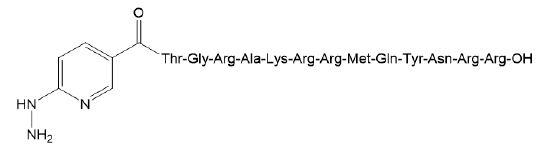Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 19 January, 2022
Accepted date: 16 February, 2022
Published date: 23 February, 2022
Citation: Nishiyama Y, Uehara T, Okazaki K, Maki H, Abe K, et al. (2022) In Vivo Monitoring of Viable Bacteria by SPECT using 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41 in Infected Mice. J Appl Microb Res. Vol: 5 Issu: 1 (01-07).
Copyright: © 2022 Nishiyama Y et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic bacterial refractory infections remain critical health care issues. When studying chronic infections, it is important but currently difficult to evaluate bacterial behavior in live individual animals over time. Ubiquicidin (UBI) 29-41 labeled with 99mTc has been tested for the specific imaging of bacterial and fungal infections in various species. In this study, we investigated the feasibility of monitoring viable bacterial counts by single-photon emission computed tomography (SPECT) in the thigh muscle of mice infected with Staphylococcus aureus using two types of 99mTc-labeled 6-hydrazinonicotinic acid (HYNIC)-UBI 29-41.
Method: 99mTc -HYNIC(Tricine)2-UBI 29-41 and 99mTc -HYNIC(GH)2- UBI 29-41 were synthesized, and their biodistribution was evaluated in normal mice. Each labeled peptide was injected into mice with S. aureus thigh infection after treatment with ciprofloxacin, and SPECT imaging was performed 2 h after the injection. The accumulation of labeled peptides at the thigh was assessed by SPECT, the number of viable bacteria was counted, and their correlation was evaluated. To evaluate bacteria regrowth, the same individual was administered the peptide twice and evaluated over time.
Results: The apparent molar activity of 99mTc-HYNIC(Tricine)2-UBI 29-41 was 400 times higher than that of 99mTc-HYNIC(GH)2-UBI 29-41. 99mTc-HYNIC(GH)2-UBI 29-41 showed high organ distribution and was 2–10 times more distributed than 99mTc-HYNIC(Tricine)2-UBI 29-41 in all organs at 0.5, 2, and 3 h after the injection of labeled peptides in normal mice. SPECT imaging revealed that the accumulation of labeled peptides in the bacterial infection site (left thigh) was higher than that in the non-infection site (right thigh) for both peptides. For 99mTc- HYNIC(GH)2-UBI 29-41, the target-to-non-target ratio (T/NT) was 3.3 when the viable bacterial count was 108 colony-forming units (cfu)/thigh and decreased to 1.2 when the viable bacterial count was 103 cfu/thigh. For 99mTc-HYNIC(Tricine)2-UBI 29-41, the T/NT ratio was significantly higher when the viable bacterial count was 108 cfu/thigh, with a value of 10.0. However, the T/NT ratio was 1.3 at 103 cfu/thigh, which is similar to the value for 99mTc-HYNIC(GH)2-UBI 29-41 with the same bacterial count. A high correlation was found between the T/NT ratios of each labeled peptide and the viable bacterial count at the infection site. The correlation was confirmed under antibacterial treatment conditions.
Bacterial regrowth after the cessation of antimicrobial agents was also monitored in live individual animals.
Conclusion: SPECT imaging with 99mTc-HYNIC-UBI 29- 41 enabled the monitoring of viable bacterial counts in the range of 103–108 cfu/thigh in thigh-infected mice over time.
Keywords
Antimicrobial peptide, Staphylococcus aureus, Monitoring viable bacteria, Single-Photon Emission Computed Tomography (SPECT).
Abbreviations
Ubiquicidin (UBI), Single-Photon Emission Computed Tomography (SPECT).
Highlights
Monitoring viable bacteria in vivo
Monitoring individual animals over time
Correlation between peptide accumulation and viable bacterial counts
Abstract
Background: Chronic bacterial refractory infections remain critical health care issues. When studying chronic infections, it is important but currently difficult to evaluate bacterial behavior in live individual animals over time. Ubiquicidin (UBI) 29-41 labeled with 99mTc has been tested for the specific imaging of bacterial and fungal infections in various species. In this study, we investigated the feasibility of monitoring viable bacterial counts by single-photon emission computed tomography (SPECT) in the thigh muscle of mice infected with Staphylococcus aureus using two types of 99mTc-labeled 6-hydrazinonicotinic acid (HYNIC)-UBI 29-41.
