Journal Name: Journal of Applied Microbiological Research
Article Type: Analysis
Received date: 13 August, 2020
Accepted date: 02 October, 2020
Published date: 09 October, 2020
Citation: Riva A, Longo V, Berlanda D, Allegrini P, Masetti G, et al. (2020) Healthy Protection of Bergamot is linked to the Modulation of Microbiota. J Appl Microb Res. Vol: 3 Issu: 2 (45-51).
Copyright: © 2020 Riva A et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The present study aimed to evaluate the effects of a new food-grade bioavailable delivery system of bergamot on human gut microbiota, in order to demonstrate the potential correlation of microbiota modulation in cardiovascular health. The identification of human gut microbiota modification was performed after ex-vivo incubation with bergamot phytosome (Vazguard™) of individual faecal slurries from healthy women (45–53 years) as follows: after incubation at 37°C in anaerobic condition, DNA was extracted and a 16S Metagenomic Sequencing Analysis performed.
Results: Twenty-five different phyla were identified, among which 4 were modulated: Firmicutes Proteobacteria, Bacteroidetes, Actinobacteria. The decreased Firmicutes/Bacteroidetes ratio and the increase of Proteobacteria were observed indicating a positive modulation of microbiota possibly linked to cardiovascular health. 418 different genera were also identified, among which several of them were mildly modulated.
Conclusion: For the first time, a gut microbiome modulation was associated to the new delivery system of bergamot phytosome, suggesting its clinical efficacy for cardiovascular health that will be further investigated. New potential applications in weight control and gastrointestinal benefits were suggested.
Keywords
microbiota, Bergamot Phytosome, Vazguard, metagenomics sequence, cardiovascular health.
Abbreviations
HPLC-DAD-MS: High-Pressure Liquid Chromatograph-Diode Array Detector-Mass Spectrometry
LC-NMR: Liquid Chromatography with Nuclear Magnetic Resonance Spectroscopy
tChol: total cholesterol
LDL: low-density Lipoproteins
TG: triglycerides
HDL: high-density Lipoproteins
CVD: Cardiovascular
F/B ratio : Firmicutes/Bacteroidetes ratio
LDL-C : lipoprotein-cholesterol
Abstract
Background: The present study aimed to evaluate the effects of a new food-grade bioavailable delivery system of bergamot on human gut microbiota, in order to demonstrate the potential correlation of microbiota modulation in cardiovascular health. The identification of human gut microbiota modification was performed after ex-vivo incubation with bergamot phytosome (Vazguard™) of individual faecal slurries from healthy women (45–53 years) as follows: after incubation at 37°C in anaerobic condition, DNA was extracted and a 16S Metagenomic Sequencing Analysis performed.
Results: Twenty-five different phyla were identified, among which 4 were modulated: Firmicutes Proteobacteria, Bacteroidetes, Actinobacteria. The decreased Firmicutes/Bacteroidetes ratio and the increase of Proteobacteria were observed indicating a positive modulation of microbiota possibly linked to cardiovascular health. 418 different genera were also identified, among which several of them were mildly modulated.
Conclusion: For the first time, a gut microbiome modulation was associated to the new delivery system of bergamot phytosome, suggesting its clinical efficacy for cardiovascular health that will be further investigated. New potential applications in weight control and gastrointestinal benefits were suggested.
Keywords
microbiota, Bergamot Phytosome, Vazguard, metagenomics sequence, cardiovascular health.
Abbreviations
HPLC-DAD-MS: High-Pressure Liquid Chromatograph-Diode Array Detector-Mass Spectrometry
LC-NMR: Liquid Chromatography with Nuclear Magnetic Resonance Spectroscopy
tChol: total cholesterol
LDL: low-density Lipoproteins
TG: triglycerides
HDL: high-density Lipoproteins
CVD: Cardiovascular
F/B ratio : Firmicutes/Bacteroidetes ratio
LDL-C : lipoprotein-cholesterol
Background
Intestinal microbiota is a complex ecosystem of microorganisms (such as bacteria, archeaebacteria, fungi and protists) resident in the gut [1]; their density and composition increase from stomach to colon and in the colon, about 1011 to 1012/ml are the estimated bacteria content [2,3]. A great number of studies have been dedicated in the last 15 years to explore human microbiota and its relationship with human diseases, such as cardiovascular and neurological disorders, inflammation, cancer, diabetes, and obesity [4], in order to identify modifications in gut bacterial composition possibly involved in a disease onset. The high level of a mixture of flavonoids contained in the juice, albedo and flavedo of bergamot fruit is responsible for bergamot antioxidant and antisenescence properties [5]. Other beneficial effects such as hypoglycaemic and hypolipidaemic have been described for bergamot in metabolic syndrome and in cardiovascular diseases (CVD) prevention [6,7]. A lecithin food-grade delivery system (Phytosome®) containing the Bergamot Polyphenolic Fraction (BPF) from Citrus Bergamia Risso et Poiteau from Calabria was developed by Indena SpA to ameliorate the oral absorption of flavonoids. The structural phytochemical composition of bergamot was deeply analysed by HPLC-DAD-MS and LCNMR [8], and a recent clinical trial in individuals with type 2 diabetes mellitus and hyperlipaemia showed a reduction of cardiovascular risk by modulating total cholesterol (tChol), low-density Lipoproteins (LDL), triglycerides (TG), highdensity Lipoproteins (HDL) and blood glucose after just 30- day supplementation [9]. The aim of the present work is to study for the first time the effect of bergamot phytosome on human gut microbiota to find possible associations with its benefits on cardiovascular health.
