Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 20 March, 2019
Accepted date: 25 March, 2019
Published date: 01 April, 2019
Citation: Micro-Pathogen Elicitor Hrip1 Protein Isolated from Alternaria tenuissima Induced Disease Resistance against Tomato Yellow Leaf Curl Virus (TYLCV) in Tomato (Solanum lycopersicum)
Copyright: © 2019 Sokea T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In previous research, Hrip1 is a new great protein candidate derived from A. tenuissma, necrotrophic fungus induced plant immunity against pathogens and transgenic plant was response to drought tolerance. To further elucidate a basic molecular function of micro-pathogen protein elicitor was expressed in a broth culture medium. Recombinant protein treatment induced tomato plant cell death to activate a plant defense response mechanism which is an important role for plant protection to alleviate viruliferous infestation in the tomato plant. In case of study is more benefited to the response against pathogen attack using the recombinant protein elicitor Hrip1 was an expression of the protein in a broth culture medium and purified from culture filtrated by a Hiscolumn chromatography containing His-Tag resin. The protein fraction confirmed by SDS-PAGE gel and molecular weight 20kDa. The Hrip1 was agro-infiltrated in tomato leaves induce definitely hypersensitive response (HR) activities that are a response to plant pathogenic defense, including accumulation of reactive oxygen species (ROS) and expression of a defense-related gene. Treatment of Hrip1 elicitor induced a progressive and significant increase of enzymes in site treated tissues. The qualitative analysis of TYLCV gene expression was performed a realtime qualitative polymerase chain reaction (RT-qPCR) showed there reduced in TYLCV concentration after post-inoculation of Agrobacterium tumefaciens strain was compared with Hrip1 treatment and buffer as control. In Hrip1-treated tomato plants reduced the severity of the disease to 60.07% was correlated with bacterial development suppression to 74.60% in duration of TYLCV infection into the tomato plant.
Keywords
Hrip1, Alternaria tenuissima, Elicitor protein, Tomato, TYLCV, RT-Qpcr, Defense-related genes, ROS, Resistance response.
Abstract
In previous research, Hrip1 is a new great protein candidate derived from A. tenuissma, necrotrophic fungus induced plant immunity against pathogens and transgenic plant was response to drought tolerance. To further elucidate a basic molecular function of micro-pathogen protein elicitor was expressed in a broth culture medium. Recombinant protein treatment induced tomato plant cell death to activate a plant defense response mechanism which is an important role for plant protection to alleviate viruliferous infestation in the tomato plant. In case of study is more benefited to the response against pathogen attack using the recombinant protein elicitor Hrip1 was an expression of the protein in a broth culture medium and purified from culture filtrated by a Hiscolumn chromatography containing His-Tag resin. The protein fraction confirmed by SDS-PAGE gel and molecular weight 20kDa. The Hrip1 was agro-infiltrated in tomato leaves induce definitely hypersensitive response (HR) activities that are a response to plant pathogenic defense, including accumulation of reactive oxygen species (ROS) and expression of a defense-related gene. Treatment of Hrip1 elicitor induced a progressive and significant increase of enzymes in site treated tissues. The qualitative analysis of TYLCV gene expression was performed a realtime qualitative polymerase chain reaction (RT-qPCR) showed there reduced in TYLCV concentration after post-inoculation of Agrobacterium tumefaciens strain was compared with Hrip1 treatment and buffer as control. In Hrip1-treated tomato plants reduced the severity of the disease to 60.07% was correlated with bacterial development suppression to 74.60% in duration of TYLCV infection into the tomato plant.
Keywords
Hrip1, Alternaria tenuissima, Elicitor protein, Tomato, TYLCV, RT-Qpcr, Defense-related genes, ROS, Resistance response.
Introduction
Tomato (Solanum lycopersicum) is one of the important numerous plant cultivated in the earth. It is used in the kind of many formulas and the best food standards quality. Currently, tomato plants are surrounding a severe infestation of growth, reproduction, yield and existence due to infection by geminivirus family. One of the geminivirus species inhibiting tomato cultivation in the earth that is caused by a tomato yellow leaf curl virus (TYLCV) and transmitted virus to plants by a vector of the Bemisia tabaci whitefly was affected tomato yield [1,2]. The TYLCV disease is most of the destructive plant viruses on destroying tomato plants globally. It was widespread of many countries in universal including Southern, Central and Northern parts of America, Southern Asia, Mediterranean basin, and Africa [3].
