson B, Cartmell E (2015) The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Critical Reviews in Environmental Science and Technology 45: 1827-1879. [ Ref ]