Method: 99mTc -HYNIC(Tricine)2-UBI 29-41 and 99mTc -HYNIC(GH)2- UBI 29-41 were synthesized, and their biodistribution was evaluated in normal mice. Each labeled peptide was injected into mice with S. aureus thigh infection after treatment with ciprofloxacin, and SPECT imaging was performed 2 h after the injection. The accumulation of labeled peptides at the thigh was assessed by SPECT, the number of viable bacteria was counted, and their correlation was evaluated. To evaluate bacteria regrowth, the same individual was administered the peptide twice and evaluated over time.
Results: The apparent molar activity of 99mTc-HYNIC(Tricine)2-UBI 29-41 was 400 times higher than that of 99mTc-HYNIC(GH)2-UBI 29-41. 99mTc-HYNIC(GH)2-UBI 29-41 showed high organ distribution and was 2–10 times more distributed than 99mTc-HYNIC(Tricine)2-UBI 29-41 in all organs at 0.5, 2, and 3 h after the injection of labeled peptides in normal mice. SPECT imaging revealed that the accumulation of labeled peptides in the bacterial infection site (left thigh) was higher than that in the non-infection site (right thigh) for both peptides. For 99mTc- HYNIC(GH)2-UBI 29-41, the target-to-non-target ratio (T/NT) was 3.3 when the viable bacterial count was 108 colony-forming units (cfu)/thigh and decreased to 1.2 when the viable bacterial count was 103 cfu/thigh. For 99mTc-HYNIC(Tricine)2-UBI 29-41, the T/NT ratio was significantly higher when the viable bacterial count was 108 cfu/thigh, with a value of 10.0. However, the T/NT ratio was 1.3 at 103 cfu/thigh, which is similar to the value for 99mTc-HYNIC(GH)2-UBI 29-41 with the same bacterial count. A high correlation was found between the T/NT ratios of each labeled peptide and the viable bacterial count at the infection site. The correlation was confirmed under antibacterial treatment conditions.
Bacterial regrowth after the cessation of antimicrobial agents was also monitored in live individual animals.
Conclusion: SPECT imaging with 99mTc-HYNIC-UBI 29- 41 enabled the monitoring of viable bacterial counts in the range of 103–108 cfu/thigh in thigh-infected mice over time.
Keywords
Antimicrobial peptide, Staphylococcus aureus, Monitoring viable bacteria, Single-Photon Emission Computed Tomography (SPECT).
Abbreviations
Ubiquicidin (UBI), Single-Photon Emission Computed Tomography (SPECT).
Highlights
Monitoring viable bacteria in vivo
Monitoring individual animals over time
Correlation between peptide accumulation and viable bacterial counts
Introduction
Bacterial refractory infections continue to pose a serious global health concern. In addition to the development of antibacterial resistance, even though pathogenic bacteria remain sensitive to antibiotics, failed eradication of causative bacteria sometimes leads to relapse [1]. Both physiological and genetic changes in bacteria are thought to contribute to drug resistance or tolerance and persistence in chronic infections [2]. Some phenotypes are fastidious, as they are only maintained in vivo and somehow disappear once the bacteria are removed from the host.
Therefore, establishing a novel method to evaluate bacterial behavior during drug treatment in individual animals over time is essential to accelerate next-generation antibacterial drug discovery research. In conventional acute infection models, counting viable bacteria by euthanizing animals at each time point makes it difficult to monitor bacterial behavior in individual animals. In contrast, imaging technology provides a useful non-invasive method to evaluate bacterial behavior.
Two imaging methods show potential for evaluating infections: optical imaging and radioactivity-based methods. Van Oosten et al. reported the use of luciferaseengineered Staphylococcus aureus and fluorescently labeled vancomycin (vanco-800CW) in a mouse myositis model [3]. Bioluminescence was used to indicate the localization of S. aureus and allowed the overlap with vanco-800CW to be determined. Ning et al. demonstrated the in vivo detection of Escherichia coli, S. aureus, Pseudomonas aeruginosa, and Bacillus subtilis using maltodextrin-based imaging probes in a rat myositis model [4]. Tang et al. reported infection imaging using concanavalin A as a bacteria-targeting ligand, a nanoparticle carrier, and a near-infrared fluorescent dye in a mouse wound model [5]. Optical imaging is considered useful for detecting bacterial infections; however, it can only be used if the infection model is close to the surface, such as in subcutaneous and thigh infections. Furthermore, the optical imaging sensitivity of bacterial counts was reported to be approximately 105 cfu/thigh. Empirically, the number of viable bacteria is reduced to around 102–103 cfu/ thigh following treatment with antibacterial agents [3-5]. However, if relapse occurs when treatment is discontinued, optical imaging is considered to have insufficient sensitivity to monitor the bacteria.
The other imaging technology is based on radioactivity. Radioactivity-based methods can be used to evaluate deep infections, such as lung infections, in addition to those near the surface, and they generally have higher sensitivity than optical imaging. Therefore, we selected single-photon emission computed tomography (SPECT) with 99mTc-labeled probes to monitor the same animal using radioactivity.
