Journal Name: Journal of Applied Microbiological Research
Article Type: Analysis
Received date: 01 April, 2019
Accepted date: 03 April, 2019
Published date: 10 April, 2019
Citation: Aninagyei E, Tetteh ETT, Banini J, Nani E, Adu P, et al (2019) Evaluation of Haemato-Biochemical Parameters and Serum Levels of 8-Iso-Prostaglandin F2? Oxidative Stress Biomarker in Sickle Cell-Malaria Comorbidity. J Appl Microb Res. Vol: 2 Issu: 1 (32-40).
Copyright: © 2019 Aninagyei E, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Blood (5ml) was collected from suspected malaria patients (n=2272) into K3-EDTA tube. Samples were grouped as malaria infections in normal haemoglobin (malaria-HbAA), malaria infections in sickle cell disease (malaria-SCD), malaria infections in sickle cell trait (malaria- SCT) and non-malaria with normal haemoglobin (controls). The aim of the study was to evaluate the effect of Plasmodium falciparum infections on haematological, biochemical profiles and oxidative stress in sickle cell patients as paucity of data exist in this regard. Malaria parasites were identified and quantified according to WHO guidelines. All eligible samples were assayed for haematological and biochemical parameters, T-cell profiling and levels of 8-iso-prostaglandin F2α oxidative stress biomarker. The study found that geometric mean of parasite density was higher in malaria-HbAA than malaria-SCD (20394 parasites/μl vs. 9990 parasites/μl, p=0.001) whilst mean body temperature was higher in malaria-SCD and malaria-HbAA than control samples and malaria-SCT. Mean leukocytes were significantly elevated in co-morbidity states while lymphopenia and neutrophilia were associated with malaria-HbAA and malaria-SCD. However, CD3+, CD4+ and CD8+ T cells were significantly higher in malaria-HbAA than malaria-SCD. Eosinophilia was also associated with malaria-SCT while monocytosis was seen in malaria- SCD. Severely low red blood cells, low haemoglobin concentrations and low red cell indices were seen in malaria-SCD with corresponding significant elevations in plasma haemoglobin, % haemolysis, serum potassium, serum bilirubin and lactate dehydrogenase in malaria- SCD. Levels of means 8-iso-prostaglandin F2α in all the patient groups were significantly higher than the control sample with compounding levels seen in malaria-SCD group. In conclusion, severe haemolysis was observed in malaria-SCD co-morbidity which associated with compounding oxidative stress. Exhaustive clinical assessment must be done to prevent or reduce oxidant related pathologies in SCD patients.
Keywords
8-epi-prostaglandin F2α; Oxidative stress; Haematological profile; Malaria-SCD co-morbidity.
Abstract
Blood (5ml) was collected from suspected malaria patients (n=2272) into K3-EDTA tube. Samples were grouped as malaria infections in normal haemoglobin (malaria-HbAA), malaria infections in sickle cell disease (malaria-SCD), malaria infections in sickle cell trait (malaria- SCT) and non-malaria with normal haemoglobin (controls). The aim of the study was to evaluate the effect of Plasmodium falciparum infections on haematological, biochemical profiles and oxidative stress in sickle cell patients as paucity of data exist in this regard. Malaria parasites were identified and quantified according to WHO guidelines. All eligible samples were assayed for haematological and biochemical parameters, T-cell profiling and levels of 8-iso-prostaglandin F2α oxidative stress biomarker. The study found that geometric mean of parasite density was higher in malaria-HbAA than malaria-SCD (20394 parasites/μl vs. 9990 parasites/μl, p=0.001) whilst mean body temperature was higher in malaria-SCD and malaria-HbAA than control samples and malaria-SCT. Mean leukocytes were significantly elevated in co-morbidity states while lymphopenia and neutrophilia were associated with malaria-HbAA and malaria-SCD. However, CD3+, CD4+ and CD8+ T cells were significantly higher in malaria-HbAA than malaria-SCD. Eosinophilia was also associated with malaria-SCT while monocytosis was seen in malaria- SCD. Severely low red blood cells, low haemoglobin concentrations and low red cell indices were seen in malaria-SCD with corresponding significant elevations in plasma haemoglobin, % haemolysis, serum potassium, serum bilirubin and lactate dehydrogenase in malaria- SCD. Levels of means 8-iso-prostaglandin F2α in all the patient groups were significantly higher than the control sample with compounding levels seen in malaria-SCD group. In conclusion, severe haemolysis was observed in malaria-SCD co-morbidity which associated with compounding oxidative stress. Exhaustive clinical assessment must be done to prevent or reduce oxidant related pathologies in SCD patients.
Keywords
8-epi-prostaglandin F2α; Oxidative stress; Haematological profile; Malaria-SCD co-morbidity.
