Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 14 March, 2020
Accepted date: 21 April, 2020
Published date: 28 April, 2020
Citation: Fabian NV et al. (2020) Isolation and Screening of Heavy Metal Resistant Ammonia Oxidizing Bacteria from Soil and Waste Dump: A Potential Candidate for Bioremediation of Heavy Metals. J Appl Microb Res. Vol: 3 Issu: 1(15-24).
Copyright: © 2020 Fabian NV. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The study was undertaken to examine the response of ammonia oxidizing bacteria to different heavy metal salt in an elevated concentration. Surface soil samples at depth of 0-15 cm were collected at random from Akwa Ibom State University in Akwa Ibom State, soil sample from University of Nigeria, Nsukka and from solid waste disposal site in Uyo, Akwa Ibom State. The response of heavy metal salt on Ammonia Oxidizing bacteria(AOB) isolated from soil samples were investigated by supplementing different heavy metal salts namely, copper(Cu),nickel (Ni), lead (Pb) and cadmium (Cd) at four loading rates(100,200,500,1000 μg/ml) in mineral salt broth with Ammonia Oxidizing Bacteria (AOB) isolate. The cultures were incubated for 7 days. Growth of AOB was measured by withdrawing samples from the medium every 24 hours and absorbance of the turbidity measured at 600 nanometre using spectrophotometer. All bacteria showed high tendency to decrease optical density while increasing metal concentration in the medium. Tolerance for the metal ions was dependent on concentration, time and the isolate tested. All the Ammonia oxidizing bacterial (AOB) showed a high level of tolerance for the metals tested, and exhibited good growth at all metal salt concentrations tested. Nitrifying bacteria; Achromobacter xylosoxidans and Achromobacter insolitus, were able to carry out biosorption of copper, nickel, lead and cadmium as seen in this study. Achromobacter insolitus had the highest biosorption capacity for copper and cadmium at 90.04% and 89.21%, respectively within a period of 28 days. Achromobacter xylosoxidans had the highest biosorption capacity for nickel and lead at 96.51% and 92%, respectively within a period of 28 days. These make the nitrifying bacteria attractive potential candidates for further investigations regarding their ability to remove metals from contaminated soil.
Keywords
Heavy metal, Tolerance, Nitrification, Ammonia Oxidizing Bacteria.
Aim of the Study
To study tolerance of ammonia oxidizing bacteria growth to heavy metal
Significance of the Study to the field
Nitrifying bacteria remain good option for bioremediation of soil and waste dump, since it is regarded as eco-friendly and efficient in biosorption of heavy metal. The study is significant to the field of Environmental microbiology by adding to knowledge in bioremediation
Abstract
The study was undertaken to examine the response of ammonia oxidizing bacteria to different heavy metal salt in an elevated concentration. Surface soil samples at depth of 0-15 cm were collected at random from Akwa Ibom State University in Akwa Ibom State, soil sample from University of Nigeria, Nsukka and from solid waste disposal site in Uyo, Akwa Ibom State. The response of heavy metal salt on Ammonia Oxidizing bacteria(AOB) isolated from soil samples were investigated by supplementing different heavy metal salts namely, copper(Cu),nickel (Ni), lead (Pb) and cadmium (Cd) at four loading rates(100,200,500,1000 μg/ml) in mineral salt broth with Ammonia Oxidizing Bacteria (AOB) isolate. The cultures were incubated for 7 days. Growth of AOB was measured by withdrawing samples from the medium every 24 hours and absorbance of the turbidity measured at 600 nanometre using spectrophotometer. All bacteria showed high tendency to decrease optical density while increasing metal concentration in the medium. Tolerance for the metal ions was dependent on concentration, time and the isolate tested. All the Ammonia oxidizing bacterial (AOB) showed a high level of tolerance for the metals tested, and exhibited good growth at all metal salt concentrations tested. Nitrifying bacteria; Achromobacter xylosoxidans and Achromobacter insolitus, were able to carry out biosorption of copper, nickel, lead and cadmium as seen in this study. Achromobacter insolitus had the highest biosorption capacity for copper and cadmium at 90.04% and 89.21%, respectively within a period of 28 days. Achromobacter xylosoxidans had the highest biosorption capacity for nickel and lead at 96.51% and 92%, respectively within a period of 28 days. These make the nitrifying bacteria attractive potential candidates for further investigations regarding their ability to remove metals from contaminated soil.
Keywords
Heavy metal, Tolerance, Nitrification, Ammonia Oxidizing Bacteria.
Aim of the Study
To study tolerance of ammonia oxidizing bacteria growth to heavy metal
Significance of the Study to the field
Nitrifying bacteria remain good option for bioremediation of soil and waste dump, since it is regarded as eco-friendly and efficient in biosorption of heavy metal. The study is significant to the field of Environmental microbiology by adding to knowledge in bioremediation
Introduction
Environmental pollution has been on the rise in the past few decades owing to increased human activities on energy reservoirs, unsafe agricultural practices and rapid industrialization [1]. Amongst the pollutants that are of environmental and public health concerns due to their toxicities are: heavy metals, nuclear wastes, pesticides, greenhouse gases, and hydrocarbons. These toxic pollutants are discharged from specific locations worldwide and thus pollute specific regions. These pollutants have different toxicity and chemical behaviour [2]. Environmental contamination caused by heavy metals (HM) has received increased attention worldwide [3-6]. Heavy metal (HM) is any metallic element that has a relatively high density and is toxic or poisonous at low concentrations. Heavy metals are elements with atomic number higher than 20, an atomic mass greater than 40 g and a specific weight of more than 5 g/cm3 [7,8]. These elements often find their way into soil through environmental contaminants including the atmospheric pollutants in industrial regions (emissions from the rapidly expanding industrial areas), unlimited use of agricultural fertilizers, mine tailings, disposal of high metal wastes, leaded gasoline and paints, animal manures, sewage sludge, pesticides, wastewater irrigation, coal combustion residues, spillage of petrochemicals, atmospheric deposition, municipal and industrial sewage systems in a nonreturnable fashion [9-12].
Activities such as the use of agrochemicals and longterm application of urban sewage sludge, industrial waste disposal, waste incineration, and vehicle exhausts are the main sources of HM in agricultural soils [13]. Heavy metals in the soil include mercury (Hg), lead (Pb), chromium (Cr), arsenic (As), zinc (Zn), cadmium (Cd), uranium (U), selenium (Se), silver (Ag), gold (Au) , copper (Cu) and nickel (Ni) [11]. The danger of heavy metals is intensified by their almost indefinite persistence in the environment due to their absolute nature which cannot be degraded [14]. Metals are non-biodegradable but can be transformed through sorption, methylation, complexation and changes in valence state [15].
Toxic metals apply their toxicity in the displacement of essential metals from their normal binding sites of biological molecules, inhibition of enzymatic functioning and disruption of nucleic acid structure, oxidation stress, genotoxicity and interfering with signalling pathways [11,16]. Ecologically, the accumulation of heavy metals in soils is extremely hazardous because soil is a major link in the natural cycling of chemical elements; it is also a primary component of the trophic chain [17-19].
Industrial operations such as electroplating, steel manufacturing, leather tanning, wood preservation, ceramics, glass manufacturing, chemical processing and fertilizer applications release alarmingly higher amounts of heavy metals into the natural environment [12]. Pollution by heavy metal is a threat to the environment and it remediation is a major challenges to environmental research. Heavy metal pollution is a serious global environmental problem as it adversely affects biotic and abiotic components of the ecosystem and alters the composition and activity of soil microbial communities [5]. The non-biodegradability of heavy metals makes it hard to remove them from contaminated biological tissues and soil and this is a major concern for global health because of their lethal nature.