Methods
Samples collection
Faecal samples from 3 healthy women aged 45–53 years were collected in sterile containers, stored at -20°C and then transferred at -80°C until analysis. All subsequent methodologies were performed under anaerobic conditions to preserve the microbiota environmental. Donors had not used antibiotics in the previous 12 months.
Phytosome digestion
Bergamot Phytosome® (Vazguard®, Indena SpA) was used in gut microbiota experiments. A simulated gastric and duodenal human digestion of bergamot phytosome (1000 mg/L) was performed in vitro before incubation with faecal slurries, mimicking in vivo conditions. Briefly, phytosome was subjected to simulated gastric digestion by incubation at 37°C for 60 minutes under shaking at pH 2 with HCl 1 M (to simulate gastric conditions before meal under a fasting state) in the presence of the gastric enzyme pepsin [10]. Further incubation under shaking for 150 minutes at pH 7.0 with Sodium Carbonate 1 M and in the presence of bile extract pancreatin (ratio 6:1) was performed to simulate pancreatic digestion [11]. The bile extract contains glycine, taurine and the main biliar salts, while pancreatin was utilized as source of pancreatic lipases and colipases.
Ex-vivo incubation and samples analysis
Faecal slurries (1% w/v) from each individual were used to inoculate the batch-culture system containing the nutrient medium and the digested bergamot phytosome (test). A batch culture system without bergamot phytosome and a non-treated faecal slurry sample were also included in the experiments as negative background control. After 16h of incubation at 37°C under anaerobic condition, samples were centrifuged and DNA extracted. 16S Amplicon barcoded library were prepared and DNA sequence obtained by Next Generation Sequence utilizing the Miseq platform (Illumina Inc, CA, USA).
Bioinformatics analysis
The 16S Metagenomics analysis were performed by using taxonomic classification of 16S rRNA targeted amplicon reads using an Illumina-curated version of the GreenGenes taxonomic database. The classification was performed by using the Illumina 16S Metagenomics workflow, as described in Wang Q et al. [12].
Statistical analysis
Data were analysed by Student’s t test.
Results
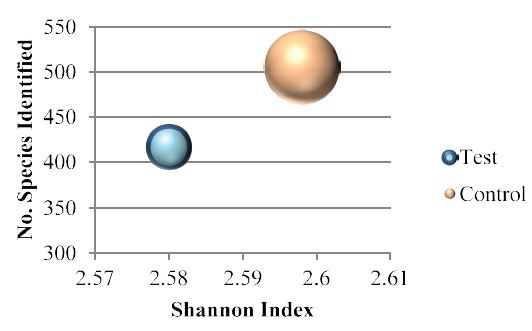
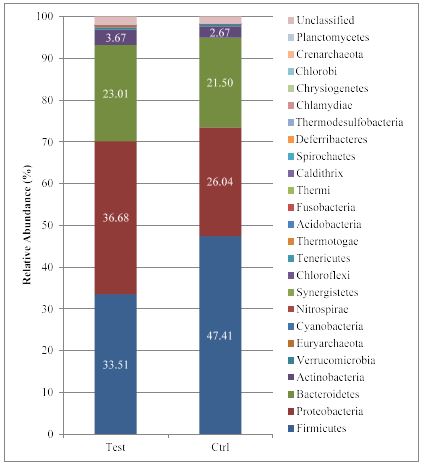
The 16S Metagenomic results were analysed in order to highlight the potential gut microbiota modulation produced by incubation of faecal samples with the bergamot phytosome. Shannon Index as a measure of the entropy of Species-level classifications [13] for both test and control groups (Figure 1) was comprised between 1.5 and 3.5, indicating a good evenness on samples. By using Illumina 16S Metagenomics workflow analysis, 25 different phyla were identified, with Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria as the four major phyla, as described in figure 2. The bergamot phytosome induced a decrease in the Firmicutes/Bacteroidetes ratio from 2.27 (Control) to 1.79 (test). Considering that an increase of the F/B ratio is correlated with obesity [3,14], bergamot phytosome is endowed with a great potential for counteracting body fat accumulation.