Introduction
The asexual stages of Plasmodium falciparum are intraerythrocytic thus inducing haematological alterations such as anaemia, thrombocytopenia and neutrophilia [1]. Parasites density influence severity of the haematological changes. These changes depend on factors such as level of malaria endemicity, presence of haemoglobinopathies, nutritional status and level of malaria immunity [2,3]. Malaria is meso-endemic in Ghana with recent nationwide prevalence of 43.4% [4]. On the other hand, sickle cell disease (SCD) is also prevalent in Ghana [5]. Sickle cell haplotype HbS leads to polymerization of deoxygenated sickle haemoglobin within inelastic red blood cells which cause occlusion of microvasculature, resulting in acute complications, chronic organ damage, high rate of morbidity and mortality [6]. These mechanisms have adverse effect on the quality and quantity of formed blood cells in affected individuals. SCD has been found to be associated with anaemia, low RBC count, low packed cell volume (PCV), low mean cell volume (MCV), low mean cell hemoglobin (MCH) [7] and leukocytosis [8]. The trends in the haematological profiles associated with SCD and malaria are similar. There is evidence of altered haematopoiesis which affect all the three haematological cell lines in SCD and malaria.
Oxidative stress is the overproduction of free radicals beyond the physiological detoxification ability of the body [9]. A principal consequence of Plasmodium infections is the development of anaemia [10] which is known to release reactive oxygen species (ROS) as a result of activation of the immune system of the body. Plasmodium infections and sickle cell disease are associated with oxidative stress due to production of ROS which result in haemoglobin degradation [11]. Oxidative stress is suspected to play a key role in disease pathogenesis, complications and mortality [12] consequently, a number of studies have focused on the measurement of oxidative stress, many of which are through specific biomarkers that indicate the oxidative damage [13]. Lipid, protein and DNA biomolecules are the main targets of free radicals that subsequently transformed into the reactive species reflecting oxidative stress in the corresponding molecules. Malondialdehyde (MDA), a product of lipid peroxidation, has been widely used as an indicator of oxidative stress. However, MDA measured by thiobarbituric acid assay overestimates actual MDA levels by more than 10- fold due probably to cross-reactivity with other aldehydes [14]. However, the isopentane, 8-iso-prostaglandin F2α (8-iso-PGF2α), is a more stable product of lipid peroxidation and has been described at the gold standard biomolecule for assessing oxidative stress [15].
In Ghana, malaria and SCD are prevalent [16] and their co-morbidity have been previously reported [17]. However, there is paucity of data on haematological, biochemical profiles, immunity levels measured by CD4 and CD3 cells and degree of oxidative stress in sickle cell and malaria comorbidities. Therefore, this study was designed to evaluate how sickle cell and malaria comorbidity impact on these parameters.
Materials and Methods
Study site
This multi-center study took place in 3 district hospitals and 3 health centers in the Greater Accra region of Ghana. The hospitals (latitude, longitude) were Ga West Municipal Hospital, Amasaman (5.7020708,-0.2992889), Ashaiman Polyclinic (5.6856,-0.0398) and Ada East District Hospital (5.8956754,0.5340865). The health centres (latitude, longitude) were Mayera Health Centre (5.720578,- 0.2703561), Oduman Health Centre (5.64171,-0.3302) and Obom Health Centre (5.7361,-0.4395).
Study design
This cross-sectional study was conducted in suspected malaria patients from November 2017-August, 2018. The following clinical information; age, sex, temperature, body weight and clinical presentations was collected from each patient before sample collection. Maximum of three samples were collected each day from the study sites. Five (5) mL of whole blood was collected from each consented patient (2 mL was dispensed into pediatric EDTA tubes and 3 mL dispensed into plain tube). Specimens were transported from study sites to Ga West Municipal Hospital laboratory daily. Haematological profile was carried out on same day of sample reception. Blood films were prepared in triplicate. Screening for sickle cell and phenotyping and infectious makers were done with whole blood while serum previously stored at -30°C was used for biochemical and oxidative stress bioanalysis.
Study subjects, sample size and sample processing
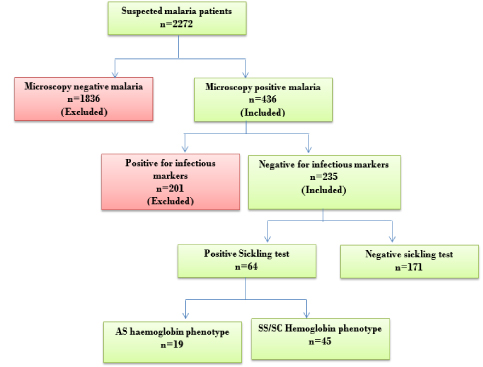
Figure 1 details participant recruitment plan used for the present study. Patients recruited for the study were physician suspected malaria cases. Sample size was determined based on single population formula using confidence interval of 95% and estimated proportion of 1 in 4 malaria infections occurring in sickle cell disease. The sample size was estimated to be 289. Measurement of 8-isoprostaglandin F2α oxidative stress biomarker was done in age and sex matched patients.
Figure 1: Flow chart for sample collection, analysis and cumulative figures.
Inclusion and exclusion criteria
Patients included in the study were microscopy diagnosed malaria patients, aged 0-20 years, who consented or whose parents consented to be part of the study. Individuals who were known SCD patients or visited the health centre on account of sickle cell crisis and malaria patients co-infected with hepatitis B virus, hepatitis C virus, syphilis and HIV 1&2 were excluded from the study.