The tomato plant was 10-15 days after infection indicated that the plants were completely infected with the virus disease. Infected plants are stunted or dwarfed since only new growth leaves produced after TYLCV infection is depleted in a magnitude of the leaf. Small tomato leaves are trundled towards upside and towards inside and leaves are often curved down and are taut, denser than normal have a leathery surface, and are crumpled. The tomato young leaves are slightly yellowish [4]. To date, techniques of cultivation and integrated pest management, such as resistant seed, fertilization, irrigation, crop rotation, sanitation, and chemical applications are the only practices that can reduce the severity of disease development in plants [5]. The antiviral action of native whey protein and transformed fractions α-lactalbumin, β-lactoglobulin, and lactoferrin were suppressed the TYLCV on infection of tomato plants [4]. It is, therefore, important to investigate to study new methods in controlling disease in crops. Recently studies on biological control have revealed that protein isolate from micro-pathogens has induced plant immune system to respond plant resistance against plant pathogens and insects in the plant. Hence, the pathogen elicitors induced plant resistance response has become a significant of a new plant disease management strategy for plant protection.
Plant cultivation in a permissive environmental condition is surrounding in a various abiotic and biotic stress condition that is a response to impair plant growth, reproduction, and loss of agricultural production yield. To escape from its stress, plants have evolved a variety of strategic reinforcement to protect themselves to alive [6]. Many plant-associated microbes are pathogen agents that respond to infection using various types of plant innate immune systems. It recognizes and responds to molecules common to numerous classes of microscopic organism, with non-pathogens and other it responds to micro pathogen virulence factors, either directly or via their effects on host targets [7]. They are also referred to as microbe/pathogenassociated molecular patterns (MAMPs/PAMPs), as they are not restricted to pathogenic microbes. This first level of recognition is referred to as PAMP-triggered immunity (PTI) [8]. In working together of pathogens, host-microbe interplays acquired the capability to convey to effector proteins to the plant cell to repress PAMP-triggered immunity, permitting, disease development and pathogen. To answer the transfer to pathogen effector proteins, plants acquired the inspection of proteins (R proteins) to either indirectly or directly display the attendance of the pathogen effector proteins [9]. Commonly, PTI and ETI give rise to similar responses to disease resistance, even though ETI is qualitatively stronger and faster and often involves a form of localized cell death named the hypersensitive response (HR) [10]. Plants generally counter to the attack to plant micro pathogens and unsuitable host bacteria, fungus, and virus by inducing pathogenesis-related (PR) genes and localized cell death (LCD) at the location of disease infection in plant, a development of mutually well-known as the (HR). Reactive oxygen species (ROS) are produced in various subcellular compartments shortly after pathogen recognition and proposed to signal subsequent to the orchestration of the HR [11]. In addition, the involvement of phytohormones, transcription factors, kinase cascades, and ROS can lead to a cross-tolerance and improvement of a plant’s resistance against pathogenic infection [12].
By concerning on agricultural production, many scientists have researched on plant protection derived from micro-pathogens of protein elicitor as fungi, bacteria, and viruses to induced plant defense response to attack the plant micro pathogen agents themselves and promoted plant growth for healthy plant and reinforcement on attacking pathogens and insects. For instance, PevD1 and VdCP1 protein elicitors from Verticillium dahliae of fungal plant pathogen induced defense responses in plants and improve pathogen resistance. PevD1 is a protein from Verticillium dahliae and activated the hypersensitive response (HR) and systemic acquired resistance (SAR) to the TMV, Botrytis cinerea, Pseudomonas syringae pv. tabaci and V. dahliae in tobacco and cotton plant [13-16]. In addition to this, a novel MoHrip1 and MoHrip2 protein elicitor identified from rice blast fungus Magnaporthe oryzae conferred on defense responses in tobacco and rice plant after protein treatment to suppress rice blast disease development M. oryzae by activating defense responses against pathogenic infection and reduced application of chemical pesticides and thus benefit human health and environment [17,18]. The MoHrip1 also encouraged plant growth by regulating the contents of SA and GA directly or indirectly [19].