Several radiolabeled agents, such as antibodies, antibiotics, and peptides, have been evaluated for imaging infections [6]. Among them, ubiquicidin (UBI) 29-41 was selected as the probe to be examined in this study. UBI 29-41 is one of the infection probes used clinically that binds to a broad spectrum of Gram-positive and Gram-negative bacteria and fungi [7]. UBI 29-41 is a cationic human antimicrobial peptide fragment with six positively charged residues (5 Arg + 1 Lys) that accumulates at the negatively charged surfaces of microorganisms. Nibbering et al. reported in vivo studies using UBI 29-41 in mice and rats infected with S. aureus [8]. They monitored the efficacy of antibiotics and reported a high correlation between the accumulation of UBI 29-41 at the infection site and the dose of antibiotics administered.
It has been reported that the nature of the coligand affects the biodistribution of 99mTc-labeled 6-hydrazinonicotinic acid (HYNIC)-chemotactic peptides [9]. In this report, we synthesized two types of labeled UBI 29-41 with α-Dglucoheptonic acid (GH) and tricine as coligands. Using these two labeled peptides, we evaluated the feasibility of in vivo monitoring of viable bacterial counts by SPECT.
Materials and Methods
Antibiotics and synthetic antimicrobial peptides
Ciprofloxacin (CPFX; hydrochloride, ≥ 98% activity) was purchased from LKT Laboratories, Inc. (MN, USA). Cyclophosphamide (CY; hydrate, 94% activity) was purchased from Shionogi & Co., Ltd. (Osaka, Japan). Brain- Heart Infusion (BHI) broth and agar were purchased from BD. (NJ, USA) UBI 29-41 denotes TGRAKRRMQYNRR (1693 Da). HYNIC-UBI 29-41 was synthesized at Toray Research Center (Tokyo, Japan, Figure 1).
Labeling procedure and quality control
99mTc-HYNIC(GH)2-UBI 29-41 was labeled as follows: 2 mg of the GH kit (freeze-dried GH 2 mg and SnCl2/2H2O 1.2 μg) was dissolved in 1 mL of 99mTcO4 − (185 MBq/mL, Nihon Medi-Physics Co. Ltd.), and the sample was incubated for 10 min at room temperature to synthesize 99mTc-GH. Then, 200 μL of the 99mTc-GH solution and 200 μL of 400 μM HYNICUBI aqueous solution were mixed and incubated for 60 min at room temperature. 99mTc-HYNIC(Tricine)2-UBI 29-41 was labeled as follows: 125 μL of 99mTcO4 − was mixed with 125 μL of 40 mg/mL tricine in 10 mM acetate buffer (pH 4). Then, 10 μL of 25 μM HYNIC-UBI aqueous solution and 6 μL of 1 mg/ mL SnCl2/2H2O in 0.1 N HCl were added, and the sample was incubated for 10 min at 90°C [10]. In both labeled reactions, the amount of HYNIC-UBI was sufficient to bind all 99mTc. Following labeling, each reaction mixture was analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC). The sample was applied to an XBridge® C18 5-μm column (4.6 × 150 mm, Waters) attached to a chromatograph equipped with an online UV detector set at 254 nm and a NaI (Tl) crystal gamma detection system (Gabi star, Raytest, Straubenhardt, Germany). 99mTc-HYNIC(GH)2- UBI 29-41 was detected using a linear gradient of two eluents at a flow rate of 1.2 mL/min: 0.1% (v/v) trifluoroacetic acid (TFA)/water (solvent A) and 0.1% TFA/acetonitrile (solvent B). The gradient was applied as follows: 95%–20% A in 15 min and 95% A for 5 min. 99mTc-HYNIC(Tricine)2-UBI 29- 41 was detected using a linear gradient of two eluents at a flow rate of 1.0 mL/min: 0.01% (v/v) TFA/water (solvent A) and 0.01% TFA/acetonitrile (solvent B). The gradient was applied as follows: 95%–70% A in 20 min and 95% A for 5 min.
Figure 1: Structural formula of HYNIC-UBI 29-41.
Microorganism
S. aureus 25923 (American Type Culture Collection) susceptible to CPFX (minimum inhibitory concentration < 1 μg/mL) was used. S. aureus 25923 was cultured on Brain– Heart Infusion Agar for 24 h at 37°C. The colony suspension was washed, counted by optical density, and used in the in vitro binding assay. The viable cell count was also determined by plating on Brain–Heart Infusion Agar. For the in vivo assay, a stock solution of S. aureus 25923 stored at −80°C was used.