Figure 1: Shannon Index.
Figure 2: Relative Phyla abundance in control and treated faecal samples.T.
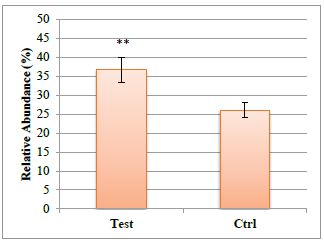
A significant increase of Proteobacteria in bergamot phytosome treated samples was also observed (p<0.01 vs control), as reported in figure 3.
The Actinobacteria is the less represented phylum. Also in this case, an increased level was observed after bergamot phytosome incubation.
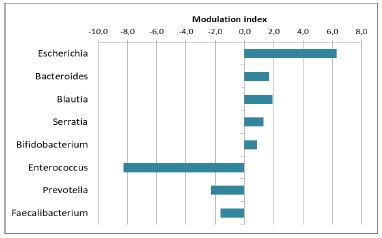
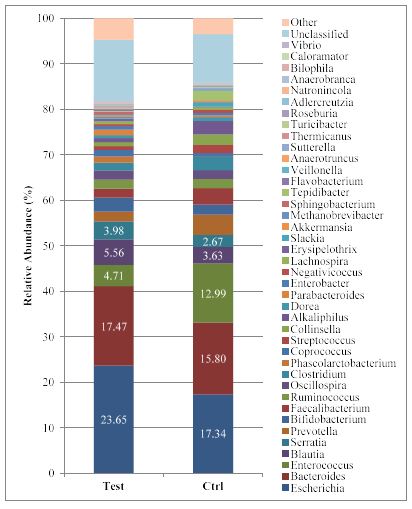
The analysis allowed identification of 418 different genera. Among them, 8 major genera (representing the 62% of those detected) were modulated, i.e. Escherichia, Serratia, Bacteroides, Prevotella, Enterococcus, Bifidobacterium, Blautia and Faecalibacterium (Figure 4). More in detail (Figure 5), an increase was observed in Blautia, Bifidobacterium, Escherichia, Serratia and Bacteroides while a reduction was observed for Enterococcus, Prevotella, and Faecalibacterium. Among them the observed difference for Escherichia (bergamot-treated 23.65 ± 2.32; control 17.34 ± 2.00) and Serratia (bergamot-treated 3.98 ± 0.38; control 2.67 ± 0.24) was statistically significant (p<0.05).
Discussion
For the first time, the effects of a new food-grade bioavailable delivery system of bergamot formulated in phytosome on human gut microbiota were investigated and then correlated to cardiovascular health, obesity and gastrointestinal disorders, thus supporting bergamot phytosome benefits in these areas, due to its potential to modulate related human gut microbiota after incubation with individual faecal slurries from healthy volunteers.
Cardiovascular diseases (CVDs) are the first cause of death at global level: the World Health Organization declared that about 17.9 million people died from CVDs in 2016, corresponding to 31% of all global deaths (https://www. who.int/news-room/fact-sheets/detail/cardiovasculardiseases-( cvds).
Several factors, like smoking and alcohol use, poor diet, and obesity represent the major risks for CVDs [15], so prevention measures are of high importance in limiting the onset of diseases. A large number of studies over ten years [16,17] suggested a link between obesity and gut microbiota, i.e. the plethora of microorganisms living in the gastrointestinal tract [1].
Figure 3:Proteobacteria levels in control and treated faecal samples.
Figure 4:Summary of the genera modulated by bergamot phytosome.
In animal models, adiposity and weight increase can be affected by intestinal microbes. Mice of identical genotype exposed to a different environments and fed by a high-fat diet develop a distinct arrangement of gut microbiota, which influences the development of obesity and metabolic syndrome [18]. An alteration in the gut barrier induced by microbiota changes due to a high-fat diet in mice was also previously demonstrated [19,20], and that condition led to metabolic diseases.