Laboratory analysis
Malaria detection and quantification: Thick and thin blood film was done for each specimen, in triplicate, on the same glass slide. The dried thin film was fixed in absolute methanol briefly, air dried and stained with 10% Giemsa. The dried smear was then examined for presence of Plasmodium parasites. The parasites were subsequently identified to the species level and quantified per μl of blood according to WHO guidelines. Parasites were quantified per 200 WBCs counted using the patients’ total WBC per μL of blood. A total of 500 WBCs were counted in negative infections [18]. Each slide was double checked by a blinded certified malaria microscopist and in cases of discordant results; a third opinion was final.
Infectious marker screening: The specimens were screened for hepatitis B virus, hepatitis C virus, syphilis and HIV I&II pathogens to eliminate any possible effect on the haematological and biochemical parameters as well as the T cell and the oxidative stress biomarker. The microbiological screening was done with rapid immunochromatographic test kits. HIVI&II and syphilis were screened with First Response® test kit (Premier Medical Corporation Ltd, India) whilst the hepatitis B and C were screened with FaStep Rapid Diagnostic Test (Houston, USA).
Haematological profiling: Haematological profiling was done using Urit 5200 (China) fully automated haematology analyzer. The 5-part differential analyzer works on the principle of laser beam multi-dimensional cell classification flow cytometry for white cell differentiation, white and red blood cell estimation. Platelets were counted by optical and electrical impedance principles and haemoglobin concentration was measured by cyanide-free colorimetric method. All other parameters were calculated.
Enumeration of CD3 CD4 and CD8 cells: The measurement of CD4 and CD8 T-cells is initiated by staining the cells with phycoerythrin (PE) fluorescent dye while CD3 cells are stained by phycoerythrin–Cy5 (PE-Cy5) fluorescent dye. The fluorescent molecules are taken up by the cells and the cells are individually illuminated by light of a defined wavelength. The light activates the fluorescent molecules so that they emit light of a characteristic wavelength. This fluorescent light is filtered out and its intensity is measured by a ploidy analyzer for each single cell. The fluorescence light intensity emitted by a labeled cell is proportional to its CD4 content (BD FACScount user manual). CD4 and CD3 cells were assayed by flow cytometry using BD FACScount flow cytometer (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. The CD4/CD3 reagent tube was brought to room temperature, vortexed up and down for about 5 seconds each and then opened with the coring station. Exactly 50μl of whole blood was added to a reagent tube vortexed upright and incubated in the workstation for 1 hour. After incubation, 50μl of formalin was added, vortexed and analyzed immediately on the FACScount. Absolute values of CD4, CD3 T cells and the CD4/CD3 ratio were then automatically printed out by an inbuilt thermal printer.
Biochemical assay: Plasma electrolytes (sodium, potassium and chloride) were measured by FT-320 electrolyte analyzer (China), lactate dehydrogenase and serum bilirubin were measured by PKL-125 (Italy) fully automated chemistry analyzer using ELItech kinetic and end-point reagents respectively (France). Plasma haemoglobin was measured using fully automated haematology analyzer by assaying for haemoglobin in plasma immediately after spinning EDTA anticoagulated samples at 2000rpm for 5 minutes. Percentage haemolysis was calculated by using formulae used by Sawant et al [19].
Sickle cell screening and phenotyping: Sickle cell screening was done with 2.0% (weight/volume) sodium metabisulphite reduction method while sickle cell phenotypes were separated by electrophoresis in alkaline medium (pH 8.6). Haemoglobin phenotyping was done alongside pooled HbA, HbS, HbC and HbF controls. Electrical voltage of 250 V and current of 50 mA were employed to obtain complete separation of haemoglobin variants for a maximum of 30 min. Results were read immediately against the controls.
Sandwich-ELISA for 8-iso-prostaglandin F2α levels: Reagents and consumables for 8-iso-prostaglandin F2alpha was obtained from YH Biosearch® assay kit (Shanghai Yehua Biological Technology Company Limited, Shanghai, China). Measurement of 8-iso-prostaglandin F2alpha was done according to manufacturer’s protocol, with these modifications; incubation of pre-diluted samples with coated anti-8-iso-prostaglandin F2α and after addition of Horseradish Peroxidase-conjugated antibody specific for human 8-epi-prostaglandin F2alpha was done for 45 minutes at room temperature. Again, the chromogen solution was incubated for 30 minutes at room temperature after dispensing into the microelisa wells. The optical density (OD) was measured by Mindray MR-96A ELISA plate reader (Shenzhen, China) at a wavelength of 450 nm. The concentration of 8-iso-prostaglandin F2α was obtained by comparing the OD of the samples to the standard curve.
Statistical analysis
Raw data were entered into Microsoft Excel 2010. Statistical analyses were done with Stata 15.0 statistical software (Stata Corp LLC, USA). One-way ANOVA was used to compare means in more than two groups. Multiple comparison was done using Tukey post hoc analysis.
Result
Results of microbiological screening
A total of 2272 suspected malaria patients were randomly selected for this study; 1069 (47.05%) were mono-infected with hepatitis B virus, hepatitis C virus, Salmonella spp, HIV, group A streptococcus and P. falciparum. While 414 (18.22%) patients had dual infections, of which P. falciparum and hepatitis B, Salmonella spp and group A streptococcus dominated (Table 1). Of the total number, those infected with only P. falciparum (n=235) were further analyzed with respect to the objectives of this study. Controls selected for comparison (n=100) were randomly selected from patients whose pyrexia was of unknown origin as far as the identified pathogens were concerned.