Nitrification is a biochemical process of oxidation of ammonia (NH4+) to nitrite (NO2-), then finally to nitrate (NO3-) by nitrifying bacteria. Nitrification is catalysed by two types of reactions. The first type of reaction is the oxidation of ammonia to nitrite by ammonium oxidizing bacteria (AOB). The second type of reaction involves the oxidation of nitrite to nitrate by nitrite-oxidizing bacteria (NOB) [20]. Ammonium-oxidizing microorganisms are organism that carries out the first step in nitrification reaction (biochemical process of oxidation of ammonia (NH4+). They include ammonia oxidizing bacteria (AOB) (Nitrosomonas, Nitrosococcus, Nitrosospira, Nitrosolobus, Nitrosovibrio), ammonia-oxidizing archaea (AOA), and heterotrophic bacteria (Arthrobacter globiformis, Aerobacter aerogenes, Thiosphaera pantotropha, Streptomyces grisens, and various Pseudomonas spp) and fungi (Aspergillus flavus) [8]. Recent research on the metabolic pathways of heterotrophic ammonia oxidation has been conducted using Paracoccus denitrificans [21], Alcaligenes faecalis, Pseudomonas putida [22], and a few other bacterial species. Some studies have suggested that the biochemical mechanisms of heterotrophic nitrification differ among strains [23]. Nitrospira in the Nitrogen Oxidizing Bacteria group have been reported as complete ammonia oxidizing bacteria (comammox) that perform the complete nitrification of ammonia to nitrate [24,25] Ammonia oxidizing microbes (AOM) obtain their energy by oxidation of ammonia (NH3) to nitrite (NO2-). These organisms utilize a few key enzymes such as ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO) to bring about the conversion. The presence of ammonia mono-oxygenase subunit-A gene (amoA) encodes ammonia mono-oxygenase (AMO), a key enzyme that catalyses the first step in ammonia oxidation. AOB was first reported in 1890 by Winogradsky and several groups began isolating and cultivating AOB from a variety of environments such as marine waters, estuarine soils and waste water treatment systems [26].
Nitrogen is an essential element for plants [27]. Nitrifying bacteria play important role in soil fertility, make available nitrate nitrogen to plants (common soil nutrient element required in large quantity by plants), aid in waste treatment plant,biogeochemical cycling of nitrogen compounds and purification of the air. Nitrifying bacteria in polluted soil initiate a syntrophic pathway that provides intermediates for heterotrophic bacterial activity and thus are excellent candidates for remediation [28], with bacteria and fungi being the most important organisms for reclamation, immobilization or detoxification of metallic and radionuclide pollutants. Some bio minerals or metallic elements deposited by microbes have catalytic and other properties in nanoparticle, crystalline or colloidal forms [29].
Microorganisms are very sensitive; they react quickly to any kind of changes (natural and anthropogenic) in the environment, and quickly adapt themselves to new conditions. Microorganisms take heavy metals into the cell in significant amounts. This phenomenon leads to the intracellular accumulation of metal cations of the environment and is defined as bioaccumulation [30]. Some bacterial plasmids contain specific genes for resistance to toxic heavy metal ions [4,31-33] and ability to solubilize phosphate (biofertilizers) [3,34]. Some microorganisms can adjust their metabolic activity or community structure to adapt to the harmful shock loadings. Microorganisms play important role in stress environment and the derived ecosystem functions [27,35].
Microorganisms can mobilize or immobilize metals by biosorption, sequestration, production of chelating agents, chemoorganotrophic and autotrophic leaching, methylation and redox transformations. These mechanisms stem from prior exposure of microorganisms to metals which enable them to develop the resistance and tolerance useful for biological treatment [36]. Microbe-metal interaction in soil/waste disposal is of interest to environmentalists in order to use adapted microorganisms as a source of biomass for bioremediation of heavy metals [4,35,37]. Metals detoxification through resistance and tolerance, this resistance can be attributed to mechanisms of exclusion or tolerance [38]. In an endeavour to safeguard the susceptible cellular components, a cell is capable of building up resistance to metals. Bruins et al. [39] hypothesized five mechanisms for resistance to metal toxicity. These are: (1) active or dynamic transport, (2) development of a permeability barrier, (3) enzymatic detoxification, (4) reduction in sensitivity and (5) sequestration. Microbes are capable of using one or more of these methods to eliminate nonessential metals and normalize essential metals concentrations in their cells. AOB can adapt to energy stress conditions, including low nitrogen levels and low pH [40]. Ammonia oxidizers groups are often considered as models for unravelling the significance of microbial diversity on the responses of soil processes to environmental stress [41,42].
Materials and Methods
Sample collection
Surface soil samples at depth of 0-15 cm were collected at random from Akwa Ibom State University in Akwa Ibom State, and soil sample from University of Nigeria, Nsukka. And from solid waste disposal site in Uyo, Akwa Ibom State. The soil was collected using sterile auger borer and into sterile polyethylene bag, merged to form a composite soil sample and transferred to the laboratory for analysis.
Microbiological Analysis
Preparation of samples for analyses: Precisely, 5 g of the sieved soil sample was suspended in 45 ml of sterile phosphate buffer containing 139 mg of K2HPO4 and 27 mg KH2PO4 per litre (pH 7.0) and shake at 100 rpm for 2 hrs in order to liberate the organisms into the liquid medium [28,43].
Preparation of media: Media preparation was carried out using Winogradsky broth medium for serial dilution of soil samples and Winogradsky solid medium for the inoculation of serially diluted soil suspension.
Preparation of Winogradsky broth: Winogradsky broth medium phase 1 (used for the isolation of nitrifying bacteria responsible for oxidizing ammonium to nitrite) was prepared with the following composition (g/l) in sterile distilled water: (NH4)2SO4, 2.0 ; K2HPO4, 1 ; MgSO4. .7H2O, 0.5; NaCl, 2.0 ; FeSO4 .7H2O, 0.4 ; CaCO3, 0.01. Each of ten test tubes filled with 9 ml of the Winogradsky broth media 1, autoclaved at 121ºC at 15 psi for 15 minutes and allowed to cool. The test tubes was used to carry out ten-fold serial dilutions of the soil suspension [28].
Preparation of Winogradsky agar media:Winogradsky agar media for nitrification phases I was prepared by adding 15.0 g agar to 1000 ml of fresh broth and sterilized at 121ºC at 15 psi for 15 minutes and allowed to cool to about 45ºC before dispersing into sterile Petri dishes [28].
Isolation of nitrifying bacteria from soil sample
All the plates were aseptically inoculated with 0.1 ml of the appropriate dilution of the soil suspension using spread plate technique. All the inoculated Petri dishes were incubated aerobically at room temperature (28 + 2ºC) for 1week and examined for growth.
Purification of isolates
Discrete colonies that developed on Winogradsky agar media for nitrification phases 1 after 1week of incubation was aseptically sub-cultured repeatedly on corresponding freshly prepared Winogradsky agar medium. All the inoculated Petri dishes were incubated aerobically at room temperature (28 ±2ºC) for 3-5 days. The pure isolates was transferred to Winogradsky agar slants and stored in the refrigerator for further use.