A human study conducted in obese and non-obese Danish people analysed genes from gut microbial populations identified individuals with low bacterial richness having more adiposity and dyslipidaemia in respect to high bacterial richness people [21], confirming a link between gut microbiota composition and obesity.
A recent paper reported a decrease in both triglycerides and LDL-C after 8-week treatments of a nutraceutical mixture containing BPF in dislypidemic overweight patients [22]. A significant reduction of serum LDL-C and triglyceride was also demonstrated by a new bergamot phytosome formulation [9]: in that clinical study, the delivery by phytosome allowed increased oral absorption of the naringin, the major component of bergamot, in respect to standard unformulated bergamot. Actually, phytosome techniques have recently demonstrated the successful delivering of other low-soluble natural compounds, like quercetin [23] and Coenzyme Q10 [24].
In this experimental study, we explored the hypothesis that bergamot phytosome would be able to modulate the human gut microbiome. Among the 25 phyla detected, a decrease in the Firmicutes/Bacteroidetes ratio was observed, indicating involvement of bergamot phytosome in the field of weight control. In fact, it is well known that the Firmicutes/Bacteroidetes ratio value increases in the presence of an obesity status [2,3].
Figure 5:Relative Genera abundance in control and treated faecal samples.
The significant increase of Proteobacteria observed in the present study in bergamot phytosome-treated samples (p<0.01 vs control) supports a positive effect for health status: while contrasting results were present in literature, our data are in agreement with those reported by Dinakaran that observed a reduction in Proteobacteria in CVD patients [25].
When analysis of genus was performed, an increased modulation in Blautia was observed in bergamot-treated samples. Blautia, involved in complex carbohydrate digestion, is recognized as a good indicator of the intestinal health, and its increase is beneficial. On the contrary, a Blautia reduction was observed in patients affected by heart failure [26], by hepatic and Chron’s disease, and cancer [27- 29]. Very interestingly, it has just been published that Blautia genus is inversely related with visceral fat accumulation [30].
Data obtained in the present study reported an increase in Bifidobacterium in bergamot-treated samples, suggesting again a positive modulation in weight control, because Bifidobacterium is decreased in obese subjects [31]. In our experiment, a reduction in Faecalibacterium was also observed: in the same paper of Gao the reduction is found in obese subjects, indicating for the latter a non-positive balance. However, in a very recent paper [32] an increase in Faecalibacterium prausnitzii is believed to be a good indicator of a health condition. In this regard, we must to underline that a large number of study on microbiome in different conditions (such as healthy or ill population, gender differences) do not allow the formulation of unique conclusions. In the same way Escherichia and Serratia increase (observed in bergamot-treated samples) would appear a non- positive effect.
The advantage in the present study of using fresh faecal materials from healthy subjects is undoubted, in respect to animal models. The study otherwise has some limitations due to its pilot design: the limited number of subjects, and the treatment administered via ’incubation’ in laboratory samples. Further clinical studies are needed to better explore the benefits of bergamot phytosome related to microbiome modulation, even though encouraging preliminary indications in the present study suggested a potential positive modulation of microbiota for CVD by bergamot phytosome and give a strong rational for further exploration.
Conclusion
In conclusion, for the first time, a microbiome modulation was associated to bergamot phytosome, supporting preliminary evidence of its clinical benefit for cardiovascular health and opening interesting new applications.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Principles of Good Clinical Practice and the Declaration of Helsinki and was in agreement with the Ethical code of PTP Science Park (Lodi, Italy) (https://www.ptp.it/it/aboutus# governance). The collection of fecal samples from donors healthy volunteers was performed after Written informed consent in an anonimyzed way, and any information cannot be directly related to each volunteer [33].
Consent for publication
All authors of the above manuscript, as well as the responsible authorities at both institutes where the work has been carried out, have agreed to this submission, and agree to transfer the ownership of copyright to Journal of Applied Microbiological Research. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Conflict of Interest
AR, VL, DB, PA, GP are Indena’s employees.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Authors’ contribution
Conceived and designed experiments: AR, GM, SB, DB, PA, GP. Performed the experiments: GM, SB. Analyze the data: VL, GP. Wrote the paper: AR, VL, DB, SB, GP. All the Authors read and approve the final manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgement
We would like to express our gratitude to Dr. Paola Misiano for her valuable editorial support.