Table 1: Microbial causes of pyrexia in the study sites.
| Type of infection | Total patients recruited n=2272 |
|---|---|
| Number of patients infected (%) | |
| Mono-infection | 1069 (47.05) |
| Hepatitis B | 12 (0.53) |
| Salmonella IgG/IgM | 378 (16.64) |
| HIV1&2 | 17 (0.74) |
| Hepatitis C | 22 (0.97) |
| Anti-streptolysin O | 405 (17.82) |
| P. falciparum | 235 (10.34) |
| Dual infections | 414 (18.22) |
| Hepatitis B + Salmonella | 5 (0.22) |
| Hepatitis B + ASO | 11 (0.48) |
| P. falciparum + Hepatitis B | 12 (0.53) |
| P. falciparum + Salmonella | 78 (3.43) |
| P. falciparum + ASO | 111 (4.88) |
| ASO + Salmonella | 197 (8.67) |
| Pyrexia of unknown origin | 789 (34.72) |
| ASO: anto-streptolysin O, IgG-immunoglobin G, IgM-immunoglobin M | |
Characteristics of malaria-sickle cell comorbidity patients and controls used in this study
This study analyzed various variables in malaria patients in normal haemoglobin (malaria-HbAA, n=171), malaria in sickle cell disease (malaria-SCD, n=45), malaria in sickle cell traits (malaria-SCT, n=19) and normal controls (pyretic patients without malaria and any sickle cell haemoglobinopathy, n=100). The age difference among the patients was not significant (F=2.22, p=0.065) as well as insignificant difference between gender among the groups (x2=1.43, p=0.231). However, temperature recorded in the normal controls differed significantly from malaria- HbAA, malaria-SCD but not malaria-SCT. In malaria-SCT, microscopy detectable P. falciparum was not found unlike in malaria-HbAA and malaria-SCD. In malaria-SCT, all infections were detected by rapid test kit where HRP2 was detected in all the 19 co-morbidities but not pLDH. Significantly high P. falciparum parasitaemia was detected in malaria-HbAA (t=7.43, p<0.001) (Table 2).
Table 2: Demographic, temperature and parasitaemia of the patients.
| Variables | Normal control (n=100) |
Malaria-HbAA (n=171) |
Malaria –SCD (n=45) |
Malaria-SCT (n=19)c | Statistic value |
p-value |
|---|---|---|---|---|---|---|
| Age (mean±SD) | 10.33 ± 2.45 | 12.79 ± 4.91 | 11.56 ± 3.65 | 9.32 ± 5.12 | F=2.22 | 0.0651 |
| Males, n (%) | 41(41.0) | 77 (43.80) | 21 (46.70) | 7(36.8) | x2=1.43 | 0.2312 |
| Females, n (%) | 59(59.0) | 113 (56.20) | 24 (53.30 | 12(63.2) | ||
| Body temp range (mean ±SD) | 37.7 ± 1.07a | 39.1 ± 1.15ac | 39.0 ± 0.87ab | 37.9 ± 0.84bc | F=2.94 | 0.0211 |
| Parasite density range | NA | 2231-620586 | 2492-112452 | NA | ||
| GM of Parasite density | NA | 20394a | 9990a | NA | t=7.43 | <0.001 |
| Interquartile range | NA | 9519-51093 | 6329-16945 | NA | ||
| 1 p-value determined by one-way ANOVA, 2 p-value determined by Pearson Chi square. Means that share a common letter are significantly different (Using the Tukey multiple comparisons test). Plasmodium falciparum infection detected by rapid test kit, GM-Geometric mean | ||||||
Haematological parameters in malaria and sickle cell co-morbidities
Significant differences in white blood cell (WBC) count were observed in all groups with leukocytosis associated with malaria-SCD and malaria-SCT. Significantly higher WBC count was observed in control samples compared to malaria- HbAA. Controls and malaria-SCT neutrophils count differed significantly from the other groups, however, marked neutropenia was observed in malaria-HbAA and malaria- SCD with insignificant differences between their means values. Mean control lymphocyte counts were significantly higher than mean lymphocyte levels in malaria-HbAA and malaria-SCD but not malaria-SCT. Lymphocytes levels did not differ in malaria-HbAA and malaria-SCD group. Mean eosinophils count recorded in each group differed from each other. Significant eosinophilia was observed in malaria-SCD and malaria-SCT while significant low eosinophils count was seen in the control group and malaria-HbAA groups. Monocytes values also differed from group to group with marked monocytosis seen in malaria-SCD and malaria-HbAA. Low counts were seen in normal controls and malaria-SCT group. Marginal changes in basophils count was seen. With mean malaria-SCT basophils count significantly lower than basophils levels in other groups and the other groups did not differ from each other.