Physiological characterization of the isolate
Nitrite determination by Griess method: Griess Ilosvay reagent was be prepared as follows: Solution A: 0.6 g of sulphanilic acid was dissolved in 70 ml of hot distilled water, cooled, and 20 ml of concentrated HC1 was added and volume was made up to 100 ml with distilled water [47].
Solution B: 0.6 g of a-naphthylamine was dissolved in 10 ml of distilled water containing 1 ml of concentrated HC1 and the volume was made up to 100ml with distilled water. Solution C: 16.4 g of sodium acetate was dissolved in 70 ml of distilled water and the volume made up to 100 ml with distilled water. The three solutions (A, B and C) was stored in dark bottles and mixed in equal parts before use.
Ammonium oxidation test for determination of nitrite: Five millilitres of Winogradsky mineral basal medium was prepared. The tubes was sterilized by autoclaving at 121ºC at 15 psi for 15 minutes and allowed to cool. One loopful of each ammonium oxidizing bacteria isolate was added into each tube and incubated aerobically for 5 days at room temperature. At the end of the incubation period, the presence of nitrite was tested using Griess Ilosvay reagent. The reagent was added and observed for the development of purplish red/pink colouration within 5 minutes [48,49].
Inoculum preparation and standardization: Inocula were prepared by inoculating isolates onto prepared nutrient agar plates and incubating at 30ºC for 24 h. After incubation, colonies were suspended in test tubes containing sterile normal saline solution. The tubes were vortex for 2 min, and then transferred into a sterile test tube. The cells suspension was adjusted to a 0.5 McFarland standard (Optical density of 0.5 -1.0 at 600nm) using sterile normal saline to give a concentration of 108 cfu/ml, to get the final inocula.
Tolerance study
Mineral salts medium of the following composition (g/l): (NH4)2SO4, 1.0 ; KH2PO4, 1.0g; K2HPO4, 1.0g; MgSO4, 0.2 ; CaCl2,0.02 ; FeCl3.6H2O; 0.004 for ammonium oxidizing bacteria. Sterilized by autoclaving at 121ºC at 15 psi for 15 minutes and allowed to cool.
Salts of Cupper (Cu), Nickel (Ni), Cadmium (Cd), and Lead (Pb) was used as CuSO4.5H2O, NiSO4.6H2O, CdCl2.H2O and Pb(CH2COO)2)2. (OH)2, respectively.
Primary Screening of heavy metal resistant nitrifying bacteria
An amount (0.1 ml) of the standard inoculum was plated into mineral salts agar medium supplement with metal salt concentrations of 100 mg/L. The plates were incubated at room temperature for 3 - 5 days [50,51]. Ammonia oxidizing bacterial isolates was selected for tolerance study.
Experimental Set up
Analytical grades of metal salts was used to prepare stock solutions. The mineral salt medium for ammonia oxidizing bacteria was amended with the appropriate aliquot of the stock solution of the metal salt concentrations of 100 mg/L, 200 mg/L, 500 mg/L and 1000 mg/L.
Effect of heavy metal on the growth of nitrifying isolates
Changes in population of the nitrifying isolates were monitored following their exposure to heavy metals. About 1 ml of the standard inoculum was introduced into each flask containing heavy metal salts amended in mineral salt medium (MSM). The cultures were incubated aerobically at room temperature (28 ±2ºC) for 7 days. The growth was measured by withdrawing samples from the medium every 24 hours and absorbance of the turbidity measured at 600 nanometre using spectrophotometer [47].
Table 1:Morphological and Biochemical Characterization of Ammonia Oxidizing Bacterial Isolates.
| Suspected organism | Colony | Cell shape | Gram stain | Spore stain | Cat | H2S | Ind | Cit | MR | Vp | Ur | Mot | Oxid | Nit red |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Colourless mucoid raise, | Rod | - | - | + | - | - | + | - | - | + | + | + | + |
| A2 | White mucoid, raise | Rod | - | - | + | - | - | + | - | + | - | + | + | + |
| A3 | White Mucoid raise, | Rod | - | - | + | - | - | + | - | - | - | + | + | + |
| A4 | Raise, brownish | Rod | - | - | + | + | - | + | - | + | - | - | - | + |
| A5 | Colourless mucoid raise | Rod | - | - | + | + | + | - | + | - | + | + | + | + |
| + present (positive) ̶ absent (negative), Cat-Catalase, Ind-Indole, Cit-Citrate, MR-Methyl red, Vp-Voges proskauer, Ur-Urease, Mot-Motility, Oxid-Oxidase, Nit red-Nitrate reduction. | ||||||||||||||
Statistical analysis
Result reported as mean ± standard deviation. All data were subjected to statistical analysis by analysis of variance (ANOVA). The means were separated with least significant difference. The result consider significant at P < 0.05. Least significant difference test (LSD) was also being performed between each treatment and the control. Correlation (association) and regression (changes) analysis was done using statistical product and service solution (SPSS) for windows version 20.
Results
Morphological and biochemical characterization of ammonia oxidizing bacterial isolates
Table 1 shows the morphological and biological characteristic of ammonia oxidizing isolate, the five potential heavy metal tolerance Ammonia oxidizing bacteria were characterized based on their cultural, morphological and biochemical characteristic. Isolate were identified as Gram negative; Catalase positive; Indole negative; Methylene red negative; Voges proskauer negative; Urease negative and Oxidase negative. The isolates were compared with Standard description of Bergey’s Manual of determinative bacteriology. A1, A2, A3, A4 and A5 represent different Ammonia oxidizing bacteria.
Tolerance of ammonia-oxidizing bacteria to heavy metal salts
All the bacterial isolates showed high tendency to decrease in optical density with increasing metal concentration in the medium. Heavy metal salts had effects on nitrifying bacteria growth. Tolerance for the metal ions was dependent on metal salt concentration, time and the isolate tested. Toxicity of the heavy metal salts was in the order of Cd > Cu > Ni > Pb. There was a significant (p < 0.05) difference in the nitrifying bacterial response rates to heavy metal salts.
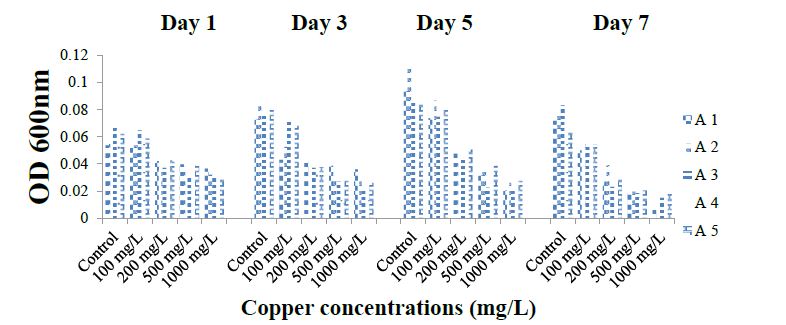
The results represented in Figure 1 shows the rate of tolerance of different copper salt concentrations by the ammonia-oxidizing bacteria (A1 to A5) at different days. As recorded at OD 600nm, the growth of A1 ranged from 0.01 ± 0.002 to 0.07 ± 0.002. Growth was highest in the absence of metal salts (0 mg/l) at 120 hours. However, increasing copper concentration upto 1000 mg/l caused a decline in growth by more than 90% at 168 hours of cultivation. Isolates A2 exhibited related trends in copper tolerance as their growth was not adversely affected even at 200 mg/l but as concentration was increased to 500 mg/l beyond, bacterial growth significantly reduced (p ≤ 0.05). For A3 to A5 accordingly, growth range values (± 0.002 standard deviation) were; 0.02 to 0.09, 0.02 to 0.10 and 0.02 to 0.08. Thus, for all isolates, the least and peak values for growth were recorded at 1000 mg/l and 0 mg/l respectively between 120 to 168 hours of cultivation and the tolerance of the isolates to copper was in the order; A1 < A4 < A3 < A10 < A2. All the isolates showed the high sensitivity to high concentration (500 and 1000 mg/l) of copper (Figure 1).