In Vitro binding to S. aureus
Ten microliters of the preparation containing each labeled peptide and 10 μL of suspension containing 5×1010 cfu/mL S. aureus were added to 80 μL of a binding buffer (20 mM phosphate buffered saline containing 0.01% Tween80 and 5 mM acetic acid, pH=5). For the serum conditions, 10 μL of mouse serum were added instead of 10 μL of the binding buffer. The suspensions were gently mixed using a vortex mixer and incubated at 37°C for 1 h. After incubation, the tubes were centrifuged at 5000 ×g for 10 min. The supernatant was removed, and the pellet was resuspended in 100 μL of binding buffer and re-centrifuged at 5000 ×g for 10 min. The supernatant was removed, and the radioactivity of the pellet was counted using a γ-counter. The radioactivity associated with the bacteria pellet was expressed as a percentage of the total 99mTc activity added.
Table 1: Biodistribution of 99mTc-HYNIC(Tricine)2-UBI 29-41 and 99mTc-HYNIC(GH)2-UBI 29-41 in mice
| Labeled peptide | Time after injection | Lung | Liver | Kidney | Left thigh | Blood |
|---|---|---|---|---|---|---|
| GH | 30 min | 4.07 ± 0.50 | 5.07 ± 0.51 | 46.16 ± 1.46 | 1.41 ± 0.18 | 3.43 ± 0.11 |
| 2 hr | 3.55 ± 0.43 | 7.37 ± 0.77 | 56.53 ± 9.20 | 1.17 ± 0.25 | 2.41 ± 0.29 | |
| 3 hr | 3.75 ± 0.24 | 8.18 ± 0.07 | 65.45 ± 4.33 | 1.11 ± 0.24 | 2.34 ± 0.11 | |
| Tricine | 30 min | 1.77 ± 0.16 * | 2.11 ± 0.12 * | 83.89 ± 5.83 * | 0.84 ± 0.06 * | 2.01 ± 0.19 * |
| 2 hr | 0.55 ± 0.04 * | 1.96 ± 0.02 * | 84.88 ± 16.08 | 0.22 ± 0.01 * | 0.40 ± 0.05 * | |
| 3 hr | 0.36 ± 0.03* | 2.08 ± 0.26* | 73.07 ± 9.81 | 0.18 ± 0.03* | 0.22 ± 0.03* | |
| Expressed as % injected dose per gram. Each value represents the mean (SD) for three animals at each interval. *Values that were significantly (P<0.05) different compared with GH. | ||||||
Animals
All procedures for animal studies were approved by the Institutional Animal Care and Use Committee of Shionogi & Co., Ltd. (Osaka, Japan). Specific-pathogen-free male ICR mice (CLEA Japan Inc., 5 weeks old) were used in the infection and SPECT studies.
Biodistribution
A 0.2-mL solution containing 30 kBq of 99mTc- HYNIC(Tricine)2-UBI 29-41 or 10 kBq of 99mTc-HYNIC(GH)2- UBI 29-41 was administered via the tail vein of mice. Animals were euthanized by exsanguination, and the thoracic cavity was opened at 0.5, 2, and 3 h post injection. Organs were excised and weighed, and the activity of probes was counted using a γ-counter. Organ uptake was calculated as a percentage of the injected dose per gram of wet tissue (%ID/ organ, Table 1).
Treatment of animal infections with antibacterial agents
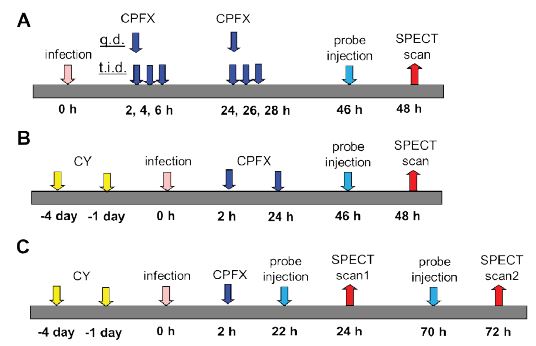
Normal and immunosuppressed mice were used. To generate immunosuppressed mice, CY was injected by intraperitoneal administration at 4 days and 1 day before infection. Mice were anesthetized with isoflurane, and 6.0×106–2.0×107 cfu of bacteria in 0.1 mL saline were aseptically injected into the left thigh muscle of each mouse. The antimicrobial procedures (original) were as follows: mice were subcutaneously administered 10–100 mg/kg of CPFX once a day or three times a day for 2 days after infection (Figure 2A, B). In experiments to monitor regrowth, mice were administered CPFX only once 2 h after infection (Figure 2C).
SPECT imaging
At 46 h after infection, 0.2 mL of a solution containing 10– 30 MBq of each labeled peptide was administered via the tail vein of mice (Figure 2A, B). In experiments for monitoring regrowth, the labeled peptide was administered at 22 h and 70 h after infection (Figure 2C).