Across the groups, mean red blood cell values differed significantly. Mean values in malaria-SCD was the lowest whilst the control values were significantly high. Similarly, mean malaria-SCD haemoglobin levels were significantly lower than values observed in all the groups while mean control values were relatively higher. However, mean haemoglobin values in malaria-HbAA and malaria-SCT did not differ from each other. Mean cell volume (MCV) values also differed from each other with lowest mean MCV observed in malaria-SCT. The control MCV (84.3±5.21fL) was higher than all other groups. On the other hand, with the exception of control mean cell haemoglobin (MCH) (31.1±3.41 pg) that differed from the other groups, MCH values observed in malaria-HbAA (25.89±3.78 pg), malaria- SCD (24.53±4.09 pg) and malaria-SCT (23.48±3.39 pg) were not statistically different from each other even though malaria-SCT values were marginally lower. Mean cell haemoglobin concentrations (MCHC) values did not differ from each other, however, MCHC values seen in malaria-SCD were lower (33.71±2.85 g/dL). Similarly, red cell distribution width values did not differ significantly from each other.
Mean platelets values observed in the control samples and malaria-HbAA were significantly different from each of the groups. The former was significantly higher and the latter significantly lower. However, malaria-SCD and malaria-SCT platelet values did not differ significantly from each other. Mean platelet volume (MPV) values in control samples were significantly different from values seen in malaria-HbAA and malaria-SCT but not malaria-SCD. MPV values seen in normal controls and malaria-SCD were higher than values seen in malaria-HbAA and malaria-SCT. Platelet large cell ratio (P_LCR) values in the control samples was significantly higher than P_LCR values seen in the other groups. Values seen in malaria-HbAA was significantly lowest (Table 3).
Table 3: Hematological parameters of malaria and sickle cell co-morbidity.
| Mean ± standard deviation | |||||
|---|---|---|---|---|---|
| Hematological parameters | Normal control (n=100) |
Malaria-HbAA (n=171) |
Malaria –SCD (n=45) |
Malaria-SCT (n=19)c | p-value1 |
| WBCx109/L | 7.17 ± 2.25a | 6.68 ± 2.42a | 12.32 ± 2.77a | 15.0 ± 4.11a | <0.001 |
| Neutrophils % | 65.03 ± 5.14a | 72.17 ± 20.1(b) | 70.44 ± 8.65(b) | 55.87 ± 11.33a | <0.001 |
| Lymphocytes % | 30.18 ± 6.32(b) | 17.53 ± 10.22(c) | 18.23 ± 8.44(c) | 29.51 ± 4.44(b) | <0.001 |
| Eosinophils % | 3.51 ± 0.75a | 2.19 ± 1.79a | 4.77 ± 0.99a | 6.33 ± 1.58a | 0.014 |
| Monocytes % | 2.14 ± 0.78a | 5.92 ± 3.30a | 7.32 ± 1.58a | 4.14 ± 0.81a | <0.001 |
| Basophils % | 0.41 ± 0.41(b) | 0.45 ± 0.24(b) | 0.32 ± 0.07a | 0.42 ± 0.11(b) | 0.043 |
| RBCx1012/L | 4.11 ± 1.08a | 3.92 ± 0.78a | 3.07 ± 0.69a | 3.21 ± 0.77a | <0.001 |
| Hb (g/dL) | 12.8 ± 2.14a | 10.83 ± 2.11(b) | 8.91 ± 1.06a | 10.33 ± 1.48(b) | <0.001 |
| Haematocrit | 34.6 ± 2.45a | 31.84 ± 6.07(b) | 26.73 ± 2.79a | 29.49 ± 2.79(b) | <0.001 |
| MCV (fL) | 84.3 ± 5.21a | 76.07 ± 10.53a | 71.33 ± 7.62a | 69.58 ± 5.51a | <0.001 |
| MCH (pg) | 31.1 ± 3.41a | 25.89 ± 3.78(b) | 24.53 ± 4.09(b) | 23.48 ± 3.39(b) | 0.041 |
| MCHC (g/dL) | 36.9 ± 1.98 | 34.07 ± 2.35 | 33.71 ± 2.85 | 35.15 ± 3.87 | 0.426 |
| RDW_CV | 15.3 ± 0.68 | 14.29 ± 1.78 | 14.48 ± 1.68 | 14.21 ± 0.93 | 0.485 |
| Pltx109/L | 202.4 ± 60.2a | 138.7 ± 37.7a | 190.0 ± 25.3(b) | 196.3 ± 34.3(b) | <0.001 |
| MPV (fL) | 9.4 ± 1.32(a) | 8.11 ± 1.40b | 9.3 ±1.28(a) | 8.87 ± 0.97b | <0.001 |
| PDW (fL) | 11.8 ± 2.66a | 12.7 ± 2.48a | 13.3 ± 1.89a | 10.7 ± 2.21a | 0.038 |
| Plateletcrit % | 0.18 ± 0.11a | 0.09 ± 0.08a | 0.11 ± 0.07(b) | 0.13 ± 0.12(b) | 0.008 |
| P_LCR % | 18.8 ± 7.84a | 10.21 ± 7.39(b) | 15.11 ± 8.06a | 11.87 ± 4.56(b) | <0.001 |
| 1p-value determined by one-way ANOVA. Means with a superscript letter differed significantly from the rest. Means with a superscript letter in parenthesis were statistically not different (Tukey multiple comparisons test). MCHC=Mean cell hemoglobin concentration, RDW_CV=Red cell distribution width coefficient of variation, RDW_SD=Red cell distribution width standard deviation, L=Litre, fL=Fentolitre, pg=pictogram, Plt=Platelets, PDW=Platelet distribution width, PCT=Plateletcrit, P_LCR=Platelet large cell ratio, Hb-haemoglobin, MCV-mean cell volume, Mean Cell Haemoglobin, Mean Cell Haemoglobin Concentration | |||||
Variations in T-lymphocytes sub-populations in malaria-sickle cell co-morbidities