Tolerance of ammonia-oxidizing bacteria isolates to nickel
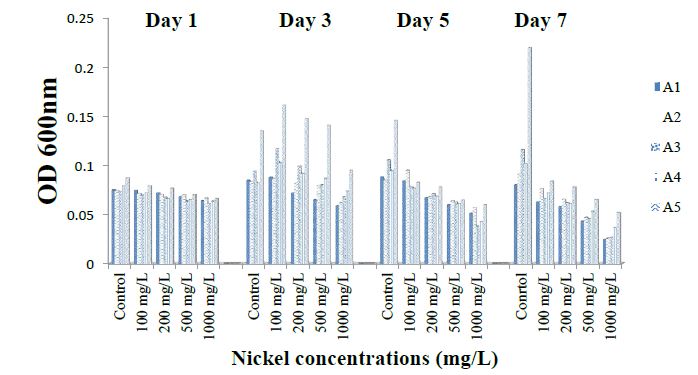
The result represented in figure 2 shows the effect of different nickel concentrations on ammonia oxidizing bacteria A1 to A5 at different days. Isolate A1 growth ranged from 0.06 ± 0.001 to 0.09 ± 0.007 within 120 hours and decreased to 0.07 ± 0.0021 after 168 hours at 0 concentrations. Isolate A1 growth ranged from 0.05 ± 0.000 to 0.06 ± 0.008 within 120 hours. Growth was highest in the absence of nickel at 120 hours. Figure 13 shows the tolerance of the A2 isolates for different concentrations of Ni. The growth of A2 range from 0.0655 ± 0.002 to 0.0835 ± 0.002 within 72 hours, increase to 0.095 ± 0.001 within 120hrs and decrease to 0.072 ± 0.013 after 168 hours at 0 concentration. A2growth range from 0.057 ± 0.003 to 0.071 ± 0.01 within 72 hours; increase to 0.064 ± 0.004 within 120hrs and increase to 0.072 ± 0.011 after 168 hours at 100ug/ml concentration. A4 growth range from 0.0305 ± 0.001 to 0.023 ± 0.007 within 72 hours, decrease to 0.0195 ± 0.006 within 120hrs and decrease to 0.014 ± 0.004 after 168 hours at 1000ug/ml concentration. A2 growth within 120 hours was observed to be; 0.095 ± 0.001; 0.064 ± 0.004; 0.0195 ± 0.006 within 120 hours at 0, 100 and 1000 mg/l concentration, respectively.
Figure 1:Tolerance of isolates to the Copper.
Figure 2: Tolerance of isolates to Nickel.
For A3 and A4 isolates accordingly, growth range values were; 0.07 ± 0.002 to 0.10 ± 0.001; 0.07 ± 0.000 to 0.09 ± 0.001 and 0.07 ± 0.002 to 0.10 ± 0.001 within 120 hours concentration. Growth of isolate A5 ranged from 0.06 ± 0.0001 to 0.08 ± 0.001 within 120 hours Growth was highest in the absence of nickel at 120 hours. However, increasing nickel concentration upto 1000 mg/l caused a decline in growth by more than 75 % at 168 hours of cultivation. Thus, for all isolates, the least and peak values for growth were recorded at 1000 and 0 mg/l, respectively, between 120 to 168 hour of cultivation and the tolerance of the isolates to nickel was in the order; A1 > 5> A2 > A4 > A3. All the isolates showed high sensitivity to high concentration (1000 mg/l) of Nickel (Ni). A1, A2 and A5 exhibited the great ability to tolerate the metal salts than A4, and A3 (Figure 2.)
Tolerance of ammonia oxidizing bacteria growth to lead
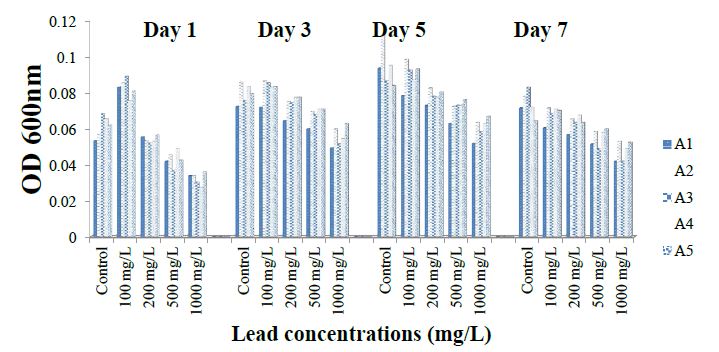
Figure 3 shows tolerance of ammonia oxidizing bacteria isolates to different lead concentrations at different day intervals. Growth was highest in the absence of metal salts at 120 hours. However, increasing lead concentration up to 1000 mg/l caused a decline in growth by more than 68 % at 168 hours of cultivation. Growth of A1 ranged from 0.05 ± 0.001 to 0.09 ± 0.001 within 120 hours. At 1000 mg/l concentration, growth for isolate A1 ranged from 0.034 ± 0.001 to 0.052 ± 0.001 within 120 hours and decreased to 0.004 ± 0.0028 after 168 hours.
Growth of A2 and A5 isolates ranged from 0.06 ± 0.000 to 0.11 ± 0.003 and 0.06 ± 0.000 to 0.08 ± 0.001 within 120 hours, respectively. The least and peak values for growth of ammonia-oxidizing bacterial isolates, the least and peak values for growth were recorded at 1000 mg/l and 0 mg/l respectively between 120 and 168 hours of cultivation and the tolerance of the isolates to lead (Pb) was in the order; A1 < A3 < A4 < A2 < A5 (Figure 3).