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, et al. (2019) What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 7: 14. [ Ref ]
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, et al. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070- 11075. [ Ref ]
Ley RE, Bäckhed F, Turnbaugh P, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022-1023. [ Ref ]
Cani PD (2018) Human gut microbiome: hopes, threats and promises. Gut 67: 1716-1725. [ Ref ]
Da Pozzo E, De Leo M, Faraone I, Milella L, Cavallini C, et al. (2018) Antioxidant and Antisenescence Effects of Bergamot Juice. Oxid Med Cell Longev 12: 9395804. [ Ref ]
Gliozzi M, Walker R, Muscoli S, Vitale C, Gratteri S, et al., (2013) Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDLcholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int J Cardiol 170: 140-145. [ Ref ]
Toth PP, Patti AM, Nikolic D, Giglio RV, Castellino G, et al. (2015) Bergamot Reduces Plasma Lipids, Atherogenic Small Dense LDL, and Subclinical Atherosclerosis in Subjects with Moderate Hypercholesterolemia: A 6 Months Prospective Study. Front Pharmacol 6: 299. [ Ref ]
Formisano C, Rigano D, Lopatriello A, Sirignano C, Ramaschi G, et al (2019) Detailed Phytochemical Characterization of Bergamot Polyphenolic Fraction (BPF) by UPLC-DAD-MS and LC-NMR. J Agric Food Chem 67: 3159-3167. [ Ref ]
Mollace V, Scicchitano M, Paone S, Casale F, Calandruccio C, et al. (2019) Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr Metab Immune Disord Drug Targets 19: 136- 143. [ Ref ]
Minekus M, Alminger M, Alvito P, Balance S, Bohn T, et al. (2014) A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct 5: 1113-1124. [ Ref ]
Hollebeeck S, Borlon F, Schneider YJ, Larondelle Y, Rogez H (2013) Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem 138: 1936-1944. [ Ref ]
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261-5267. [ Ref ]
Hill MO (1973) Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 54: 427-432. [ Ref ]
Koliada A, Syzenko G, Moseiko V (2017)Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 17: 120. [ Ref ]
Chiuve SE, Cook NR, Shay CM, Rexrode KM, Albert CM, et al. (2014) Lifestyle-based prediction model for the prevention of CVD: the Healthy Heart Score. J Am Heart Assoc 3: e000954. [ Ref ]
Sanz Y, Santacruz A, De Palma G (2008) Insights into the roles of gut microbes in obesity. Interdiscip Perspect Infect Dis 2008: 829101. [ Ref ]
Gérard P (2016) Gut microbiota and obesity. Cell Mol Life Sci 73: 147-162. [ Ref ]
Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, et al. (2015) Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab 22: 516-530. [ Ref ]
Serino M, Luche E, Gres S, Baylac A, Bergé M, et al. (2012) Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61: 543-53. [ Ref ]
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FF, Knight R, et al. (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1: 6ra14. [ Ref ]
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, et al. (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541-546. [ Ref ]
Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C (2019) Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother Res 33: 2094-2101. [ Ref ]
Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P (2019) Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur J Drug Metab Pharmacokinet 44: 169-177. [ Ref ]
Petrangolini G, Ronchi M, Frattini E, De Combarieu E, Allegrini P, et al. (2019) A New Food-grade Coenzyme Q10 Formulation Improves Bioavailability: Single and Repeated Pharmacokinetic Studies in Healthy Volunteers. Curr Drug Deliv 16: 759-767. [ Ref ]
Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, et al. (2014) Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 9: e105221. [ Ref ]
Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, et al. (2017) Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 4: 282-290. [ Ref ]
Libertucci J, Dutta U, Kaur S, Jury J, Rossi L, et al. (2018) Inflammationrelated differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am J Physiol Gastrointest Liver Physiol 315: G420-G431. [ Ref ]
Luu TH, Michel C, Bard JM, Dravet F, Nazih H, et al. (2017) Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutr Cancer 69: 267-275. [ Ref ]
Nistal E, Sáenz de Miera LE, Pomar MB, Sánchez-Campos S, García- Mediavilla MV, et al. (2019) An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev Esp Enferm Dig 111: 275-282. [ Ref ]
Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, et al. (2019) Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes 5: 28. [ Ref ]
Gao R, Zhu C, Li H, Yin M, Pan C, et al. (2018) Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity (Silver Spring) 26: 351-361. [ Ref ]
Verhoog S, Taneri PE, Roa Díaz ZM, Marques-Vidal P, Troup JP, et al. (2019) Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients 11: 1565. [ Ref ]
A. Borovecki, A. Mlinaric, M. Horvat, V. Supak Smolcic. Informed consent and ethics committee approval in laboratory medicine. Biochem Med (Zagreb).2018 Oct 15;28(3):030201. doi: 10.11613/BM.2018.030201. Review. [ Ref ]