The accumulation of each labeled peptide in the bacteriainfected site in mice was assessed by SPECT/CT (Triumph II SPECT 2H/XO SRI CT, TriFoil Imaging). Two hours after the labeled peptide injection, mice were anesthetized with isoflurane. Mice were then arranged lying face down on a SPECT/CT bed with both hind legs spread out and fixed with surgical tape. Whole body images were acquired under the following conditions: energy window, 20% at 140 keV; projection limit, 20 s; projection count, 64; and rotation angle, 360 degrees. The total time for actual imaging was only ~20 min. After whole body imaging, the mice were euthanized, and the infected (left) and normal (right) legs were extracted. Leg-only images were then acquired using the same conditions. For image processing, adjusted regions of interest were drawn over the entire infected muscle (target [T]) and contralateral muscle (nontarget [NT]). The accumulation of each labeled peptide at the infection site was expressed as the ratio of the counts in the target and nontarget muscles (T/NT).
Figure 2: Schematic diagram of the experimental protocol infected with S. aureus in normal mice (A) in immunosuppressed mice (B) at monitoring for regrowth (C).
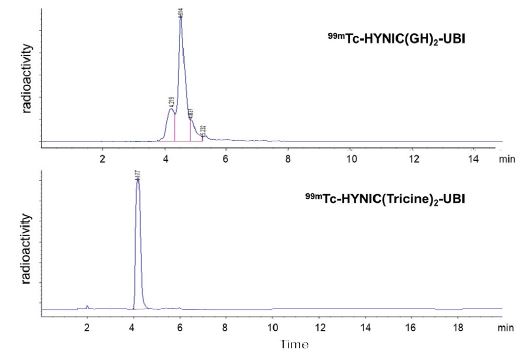
Figure 3: Typical HPLC radioactivity profiles of 99mTc-HYNIC(GH)2-UBI and 99mTc-HYNIC(Tricine)2-UBI.
Figure 4:In vitro bacterial binding of 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41 to S. aureus. Results are expressed as the mean ± SD for three tests.
Determining the number of viable bacteria
After SPECT imaging, the entire infected thigh muscles were removed and individually homogenized in Mueller– Hinton broth. Serial dilutions of the thigh homogenate were plated on Brain–Heart Infusion Agar. The plates were then incubated for 24 h at 37°C, and the numbers of colonies were counted. The viable bacterial counts in thigh (log10 CFU/thigh) were calculated from the number of colony in the plate.
Statistical Analysis
The differences between log cfu before and after treatment with CPFX in mice were evaluated using the Student’s t-test. The P values were calculated, and statistical significance was accepted within 95% confidence limits by SAS® Version 9.4. All results were reported as means and SD. The Pearson correlation coefficient (r) was used to assess the correlation between the labeled peptide accumulation and the viable bacterial count.
Results
Labeling and quality control
RP-HPLC analysis of 99mTc-HYNIC(GH)2-UBI 29-41 showed two major peaks (4.2 and 4.5 min), and that of 99mTc- HYNIC(Tricine)2-UBI 29-41 showed a single peak (4.2 min, Figure 3). Leading and trailing shoulders were detected for both peptides; however, a previous report indicated that derivatives with GH and tricine as coligands existed in many isomeric forms [11]. In both labeled reactions, the amount of HYNIC-UBI was sufficient to bind all 99mTc. The radiochemical purity was 91±9% (n=8) for 99mTc-HYNIC(GH)2-UBI 29-41 and 100% (n=8) for 99mTc-HYNIC(Tricine)2-UBI 29-41. Each labeled peptide was administrated without purification. The apparent molar activity of 99mTc-HYNIC(GH)2-UBI 29-41 was 0.85±0.085 GBq/μmol (n=8), and that of 99mTc- HYNIC(Tricine)2-UBI 29-41 was 389 GBq/μmol (n=8). It was confirmed that the stability of each labeled peptide had decreased by only a few percent at 2 h after administration to the second animal.
In Vitro binding to S. aureus
The binding of 99mTc-HYNIC(GH)2-UBI 29-41 to S. aureus (5×108 cfu/tube) without serum was 90.7±5.2% of the total 99mTc activity, and in the presence of serum, the binding was 69.5±2.7%. The binding of 99mTc-HYNIC(Tricine)2-UBI 29-41 was 90.7±1.7% without serum and decreased to 13.2±1.0% in the presence of serum (Figure 4).
Biodistribution
The biodistributions of 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41 in normal mice at 0.5, 2, and 3 h are summarized in table 1. The data showed that the highest concentrations of radioactivity were measured in the kidney for both peptides. The accumulation of 99mTc- HYNIC(GH)2-UBI 29-41 in the kidney increased over time, whereas 99mTc-HYNIC(Tricine)2-UBI 29-41 decreased. 99mTc- HYNIC(GH)2-UBI 29-41 showed high organ distribution and was 2–10 times more distributed than 99mTc- HYNIC(Tricine)2-UBI 29-41 in all organs at all time points. This difference in organ distribution is attributed to the difference in coligands. The activity at the infection site in the thigh showed no further increase after 2 h; therefore, the SPECT images were acquired 2 h after peptide injection.