Tolerance of ammonia-oxidizing bacteria isolates to cadmium
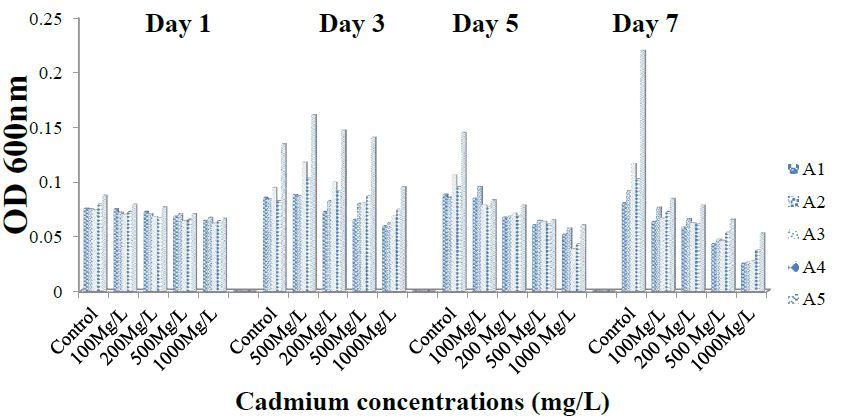
The result represented in Figure 4 shows the level of cadmium effect on the ammonia- oxidizing bacteria isolates at different day intervals. All the isolates showed high sensitivity to all the concentration (100, 200, 500 and 1000 mg/l) of cadmium. Growth was highest in the absence of cadmium (0 mg/l) at 120 hours. However, increasing metal salt concentration to 1000 mg/l caused a decline in growth by more than 97% at 168 hours of cultivation. The growth of A1 isolate ranged from 0.08 ± 0.006 to 0.09 ± 0.007 within 120 hours at 0 mg/l concentration. At 100 and 1000 mg/l concentration of cadmium, A1 growth ranged from 0.08 ± 0.010 to 0.08 ± 0.001 and 0.06 ± 0.006 to 0.05 ± 0.001, respectively. Isolate A2 growth range from 0.08 ± 0.008 to 0.08 ± 0.001 within 72 hours, increased to 0.09 ± 0.001 after 168 hours at 0 concentrations. At 100 mg/l concentration A2 growth ranged from 0.07 ± 0.015 to 0.09 ± 0.008 within 72 hours, increased to 0.10 ± 0.001 within 120 hrs and decreased to 0.08 ± 0.001 after 168 hours at 100 mg/l concentration. The growth of A2 ranged from 0.08 ± 0.001 to 0.06 ± 0.002 within 72 hours and reduced to 0.03 ± 0.002 after 168 hours.
For isolates A3 and A4 accordingly, growth ranged values were; 0.08 ± 0.003 to 0.12 ± 0.001; 0.08 ± 0.008 to 0.09 ± 0.002; 0.08 ± 0.005 to 0.12 ± 0.003; 0.07 ± 0.014 to 0.11 ± 0.003 and 0.08 ± 0.005 to 0.10 ± 0.000. Growth of A5 ranged from 0.09 ± 0.002 to 0.13 ± 0.002 within 72 hours, increased to 0.15 ± 0.001 within 120 hours and increased to 0.22 ± 0.02 after 168 hours at 0 concentrations. Isolate A5 growth ranged from 0.08 ± 0.001 to 0.16 ± 0.004 within 72 hours, decreased to 0.08 ± 0.001 within 120 hours and increased to 0.08 ± 0.006 after 168 hours at 100 mg/l concentration. A5 growth ranged from 0.08 ± 0.005 to 0.10 ± 0.020 within 72 hours; decreased to 0.05 ± 0.000 after 168 hours at 1000 mg/l concentration. Tolerance of the ammonia-oxidizing bacterial isolates to cadmium was in the order; A3 < A4 < A1 < A2 < A5 (Figure 4).
Biosorption of copper, nickel, lead and cadmium by different nitrifying bacteria
Biosorption of copper, nickel, lead and cadmium by different nitrifying bacteria Achromobacter xylosoxidans and Achromobacter insolitus. The results of assessment of the two isolates represented as AOB 2 and AOB 5 for biosorption of selected heavy metals via shake flask method at different day intervals for 28 days under controlled environmental conditions.
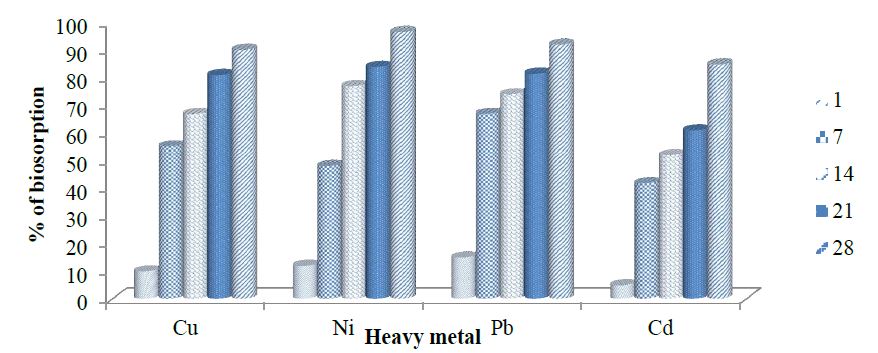
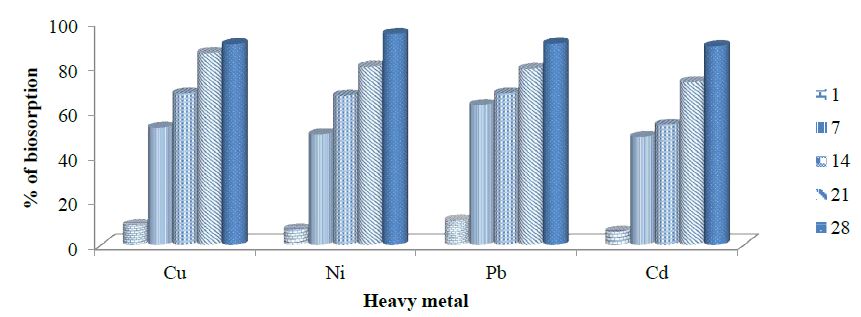
Biosorption of copper by ammonia-oxidizing bacteria (AOB 2 and AOB 5). Isolate Achromobacter xylosoxidans (AOB 2) biosorption capacity of copper range value was; 10 to 90.1. AOB 2 biosorption of nickel were; 12 % on day 1; 48% on day 7; 77% on day 14; 84% on day 21 and 96.51% on 28 days. AOB 2 mitigation of lead range from 15 to 67, 74 to 81.5 and 92 % at 1, 7, 14, 21 and 28 days, respectively. Isolate AOB 2 biosorption of cadmium was 5 % on day 1; 41.796% on day 7; 52% on day 14; 61% on day 21 and 84.82% on 28 days as presented in figure 5. Achromobacter insolitus (AOB 5) mitigation of copper ranged from 9 to 52.67, 68 to 86 and 90.04% at 1 , 7, 14, 21 and 28 days, respectively. AOB 5 biosorption of nickel was 7% on day 1; 49.64 % on day 7; 67% on day 14; 80% on day 21 and 94.67% on 28 days. Isolate AOB 5 mitigation of lead ranged from 11 to 62.75, 68 to 79 and 90.25% at 1, 7, 14, 21 and 28 days, respectively. AOB 5 biosorption of cadmium was 6 % on day 1; 48.56% on day 7; 54% on day 14; 73% on day 21 and 89.21% on 28 days as presented in figure 6.
Figure 3: Tolerance of isolates to the lead.
Figure 4: Tolerance of isolates to the cadmium.
Discussion
Effects of copper, nickel, lead and cadmium on the growth of nitrifying bacteria were evaluated in a different medium formulation; all the bacterial isolates demonstrated ability to grow in mineral salt agar medium incorporated with different heavy metal salt at different concentrations (100 , 200, 500 and 1000 mg/l).
Growth of ammonia-oxidizing and nitrite-oxidizing bacteria increased successively throughout the period of five days of exposure to various concentrations of the heavy metal salts but decreased at day 7. Increasing heavy metal concentration up to 1000 mg/l decreased the bacterial growth significantly (p ≤ 0.05). However, maximum growth was recorded for all the isolates, in the absence of heavy metal salts. In addition, there was significant (p < 0.05) difference in the nitrifying bacteria response rates to heavy metal salts (copper (Cu), nickel (Ni), lead (Pb) and cadmium (Cd) at four loading rates (100, 200, 500, 1000 mg/l).