In Table 4, the concentrations of CD3 cells, CD4 cells, CD8 cells and their ratios are presented. In all the values significant variations existed. Total T cells (CD3+ cells) (1487±687 cells/μL) were significantly higher in the normal controls compared to malaria-HbAA (891±216 cells/μL), malaria-SCD (605±198 cells/μL) and malaria-SCT (929±165 cells/μL) groups. The mean values in each group also differ significantly from mean values in other groups. Similarly, mean cytotoxic T cells (CD8+ cells) in each of the groups significantly varied from each other. Mean CD8+ cells in the control samples (604±213 cells/μL) were significantly higher whilst mean values in malaria-SCD (184±97 cells/ μL) was significantly lower. CD8+ cells in malaria-HbAA (316±102 cells/μL) differed significantly from levels observed in malaria-SCT (387±196 cells/μL cells/μL). Mean T helper cells (CD4+ T cells) levels in the control samples (833±205 cells/μL) and malaria-SCD patients (421±145 cells/μL) differed significantly from all the other groups. However, CD4+ T cell levels in malaria-HbAA (512±193 cells/μL) group did not differ significantly from malaria-SCT group (499±201 cells/μL). Mean CD4+/CD3+ ratios and mean CD4+/CD8+ ratios also differed significantly from each other. Mean CD4+/CD3+ ratios were higher in the control group (0.56±0.09 cells/μL) than in all other groups and was lower in malaria-SCD (0.29±0.05 cells/μL) than in the other groups. On the other hand, CD4+/CD8+ ratios were higher in malaria-SCD group (2.28±0.55 cells/μL) than in the other groups while lower levels were observed in malaria-SCT group (1.29±0.79 cells/μL).
Table 4: T-cell lymphocytes populations in malaria and malaria-sickle cell co-morbidities.
Mean ± standard deviation (cells/µL) |
|||||
|---|---|---|---|---|---|
| T-cells | Normal control (n=45) |
Malaria-HbAA (n=45) |
Malaria-SCD (n=45) | Malaria-SCT (n=19) |
p-value1 |
| CD3+ T cells | 1487 ± 687a | 891 ± 216a | 605 ± 198a | 929 ± 165a | <0.001 |
| CD4+ T cells | 833 ± 205a | 512 ± 193(b) | 421 ± 145a | 499 ± 201(b) | <0.05 |
| CD8+ T cells | 604 ± 213a | 316 ± 102a | 184 ± 97a | 387 ± 196a | <0.05 |
| CD4+/CD3+ ratio | 0.56 ± 0.09a | 0.35 ± 0.12a | 0.29 ± 0.05a | 0.39 ± 0.10a | <0.05 |
| CD4+/CD8+ ratio | 1.38 ± 1.67a | 1.62 ± 0.68a | 2.28 ± 0.55a | 1.29 ± 0.79a | <0.05 |
| 1p-value determined by one-way ANOVA. Means with a superscript letter differed significantly from the rest. Means with a superscript letter in parenthesis were statistically not different (Tukey multiple comparisons test). | |||||
Levels of biochemical parameters in malaria-sickle cell co-morbidities
Table 5 represents mean levels of haemolytic indicators (plasma haemoglobin, % haemolysis, serum potassium, lactate dehydrogenase and serum bilirubin), serum chloride, serum sodium and oxidative stress biomarker (8-iso-prostagladin F2α). Mean plasma haemoglobin levels were significantly elevated in all the groups except in the control group. Marked elevation of plasma haemoglobin was observed in malaria-SCD group (0.46±0.12 g/dL). Similarly, % haemolysis was elevated in the co-morbid states than in the control samples. Marked elevation was seen malaria- SCD group (3.44±0.29 %). Again, serum potassium levels were markedly elevated in co-morbid states compared to control samples (3.74±1.11 mmol/L) and highest elevation were seen malaria-SCD (7.89±1.15 mmol/L) and malaria- SCT (6.88±0.93 mmol/L). Levels of plasma haemoglobin, % haemolysis and serum potassium corresponded to serum lactate dehydrogenase (LDH) levels in the groups where LDH levels in malaria-SCD (478.45±13.74 U/L) were markedly elevated with non-significant differences between malaria-HbAA (295.63±10.45 U/L) and malaria-SCT (266.78±9.78 U/L), even though LDH levels seen in them were significantly higher compared to control values. Serum total bilirubin levels were also elevated in the co-morbid states in relation to the control group. Even though marginal differences existed between serum bilirubinaemia, levels of indirect bilirubinaemia differed significantly from each other. Whilst mean chloride levels differed between some of the groups, mean sodium concentrations did not differ from each other. Mean levels of 8-iso-PGF2α oxidative stress biomarker differed from one group to another. Elevation 8-iso-prostaglandins F2α (8-iso-PGF2α) was observed in all the groups except the control group (84.1±16.3 pg/mL). Mean serum 8-iso-PGF2α concentration seen malaria-SCD (643.8±54.54 pg/mL) was almost twice the value seen in malaria-HbAA (338.1±50.2 pg/mL). These values were significantly higher than mean malaria-SCT concentration (129.1±18.8 pg/mL).