Effect of antibiotic administration
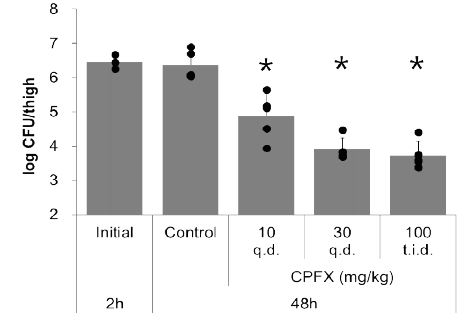
To evaluate the correlation between the labeled peptide accumulation and the viable bacterial count, it was necessary to establish various viable bacterial counts. In addition to the untreated (control) group, 2-day treatment with 10–100 mg/kg of CPFX was administered to mice. As a result, viable bacterial counts in the range of 103–108 cfu/thigh were established (Figure 5). This range was considered sufficient to evaluate treatment with antibiotics in a chronic infection model.
Detection of labeled peptide accumulation at the infection site using SPECT
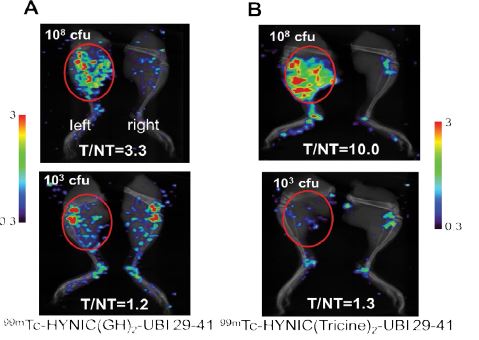
The bacterial infection site was imaged 2 h after the injection of each labeled peptide. Of the many tests performed with 103–108 cfu/thigh, representative images for each labeled peptide in S. aureus-infected mice are shown in figure 6. For 99mTc-HYNIC(GH)2-UBI 29-41, the T/NT ratio was 3.3 when the viable bacterial count was 108 cfu/thigh and decreased to 1.2 when the viable bacterial count was 103 cfu/thigh. For 99mTc-HYNIC(Tricine)2-UBI 29-41, the T/ NT ratio was significantly higher when the viable bacterial count was 108 cfu/thigh, with a value of 10.0. However, the T/NT ratio was 1.3 at 103 cfu/thigh, which is similar to the value for 99mTc-HYNIC(GH)2-UBI 29-41 with the same bacterial count.
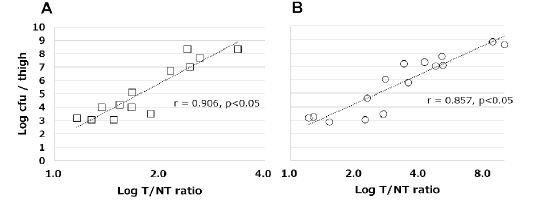
The correlations between the accumulation of each labeled peptide and the viable counts of bacterial are shown in figure 7. The accumulation of each labeled peptide showed a high correlation with the viable bacterial count: r=0.906 and P=0.002 for 99mTc-HYNIC(GH)2-UBI 29-41; r=0.857 and P=0.001 for 99mTc-HYNIC(Tricine)2-UBI 29-41.
Monitoring for regrowth by SPECT
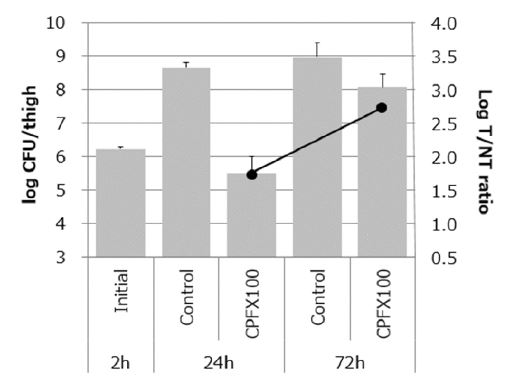
The mice were treated with CPFX 2 h after infection and first imaged at 24 h after infection when the bacteria had decreased. Afterward, individual mice were re-imaged at 72 h after infection when the remaining bacteria had regrown (Figure 2C). In the CPFX treatment group, the viable bacterial count was 105 cfu/thigh at 24 h after infection but increased to 108 cfu/thigh at 72 h. The T/NT ratio value increased from 1.8 at 24 h to 2.8 at 72 h after treatment with CPFX (Figure 8).
Figure 5: Viable bacterial count in thigh infected with S. aureus for normal mice treated with CPFX for 2 days. Results are expressed as the mean ± SD for three animals. *Values that were significantly (P < 0.05) different to the control.