Tolerance of ammonia-oxidizing bacterial isolates to copper, nickel, lead and cadmium was in the order; A1 < A4 < A3 < A5 < A2; A1 > A5> A2 > A4 > A3; A1 < A3 < A4 < A2< A5 and A3 < A4 < A1 < A2 < A5 respectively.
Among the ammonia-oxidizing bacterial, isolates A2 and A5 exhibited the greater ability to tolerate the metal salts than A3, A4, and A1. Results obtained showed that the addition of heavy metal pollutants in the environment had different impacts on eco-physiological microorganisms. Adding Cu, Ni, Pb and Cd in low concentration had stimulatory or slightly inhibitory effect, while application of high concentrations of heavy metals had a strong inhibitory effect. Most nitrifying bacteria were susceptible to heavy metals; some bacteria were resistant to the pollution, maintaining themselves even in the presence of elevated concentrations of metals (Cu, Ni, Pb and Cd). Nitrifying bacteria were more sensitive to Cd than for Pb, Cu, and Ni. Harmful effect of cadmium was very obvious in all eco-physiological.
Toxicity of heavy metals not only depends upon the concentration, but also the chemical structure, time of exposure, and the source of the metal contamination [5,52]. The study agrees with the work of Mertoglu et al. [53] which reported population shifts in an enriched nitrifying system under gradually increased cadmium loading. Frey et al., Lee et al., and Vasileiadis et al. also reported a sensitive response of the AOB community to heavy metals. Heavy metals can affect diversity of certain microbial communities and related soil processes [54-57].
Conspicuous responses of different AOB communities to metal pollution stress have been observed in agricultural soils amended with metals and industrial effluents [26,51,58]. Okpokwasili and Odokuma, demonstrated that nitrifying bacteria (Nitrobacter) predominant in waste environments were sensitive to various toxicants [59]. In another study, John and Okpokwasili, studied crude oil degradation and plasmid profile of nitrifying bacteria isolated from oil impacted mangrove sediment in the Niger Delta and found that nitrifying bacteria were able to carry out degradation of crude oil [28].
Alternatively, Frey et al. reported that soil metal contamination does not decrease the abundance of AOB while the research of Stefanowicz et al. and Qu et al. demonstrated that bacterial functional diversity significantly decreased with increasing soil pollution [54,60,61]. Bermudez et al. hypothesized that high organic matter contents of soil can bind metals and decrease their toxicity [62]. Yeung et al. studied the adaptation of nitrifying microbial biomass to nickel in batch incubations [63]. Wu et al. [64] investigated he long-term effect of field fertilization on the community structure of ammonia-oxidizing bacteria and reported no significant effect of metals on AOB abundance, this suggests that metal concentration was not the main factor affecting the abundance of ammonia-oxidizing bacteria. Moreover, Kris et al. had demonstrated that the nitrifying community display tolerance to long-term Zn stress. Some microorganisms are able to produce inducible proteins that confer tolerance to metal pollutants [65,66].
Figure 5:Biosorption of copper, nickel, lead and cadmium by ammonia oxidizing bacteria (AOB 2).
Figure 6:Biosorption of copper, nickel, lead and cadmium by ammonia oxidizing bacteria AOB 5.
Several other studies have particularly emphasized the response of nitrifying bacteria to heavy metals. Some of these studies reported a sensitive response of AOB and NOB community to Heavy metals [54-56]. Additionally, Mertens et al., Liu et al., Lee et al., and Vasileiadis et al., particularly emphasized the response of ammonia oxidizing bacteria to heavy metals such as Zn, Cu and Hg [55,56,67,68]. Heavy metals used in greater amounts result in metabolic disorders and suppress the growth of most microorganisms. Roane et al., [69] inferred that heavy metals influence the microbial population by affecting their growth, morphology, biochemical activities, ultimately resulting in decreased biomass and diversity. According to Khan et al., Siokwu and Anyanwu, Subrahmanyam et al., Vashishth et al., and Wang et al., tolerance of nitrifying bacteria to heavy metal salts is dependent on concentration, time and the isolate tested [66,70-73].
Nitrifying bacteria; Achromobacter xylosoxidans and Achromobacter insolitus, were able to carry out biosorption of copper, nickel, lead and cadmium as seen in this study. Achromobacter insolitus had the highest biosorption capacity for copper and cadmium at 90.04% and 89.21%, respectively within a period of 28 days. Achromobacter xylosoxidans had the highest biosorption capacity for nickel and lead at 96.51% and 92%, respectively within a period of 28 days.
Statistical analysis ascertains that there is a significant (p<0.05) difference in biosorption rates between medium with the bacteria isolates and control. Biosorption of heavy by nitrifying bacteria gave a positive result. Biosorption of heavy metals by different nitrifying bacteria is dependent on the characteristics of the pollutant (heavy metal). Several researchers have also reported the excellent bioremediation potentials of various soil microorganisms [74-84].
Conclusion
All the Ammonia Oxidizing bacteria were able to adapt and grow under various extreme conditions show a high level of tolerance for heavy metal tested. A2 and A5 exhibited the greatest ability to tolerate the metal salts than the others which makes the organism an attractive potential candidates for further investigations regarding their ability to remove heavy metal in bioremediation. It may be a good option for bioremediation of soil and waste since it is regarded as an eco-friendly and efficient. The understanding of microbial tolerance and adaptation to the presence of metal in the environment is critical in determining the management and potential long-term effect of that part of the environment receiving metal contamination.
The nitrifying bacteria studied showed efficient oxidizing ability for ammonium and nitrite. Nitrification in the presence of heavy metal salt depended on the heavy metal concentration, time and the isolates tested. Achromobacter xylosoxidans and Achromobacter insolitus demonstrated nitrification ability even at high concentrations of heavy metal salts. Thus, they may be used to remove high strength ammonium from digested sludge. The organisms were able to carry out biosorption of copper, nickel, lead and cadmium. The understanding of heavy metal toxicity to microorganisms in the environment is crucial in determining its long-term effects and possibility of remediation from the contaminated environments by the adapted microbial strains.
Acknowledgement
We thank the University of Nigeria Nsukka for giving us the opportunity to carry out the research and we also appreciate Rev. Fr. Prof. Vincent Nyoyoko, for funding the research.