Table 5: Variations in biochemical variables.
| Mean ± standard deviation> | |||||
|---|---|---|---|---|---|
| Biochemical parameters> | Normal control> (n=100)> |
Malaria-HbAA> (n=171)> |
Malaria –SCD> (n=45)> |
Malaria-SCT (n=19)> | p-value1> |
| Haemolytic indicators> | |||||
| Plasma Hb (g/dL) | 0.08 ± 0.003a | 0.24 ± 0.07a | 0.46 ± 0.12a | 0.37 ± 0.15a | <0.001 |
| % haemolysis | 0.078 ± 0.009a | 2.08 ± 0.76a | 3.44 ± 0.29a | 2.62 ± 1.05a | <0.001 |
| Potassium (mmol/L) | 3.74 ± 1.11a | 5.78 ± 1.02a | 7.89 ± 1.15(b) | 6.88 ± 0.93(b) | <0.001 |
| LDH (U/L) | 165.20 ± 13.12a | 295.63 ± 10.45(b) | 478.45 ± 13.74a | 266.78 ± 9.78(b) | <0.05 |
| Serum bilirubin> | |||||
| Total (µmol/L) | 9.78 ± 1.15a | 31.78 ± 3.26a | 63.45 ± 17.12a | 48.56 ±4 .56a | <0.001 |
| Direct (µmol/L) | 5.89 ± 0.98(a) | 7.89 ± 3.36b | 9.63 ± 2.45b | 5.45 ± 4.03(a) | <0.05 |
| Indirect (µmol/L) | 4.78 ± 16a | 25.2 ± 4.45a | 57.3 ± 3.12a | 42.98 ± 4.19a | <0.001 |
| Chloride (mmol/L) | 98.56 ± 7.33a | 69.96 ± 4.56(b) | 70.12 ± 3.87(b) | 73.64 ± 1.87a | 0.029 |
| Sodium (mmol/L) | 138.78 ± 2.41 | 139.21 ± 2.15 | 140.01 ± 1.99 | 138.97 ± 2.11 | 0.058 |
| 8-iso-PGF2α (pg/mL) | 84.1 ± 16.3a | 338.1 ± 50.2a | 643.8 ± 54.54a | 129.1 ± 18.8a | <0.001 |
| 1>>p-value determined by one-way ANOVA. Means with a superscript letter differed significantly from the rest. Means with a superscript letter in parenthesis were statistically not different (Tukey multiple comparisons test).> > | |||||
Discussion
Malaria has been demonstrated to induce oxidant stress due to parasite multiplication as well as host immune response to the parasite [20]. In this study we show that P. falciparum infection induces significant elevations in oxidant stress marker 8-iso-prostaglandin F2α (8-iso-PGF2α). Additionally, P. falciparum infection and SCD co-morbidity led to synergistic increase of this oxidant stress biomarker in the peripheral blood of these patients. The utility of 8-iso- PGF2α as oxidative stress biomarker indicated significant oxidative stress in malaria-HbAA, malaria-SCD and malaria- SCT compared to control subjects. These finding is suggestive that 8-iso-PGF2α may be a useful oxidative stress biomarker in malaria and sickle cell patients. Previous studies have described the interconnection between inflammation and oxidative stress and changes in one can lead to changes in the other [21]. Oxidative stress due to overproduction of reactive oxygen species (ROS) has important role in initiation and progression of chronic diseases [22], so doubling of oxidative stress in malaria-SCD, as found in this study, was not surprising. In malaria-SCT, levels of 8-iso-PGF2α, though significantly higher than control levels, was significantly less than levels observed in malaria-HbAA and malaria- SCT. This observation could be as a result of protective role being offered by sickle cell trait status to oxidative stress. Moreover, no parasite was detected in the blood of such patients, so absence of microscopic detectable malaria parasitaemia explains the suppression of oxidative stress in these patients. Strikingly, in all the malaria-SCT patients, histidine rich protein 2 (hrp2) were detected but not Plasmodium lactate dehydrogenase (pLDH). pLDH has been reported to be a glycolytic pathway enzyme secreted by only metabolically active infections [23]. In humans, 8-iso-PGF2α is not only a marker of oxidative stress but also a biologically active molecule [24]. Its persistence in the body promotes atherosclerosis and attenuates angiogenesis by activating thromboxane receptor [25]. Again, higher levels of 8-iso- PGF2α have also been observed in a number of conditions such as alcoholism and non-alcoholic liver diseases [26], pulmonary disease [27] and diabetes [28]. In view of this sickle patients with malaria must be properly managed to prevent atherosclerosis and attenuation of angiogenesis and advised against alcoholism.