Figure 6: SPECT images of [A] 99mTc-HYNIC(GH)2-UBI 29-41(10MBq) in normal mice [B] 99mTc-HYNIC(Tricine)2-UBI 29-41(30MBq) in immunosuppressed mice. Mice were infected in the left thigh (red circle) with S. aureus, acquired 2 h after the administration.
Figure 7: Correlation between viable S. aureus counts and the accumulation of [A] 99mTc-HYNIC(GH)2-UBI 29-41 in normal and immunosuppressed mice (square) [B] 99mTc-HYNIC(Tricine)2-UBI 29-41 in immunosuppressed mice (circle), expressed as the target-to-non-target(T/NT) ratio.
Discussion
The biodistributions of 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41 in normal mice both showed high renal excretion. Welling MM et al. reported that 99mTc-HYNIC(Tricine)2-UBI 29-41 showed rapid elimination mainly through the kidneys and poor distribution in all organs [12]. The clearance of 99mTc-HYNIC(GH)2-UBI 29- 41 was slower than that of 99mTc-HYNIC(Tricine)2-UBI 29- 41, and the distribution of 99mTc-HYNIC(GH)2 -UBI 29-41 in organs was higher than that of the other peptide. The binding of 99mTc-HYNIC(Tricine)2-UBI 29-41 was influenced by the presence of serum (Figure 4). Several research groups have reported that small biomolecules labeled with tricine as a coligand were unstable and existed as multiple species in solution and that they may react with the imidazole group of histidine residues in circulating blood proteins, such as albumin [13,14]. Therefore, the low organ distribution of 99mTc-HYNIC(Tricine)2-UBI 29-41 is attributed to similar interactions. In addition, because 99mTc-HYNIC(Tricine)2- UBI 29-41 is less hydrophobic than 99mTc-HYNIC(GH)2-UBI 29-41, it shows less non-specific binding to organs.
Figure 8: Left axis (bar graph): Viable bacterial count in thigh infected with S. aureus for immunosuppressed mice treated with CPFX. Results are expressed as the mean ± SD for three animals. Right axis (line graph): The accumulation of 99mTc-HYNIC(GH)2 -UBI in the left thigh. Results are expressed as the mean for two animals.
Next, we aimed to quantify the viable bacteria by measuring the amount of labeled peptide accumulated in the bacteria using SPECT. To this end, we investigated the correlation between the labeled peptide accumulation and the viable bacterial counts. To the best of our knowledge, this correlation has only been investigated by Lupetti et al. using 99mTc-labeled fluconazole with Candida albicans at 106–108 cfu/g tissue (about 105–107 cfu/thigh), and a high correlation was observed [15]. In this study, we used a dynamic range of bacterial counts from 103–108 cfu/thigh. Empirically the number of viable bacteria is reduced to around 102–103 cfu/thigh following treatment with antibacterial agents, the lower limit was set at 103 cfu/thigh. To achieve this bacterial count, mice were treated with CPFX. The bacterial count reached a minimum of 104 cfu/thigh on the first day of treatment and reached the target of 103 cfu/thigh on the second day of treatment (Figure 5).
The accumulation of each labeled peptide was evaluated at high bacterial counts (107–108 cfu/thigh) using SPECT. The T/NT ratio of 99mTc-HYNIC(GH)2-UBI 29-41 was 2.4, and that of 99mTc-HYNIC(Tricine)2-UBI 29-41 was 10.0 (Figure 6). We then investigated peptide accumulation at the detection target of 103 cfu/thigh using the same method and determined T/NT ratios of 1.2 and 1.3 for 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41, respectively. The T/ NT ratio range of 99mTc-HYNIC(GH)2-UBI 29-41 was 1.2–3.3, and that of 99mTc-HYNIC(Tricine)2-UBI 29-41 was 1.3–10.0.
This difference in the T/NT ratio range is thought to be due to variations in the biodistributions of each peptide and their binding activity to bacteria. 99mTc-HYNIC(Tricine)2- UBI 29-41 exhibited less non-specific binding to organs than 99mTc-HYNIC(GH)2-UBI 29-41 (Table 1), which is one of the factors indicating a high T/NT ratio at a high bacterial count. However, due to the lower accumulation of 99mTc- HYNIC(Tricine)2-UBI 29-41 than 99mTc-HYNIC(GH)2-UBI 29-41, the injection dose for 99mTc-HYNIC(Tricine)2-UBI 29- 41 had to be higher to be detected by SPECT. In this study, both peptides with different properties could be detected by SPECT, but it was necessary to adjust the injection dose for each labeled peptide according to the lower limit of quantification of SPECT. Based on this thigh infection system, 99mTc-HYNIC(Tricine)2-UBI 29-41 is more suitable for quantifying bacteria than 99mTc-HYNIC(GH)2-UBI 29-41.