Hadia-e-Fatima, Ahmed A (2018) Heavy metal pollution – A mini review. J Bacteriol Mycol Open Access 6:179-181. [ Ref ]
Nibourg GAA, Hoekstra R, Van der Hoever TV, Ackermans MT, Hakvoort TBM, et al. (2013) Effects of acute-liverfailure-plasma exposure, on hepatic functionality of Hepa RG-AMC-Bioartificial Liver. Liver International 33: 516-524. [ Ref ]
Gupta DK, Chatterjee S, Datta S, Veer V, Walther C (2014) Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 108: 134-144. [ Ref ]
Sharma J (2016) Removal of heavy metals by indigenous microorganisms and identification of gene responsible for remediation. International Journal of Nano Studies & Technology S3: 1-8. [ Ref ]
Ayangbenro A, Babalola O (2017) A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. International Journal of Environmental Research and Public Health 14: 94. [ Ref ]
Liu S, Niu GZ, Liu Y, et al. (2018) Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Biores Technol 224: 25-33. [ Ref ]
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. International Journal of Environment and Pollution 121: 129-136. [ Ref ]
Hamsa N, Yogesh GS, Koushik U, Patil L (2017) Nitrogen transformation in soil: effect of heavy metals. Int J Curr Microbiol Appl Sci 6: 816-832. [ Ref ]
Cobbina SJ, Chen Y, Zhou Z, Wu X, Zhao T, et al. (2015) Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J Hazard Mater 294: 109-120. [ Ref ]
Malik Z, Ahmad M, Abassi GH, Dawood M, Hussain A, et al. (2017) Agrochemicals and soil microbes: interaction for soil health,” in Xenobiotics in the Soil Environment: Monitoring, Toxicity and Management. [ Ref ]
Srivastava V, Sarkar A, Singh S, Singh P, de Araujo ASF, et al. (2017) Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front Environ Sci 5: 64. [ Ref ]
Ramya DA, Joseph Thatheyus AJ (2018) Microscopic Investigations on the Biosorption of Heavy Metals by Bacterial Cells: A Review. Science International 6: 11-17. [ Ref ]
Mishra J, Singh R, Arora NK (2017) Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Journal of Frontiers microbiology. [ Ref ]
Gupta A, Joia J, Sood A, Sood R, Sidhu C, at al. (2016) Microbes as potential tool for remediation of heavy metals: A review. J Microb Biochem Technol 8: 364-372. [ Ref ]
Anyanwu CU, Nwankwo SC, Moneke AN (2011) Soil Bacterial Response to Introduced Metal Stress. International Journal of Basic & Applied Sciences 11: 73-76. [ Ref ]
Venkatachalam P, Jayalakshmi N, Geetha N, Sahi SV, Sharma NC, et al. (2017) Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plant Acalypha indica L. under lead stress. Chemosphere 171: 544-553. [ Ref ]
Liu Q, Liu Y, Zhang M (2012) Mercury and cadmium contamination in traffic soil of Beijing, China. Bull Environ Contam Tox 88: 154-157. [ Ref ]
Sagi Y, Yigit SA (2012) Heavy metals in Yenicacga Lake and its potential sources: soil, water, sediment and plankton. Environmental Monitoring and Assessment 184: 1379-1389. [ Ref ]
Wyszkowska J, Borowik A, Kucharski M, Kucharski J (2013) Effect of Cadmium, Copper and Zinc on Plants, Soil Microorganisms and Soil Enzymes. Journal of Elementary School 4: 769-796. [ Ref ]
Capone DG (2018) Discovery of New Nitrite-Oxidizing Bacteria Increases Phylogenetic and Metabolic Diversity within This Niche. mBio 9. [ Ref ]
Moir JWB, Wehrfritz JM, Spiro S, Richardson DJ (1996b) The biochemical characterization of a novel non-harne iron hydroxylamine oxidase from Paracoccus denitrificans GB17. Biochem J 319: 823-827. [ Ref ]
Daum M, Zimmer W, Papen H, Kloos K, Nawrath K, et al. (1998) Physiological and molecular biological characteriza-tion of ammonia oxidation of the heterotrophic nitrifier Pseudomonas putida. Current Microbiology 37: 281-288. [ Ref ]
Niel EWJ, Arts PAM, Wesselink BJ, Robertson LA, Kuenen JG (1993) Competition between heterotrophic and autotrophic nitrifiers for ammonia in chemostat cultures. FEMS Microbiology Letters 102: 109-118. [ Ref ]
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, et al. (2015) Complete nitrification by Nitrospira bacteria. Nature 528: 504-509. [ Ref ]
Hanna Koch, Maartje AHJ, van Kessel, Sebastian Lücker (2018) Complete nitrification: insights into the ecophysiology of comammox Nitrospira. Applied micobiology and biotechnology. [ Ref ]
Reddy AD, Subrahmanyam G, NaveenKumar S, Karunasagar I, Karunasagar I (2015) Isolation of Ammonia Oxidizing Bacteria (AOB) from Fish Processing Effluents. National Academy Science Letters 38: 393-397. [ Ref ]
Vimal SR, Singh JS, Arora NK, Singh S (2017) Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 27: 177-192. [ Ref ]
John RC, Okpokwasili GC (2012) Crude Oil-Degradation and Plasmid Profile of Nitrifying Bacteria Isolated from Oil-Impacted Mangrove Sediment in the Niger Delta of Nigeria. Bull Environ Contam Toxicology 88: 1020-1026. [ Ref ]
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156: 609-643. [ Ref ]
Wolejko E, Wydro U, Loboda T (2016) The Ways to Increase Efficiency of Soil Bioremediation. Ecol Chem Eng S 23: 155-174. [ Ref ]
Liu J, Cao W, Jiang H, Jing Cui J, Shi C, et al. (2018) Impact of Heavy Metal Pollution on Ammonia Oxidizers in Soils in the Vicinity of a Tailings Dam, Baotou, China. Bulletin of Environmental Contamination and Toxicology 101: 110-116. [ Ref ]
Pacwa-Płociniczak M, Płociniczak T, Yu D (2018) Effect of silene vulgaris and heavy metal pollution on soil microbial Diversity in long-term contaminated soil. Water Air Soil Pollut 229: 13. [ Ref ]
Lukina AO, Boutin C, Rowlan O (2016) Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere 162: 355-364. [ Ref ]
Ibiene AA, Okpokwasili GSC (2011) Comparative toxicities of three agro-insecticide formulations on nitrifying bacteria. Report and Opinion 3: 14-17. [ Ref ]
Singh JS, Abhilash PC, Gupta VK (2016a) Agriculturally important microbes in sustainable food production. Trend Biotechnol 34: 773-775. [ Ref ]
Viti C, Giovannetti L (2003) The Impact of Chromium Contamination on Soil Heterotrophic and Photosynthetic Microorganisms. Annals of Microbiology 51: 201-213. [ Ref ]
Singh JS, Kaushal S, Kumar A, Vimal SR, Gupta VK (2016b) Book review: Microbial Inoculants in Sustainable Agricultural Productivity- Vol. II: Functional Application. Front Microbiol 7: 2015. [ Ref ]
Klassen SP, Malean JE, Grossl PR, Sims RC (2000) Heavy metals in the environment. J Environ Qual 29: 1826-1834. [ Ref ]
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicology and Environmental Safety 45: 198-207. [ Ref ]
Valentine DL (2007) Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol 5: 316-323. [ Ref ]
Philippot L, Hallin S (2005) Finding the missing link between diversity and activity using denitrifying bacteria as a model functional community. Current opinion in microbiology 8: 234-239. [ Ref ]
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, et al. (2009) Initial community evenness favours functionality under selective stress. Nature 458: 623-626. [ Ref ]
Deni J, Penninck MJ (1999) Nitrification and autotrophic nitrifying bacteria in a hydrocarbon polluted soil. Appl Environ Microbiol 65: 4008-4020. [ Ref ]
Holt JG, Krieg NR, Sneath PHA (1994) Bergey’s Manual of determinative bacteriology. Williams & Wilkins, USA. [ Ref ]