Leucocytosis was associated with malaria and sickle cell co-morbidity. However, in malaria-SCT, leukocyte count was comparatively higher. In Asia, leukocyte count in malaria infected patients were lower compared to control samples [29] while previous study in Ghana reported otherwise [30]. In haemoglobin S patients, mean WBC value of 7.1x109/L and 4.6x109/L were reported for SCT and sickle cell disease patients respectively [31]. Normal WBCs have been reported in several studies involving sickle cell carriers and patients [32-33]. The reduced response to erythropoietin secretion in sickle cell individuals could explain this phenomenon [34]. However, in malaria-sickle cell co-morbidity, elevation of WBC may be due to significant elevation in neutrophil, esosinophil and monocytes count as a result of acute inflammatory response to Plasmodium immunogens. In malaria-SCD, lymphocyte count was a little above half the lymphocytes count in control samples. However, lymphocyte counts in malaria-SCT and malaria-HbAA were insignificantly different. Even though differences in mean lymphocyte count in malaria-HbAA and malaria-SCD were insignificant, mean CD4+ T cells count in malaria-SCD were significantly lower than value observed in malaria-HbAA. Again, CD3+ T cells and CD8+ T cells counts in malaria-SCD were significantly lower than values observed in malaria- HbAA. With this differences, immune responses in malaria- HbAA will be effective compared to malaria-SCD. This is because, CD4+ T cells play a central role in immune protection through helping B cells make antibodies, induce microbicidal activity through activation of macrophages and recruitment of neutrophils, eosinophils and basophils to sites of infection through production of cytokines and chemokines, to orchestrate the full array of immune responses [35]. Even though eosinophils and monocytes were relatively higher in co-morbidity states, corresponding decrease in lymphocytes and T cells counts will down-regulate immune response in co-morbidity states.
Significantly low RBC, haemoglobin and red cells indices were associated with all malaria infected group. Even though significantly low values were seen in the co-morbidity states, lower levels were associated with malaria-SCD than malaria- SCT. Evaluation of the biochemical parameters indicated that intravascular haemolysis accounted for these differences with respect to mean control values. In co-morbidity states, plasma haemoglobin as well as percentage haemolysis were increased. Similarly, serum potassium, LDH and serum bilirubin were also increased in the group of patients. However, these parameters were significantly increased in the SCD group compared to SCT group. Haemolysis of red cells resulted in reduction in both RBC count and haemoglobin concentration as were observed in the malaria-SCD group. This subsequently elevated percentages haemolysis and LDH. Indirect bilirubinaemia (unconjugated bilirubinaemia) were also significantly higher in the co-morbidity states with higher elevation seen in the malaria-SCD patient group. Indirect hyperbilirubinaemia associated with co-morbid states could be ascribed to impairment of hepatic function in malaria. Liver impairment in acute malaria cases could adversely affects the function of cytochrome P450 enzymes [36]. In this case impairment of hepatic functions in the face of excessive haemolysis could reduce conjugation of bilirubin, hence, the observed indirect hyperbilirubinaemia. Significant elevations were observed in platelets, mean platelet volume, platelet distribution width and plateletcrit in malaria-SCD patients. Relative thrombocytopenia was seen in the malaria-HbAA patients. Malaria related case vs. control thrombocytopenia has been reported in several studies [3,37,38]. Elevation in platelets and platelet indices suggests efficient haemostasis in malaria-SCD than malaria infections in the absence of SCD.
Conclusion
Compared to control samples and malaria-HbAA patients, malaria-SCD and malaria-SCT patients were associated with leukocytosis. Even though malaria-HbAA and malaria-SCD were associated with relative marked lymphopenia, CD3+, CD4+ and CD8+ T cells were significantly higher in malaria- HbAA group. Again, lower erythrocyte counts as well as erythrocyte indices were associated with malaria-SCD group with corresponding and significant increases in serum potassium, indirect bilirubin, LDH, plasma haemoglobin and % haemolysis. Haemostatis is expected to be effective in the co-morbidity states than malaria mono-infections. Finally, malaria co-morbidity with sickle cell disease has synergistic effect of oxidative stress.
List of Abbreviations
ANOVA-one-way analysis of variance; ELISA-enzyme linked immuno-sorbent assay; HbS-Sickle cell hemoglobin; malaria-HbAA- malaria in normal hemoglobin; malaria-SCD-malaria in sickle cell disease; MDA-malondialdehyde; MCHmean cell hemoglobin; MCV-mean cell volume; OD-optical density; SCD-Sickle cell disease; SCT-sickle cell trait; SSASub- Saharan Africa; TBAA-thiobarbituric acid assay.
Ethical Approval
Ethical approval for this study was granted by Ghana Health Service Ethics Review Committee (Approval No: GHS-ERC002/03/18). Participant consent was sought for publication.
Raw Data
All relevant data are within the paper.
Acknowledgement
We acknowledge Michael Ankwadah, Michael Gyimah, Sedem Bokor, Bridgette Tevi and Alex Nyarko for the role they played in recruiting the patients, collection of clinical data and taking specimens for the study. We are also grateful to Nicholas Sowa for his immense contributions during the laboratory measurement of the 8-iso-prostaglandin F2α oxidative stress biomarker.
Author’s Contribution
EA, PA, DOA, RKDE conceptualized, designed and coordinated the study. EA, EDT, PA performed the statistical analysis and JB, EN participated in the sample collection and processing. AE, PA drafted the manuscript, manuscript proofread by RKDE, AE-Y which was later approved by all authors.
There are no references