In this report, it was possible to monitor decreases in the number of bacteria with antibacterial agents. This suggests that bacteria killed by antibacterial agents are eliminated from the site of infection by the immune system. Moreover, it has been reported that UBI does not accumulate in sterile inflammation, and its accumulation at the infected site is thought to occur only in viable bacteria [16,17].
This study determined that SPECT imaging with 99mTc- HYNIC-UBI29-41 is a useful method to quantify viable bacteria in the range 103–108 cfu/thigh by measuring the accumulation of labeled peptides. Furthermore, even with continuous SPECT imaging in individual mice, the accumulation of the labeled peptide showed a high correlation with the viable bacterial count.
In this study, we examined one bacterial species and model. Therefore, we aim to study other bacterial species and models for research on bacterial refractory infections involving persisters and biofilms in the future.
Conclusion
SPECT imaging can be used to quantify viable bacterial counts ranging from 103 to 108 cfu/thigh by measuring the accumulation of labeled antimicrobial peptides. This approach enables the monitoring of viable bacterial counts in live individual animals over time, which is required for the investigation of chronic bacterial refractory infections.
Acknowledgment
We thank Yumi Sato for helping with 99mTc labeling and Masaaki Izawa for establishing the mouse infection model.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing Interests
The authors declare that they have no conflicts of interest.
ReAct facts (2012) Burden of antibiotic resistance. [ Ref ]
Maisonneuve E, Gerdes K (2014) Molecular mechanisms underlying bacterial persisters. Cell 157: 539-548. [ Ref ]
Van Oosten M, Schafer T, Gazendam JAC (2013) Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat Commun 4: 2584. [ Ref ]
Ning X, Lee S, Wang Z (2011) Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat Mater 10: 602-607. [ Ref ]
Tang E, Nair A, Baker DW (2014) In vivo imaging of infection using a bacteria-targeting optical nanoprobe. J Biomed Nanotechnol 10: 856-863. [ Ref ]
Van Oosten M, Hahn M, Crane LM (2015) Targeted imaging of bacterial infections: advances, hurdles and hopes. FEMS Microbiology Reviews 39: 892-916. [ Ref ]
Sathekge M, Garcia‑Perez O, Paez D (2018) Molecular imaging in musculoskeletal infections with 99mTc-UBI 29-41 SPECT/CT. Ann Nucl Med 32: 54-59. [ Ref ]
Nibbering PH, Welling MM, Paulusma-Annema A (2004) 99mTc-Labeled UBI 29-41 peptide for monitoring the efficacy of antibacterial agents in mice infected with Staphylococcus aureus. J Nucl Med 45: 321-326. [ Ref ]
Babich JW, Fischman AJ (1995) Effect of “co-ligand” on the biodistribution of 99mTc-labeled hydrazine nicotinic acid derivatized chemotactic peptides. Nucl Med Biol 22: 25-30. [ Ref ]
Welling MM, Korsak A, Gorska B (2005) Kit with technetium-99m labelled antimicrobial peptide UBI 29-41 for specific infection detection. J Label Compd Radiopharm 48: 683-691. [ Ref ]
Edwards DS, Liu S, Ziegler MC (1999) RP463: A stabilized technetium- 99m complex of a hydrazino nicotinamide derivatized chemotactic peptide for infection imaging. Bioconjug Chem 10: 884-891. [ Ref ]
Welling MM, Visentin R, Feitsma HIJ (2004) Infection detection in mice using 99mTc-labeled HYNIC and N2S2 chelate conjugated to the antimicrobial peptide UBI 29-41. Nucl Med Biol 31: 503-509. [ Ref ]
Meszaros LK, Dose A, Biagini SCG (2010) Hydrazinonicotinic acid (HYNIC) – coordination chemistry and applications in radiopharmaceutical chemistry. Inorganica Chim Acta 363: 1059-1069. [ Ref ]
Purohit A, Liu S, Ellars CE (2004) Pyridine-containing 6-hydrazinonicotinamide derivatives as potential bifunctional chelators for 99mTc-labeling of small biomolecules. Bioconjug Chem15: 728-737. [ Ref ]
Lupetti A, Welling MM, Mazzi U (2002) Technetium-99m labelled fluconazole and antimicrobial peptides for imaging of Candida albicans and Aspergillus fumigatus infections. Eur J Nucl Med 29:674-679. [ Ref ]
Akhtar MS, Iqbal J, Khan MA (2004) 99mTc-labeled antimicrobial peptide ubiquicidin (29-41) accumulates less in Escherichia coli infection than in Staphylococcus aureus infection. J Nucl Med 45: 849-856. [ Ref ]
Gandomkar M, Najafi R, Mazidi M (2008) New peptide based freeze-dried kit [99mTc-HYNIC]-UBI 29-41 as a human specific infection imaging agent. Iran J Nucl Med 16: 25-30. [ Ref ]