Cheesbrough M (2006) District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press, United Kingdom. [ Ref ]
Saha M, Sarkar A, Bandhophadhyay B (2013) Development of Molecular Identification of Nitrifying Bacteria in Water Bodies of East Kolkata Wetland, West Bengal. Journal of Bioremediation and Degradation 5: 1-5. [ Ref ]
Bhaskar KV, Charyulu PBBN (2005) Effect of environmental factors on nitrifying bacteria isolated from the rhizosphere of Setaria italica (L.) Beauv. African Journal of Biotechnology 4: 1145-1146. [ Ref ]
Joel OF, Amajuoyi CA (2010) Determination of the Concentration of Ammonia that could have Lethal Effect on Fish Pond. Journal of Engineering And Applied Sciences. [ Ref ]
Hoang PH, Nguyen Hong T, Tran Trung T, Tran TT, at al. (2016) Isolation and selection of nitrifying bacteria with high biofilm formation for treatment of ammonium polluted aquaculture water. Journal of Vietnamese Environment. 8: 33-40. [ Ref ]
Jamaluddin H, Zaki DM, Ibrahim Z, Tan S, Zainudin N, et al. (2012) Isolation of metal tolerant bacteria from polluted wastewater. Paper presented at the Pertanika Journal of Tropical Agricultural Science 35: 647-662. [ Ref ]
Pandit R, Patel B, Kunjadia P, Nagee A (2013) Isolation, characterization and molecular identification of heavy metal resistant bacteria from industrial effluents. Amala-khadiAnkleshwar, Gujarat. Inter J Environ Sci 3: 1689-1699. [ Ref ]
Johnson H, Cho H, Choudhary M (2019) Bacterial Heavy Metal Resistance Genes and Bioremediation Potential. Computational Molecular Bioscience 9: 1-12. [ Ref ]
Mertoglu B, Semerci N, Guler N, Calli B, Cecen F, et al. (2008) Monitoring of population shifts in an enriched nitrifying system under gradually increased cadmium loading. Journal of Hazardous Materials 160: 495-501. [ Ref ]
Frey B, Pesaro M, Rudt A, Widmer F (2008) Resilience of the rhizosphere pseudomonas and ammonia-oxidizing bacterial populations during phytoextraction of heavy metal polluted soil with poplar. Environ. Microbiol 10: 1433-1449. [ Ref ]
Lee S, Cho K, Lim J, Kim W, Hwang S (2011) Acclimation and activity of ammoniaoxidizing bacteria with respect to variations in zinc concentration, temperature, and microbial population. Bioresour Technol 102: 4196-4203. [ Ref ]
Vasileiadis S, Coppolecchia D, Puglisi E, Balloi A, Mapelli F, et al. (2012) Response of ammonia oxidizing bacteria and archaea to acute zinc stress and different moisture regimes in soil. Microb Ecol 64: 1028-1037. [ Ref ]
Haferburg G, Kothe E (2007) Microbes and metals: interactions in the environment. J Basic Microb 47: 453-467. [ Ref ]
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT, et al. (1999) Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metalresistant bacteria. Appl Environ Microb 65: 95-101. [ Ref ]
Okpokwasili GO, Odokuma LO (1994) Tolerance of Nitrobacter to Toxicity of some Nigerian Crude oil. Bulletin of Environmental Contamination and Toxicology 52: 388-395. [ Ref ]
Stefanowicz AM, Niklińska M, Laskowski R (2008) Metals affect soil bacterial and fungal functional diversity differently. Environ Toxicol Chem 27: 591-598. [ Ref ]
Qu J, Ren G, Chen B, Fan J, Yong E (2011) Effects of lead and zinc mining contamination on bacterial community diversity and enzyme activities of vicinal cropland. Environ Monit Asses 182: 597-606. [ Ref ]
Bermudez GMA, Moreno M, Invernizzi R, Pla R, Pignata ML (2009) Heavy metal pollution in topsoils near a cement plant: the role of organic matter and distance to the source to predict total and HCl extracted heavy metal concentrations. Chemosphere 78: 375-381. [ Ref ]
Yeung CH, Francis CA, Criddle C (2013) Adaptation of nitrifying microbial biomass to nickel in batch incubations. Journal of Applied Microbiology and Biotechnology 97: 847-857. [ Ref ]
Wu YC, Lu L, Wang BZ, Lin XG, Zhu JG, et al. (2011) Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil. Soil Science Society of America Journal 75: 1431-1439. [ Ref ]
Kris B, Jelle M, Erik S (2005) Toxicity of heavy metals in soil assessed with various soil microbial and plant growth assays: a comparative study. Environ Toxicol Chem 24: 634-640. [ Ref ]
Vashishth A, Khanna S (2015) Toxic Heavy Metals Tolerance in Bacterial Isolates based on their Inducible Mechanism. International Journal of Novel Research in Life Sciences 2: 34-41. [ Ref ]
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, et al. (2009) Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J 3: 916-923. [ Ref ]
Liu YR, Zheng YM, Shen JP, Zhang LM, He JZ (2010) Effects of mercury on the activity and community composition of soil ammonia oxidizers. Environmental Science and Pollution Research 17: 1237-1244. [ Ref ]
Roane TM, Pepper IL (2000) Pepper, “Microbial responses to environmentally toxic cadmium”. Microbial Ecology Journal 38: 358-364. [ Ref ]
Khan S, Hesham EL, Qiao M, Rehman S, He JZ (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut R 17: 288-296. [ Ref ]
Siokwu S, Anyanwu CU (2012) Tolerance for heavy metals by filamentous fungi isolated from a sewage oxidation pond. African Journal of Microbiology Research 6: 2038-2043. [ Ref ]
Subrahmanyam G, Hu HW, Zheng YM, Gattupalli A, He JZ, et al. (2014) Response of ammonia oxidizing microbes to the stresses of arsenic and copper in two acidic alfisols. Applied Soil Ecology 77: 59-67. [ Ref ]
Wang P, Di HJ, Keith C, Cameron KC, Tan Q, et al. (2017) The response of ammoniaoxidizing microorganisms to trace metals and urine in two grassland soils in New Zealand. Environ Sci Pollut Res 24:2476-2483. [ Ref ]
Ashraf R, Ali TA (2007) Effect of heavy metals on soil microbial community and mung beans seed germination. Pakistan Journal of Botany 39: 629. [ Ref ]
Black CA (2000) Method of Soil Analysis II. American Society of Agronomy. Madison. [ Ref ]
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014: 752708. [ Ref ]
Li X, Zhu YG, Cavagnaro TR, Chen M, Sun J, et al. (2009a) Do ammonia-oxidizing archaea respond to soil cu contamination similarly as ammonia-oxidizing bacteria? Plant Soil 324: 209-217. [ Ref ]
Li F, Zheng YM, He JZ (2009b) Microbes influence the fractionation of arsenic in paddy soils with different fertilization regimes. Sci Total Environ 407: 2631-2640. [ Ref ]
Liu Y, Chen M, Yongmei H (2013) Study on the adsorption of Cu (II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J 218: 46-54. [ Ref ]
Moir JWB, Crossman LC, Spiro S, Richardson DJ (1996a) The purification of ammonia monooxygenase from Paracoccusdenitrificans. FEBS Lett 387: 71-74. [ Ref ]
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ. Microbiol 63: 4704-4712. [ Ref ]
Smolders E, Brans K, Coppens F, Merckx R (2001) Potential nitrification rate as a tool for screening toxicity in metal contaminated soils. Environ Toxicol Chem 20: 2469-2474. [ Ref ]
Zimmer S, Messmer M, Haase T, Piepho PH, Mindermann A, et al. (2016) Effects of soybean variety and Bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in Germany. Eur J Agron 72: 38-46. [ Ref ]
Ladomersky E, Petris MJ (2015) Copper tolerance and virulence in bacteria. Metallomics 7: 957-964. [ Ref ]