In this research involved in plant protection, we have elucidated the recombinant Hrip1 protein from a microbial protein elicitor A. tenuissima, necrotrophic fungus induced the locale and systemic defense responses in host plants and conferred on plant disease resistance against micropathogens. Additionally, we revealed that the Hrip1-mediated plant defense-related gene and ROS play a significant role. The present study provides a basis of molecular mechanisms of Hrip1 induced disease resistance in the tomato plant.
Materials and Methods
Condition of plant cultivation
Tomato (Solanum lycopersicum) seed, Gailiangmaofen802F1 (Jiaxin Seed Limited Company) were grown from seeds in a plant growth chamber under controlled conditions at 24-26oC under cool. The seed was rinsed with sterile distilled water and it was sowed tomato seed directly in Petri dishes on filter papers moisten with distilled water. Germination of tomato seeds was transferred into 12cm diameter pots filled with a compost soil mixture (virus free) and transfer to an insect free plant growth chamber. One tomato plant seedling transplanted per pot in a plant growth chamber at white fluorescent lights, 50-100 μEm-2sec-1, with a photoperiod of 16h light and 8h darkness [20]. After for 3-4 week-old tomato seedling grow very well for induced HR activities and for bioassays.
Expression and protein purification
The Hrip1 gene cloning was cultured in 50ml of 2.0% dextrose, 1.0% yeast extract and 2.0% peptone (YPD) liquid medium with shaking at 200rpm for overnight at 30oC and transferring 10% of YPD culture into 1000ml BMGY liquid medium of 100mM KH2PO4, 100mM K2HPO4, pH 6.0, 10ml of glycerol and 13.4g yeast nitrogen base was cultured overnight until its absorbance (OD600) was 0.6. The cell pellets were harvested using a centrifuge at 4000rpm for 5min at room temperature and re-suspended in 100ml of BMMY liquid medium of 100mM KH2PO4, 100mM K2HPO4, pH 6.0 and 1.34g yeast nitrogen base incubate at platform shaker 200rpm at 30°C and continually cultivation for 72h and further inoculated sterile 100% methanol (CH3OH) to a final concentration of 0.5% every 24 [16]. The protein supernatant filtrate with a syringe filter passed through a 0.22μm membrane and 25mm diameter to remove impurities (Millipore, Corp., Billerica, MA, USA) and purification using a His-column chromatography containing His-Tag resin (TransGen Biotech, Beijing), loading buffer with elution buffer B (50mM Tris-HCl, 200mM NaCl, 500mM Imidazole, pH 8.0) to remove possible residual impurities or unbound proteins and binding buffer A (50mM Tris-HCl, 200mM NaCl, 20mM Imidazole, pH 8.0) balances the column is stable. The protein fraction was centrifuged using a desalting tube, Millipore column (10000MWCO) washed 3 times with buffer (50mM Tris-HCl, pH 8.0). The fraction of soluble protein concentration was confirmed by SDS-PAGE assay and Easy II Protein Quantitative Kit (BCA) method for checking protein concentration and stored at -80oC refrigerator to other use.
SDS-PAGE assay
The protein fraction was confirmed by 12% of sodium dodecyl sulfate gel electrophoresis (SDS-PAGE) loaded in well with helping of a micropipette. After loading sample was connected to power a supply (DC) of gel electrophoresis, and it was stained with Coomassie blue R-250 staining buffer (GenStar, Beijing, China). The standard protein ladder of SDS-PAGE was used to identify the purity and protein molecular weight range was run along with the sample.
Induction of HR
Tomato (Solanum lycopersicum) were grown from seeds in a plant growth chamber under controlled conditions the three lower leaves on each plant are fully infiltrated on the abaxial surface with a 1mL tuberculin syringe without the needle and taking 50μl of Hrip1 protein solution (50μM) as a treatment and buffer 50μl as control. Induction of HR was agroinfiltrated one spot to tomato leaves, cover 10- 20mm2 (One sample from every three replicates). After 48h post agroinfiltrated, HR symptom was apparent slightly in the areas. Tomato leaves detached after treatment protein and control at 6h, 18h, 24h, 48h, 72h, and 96h respectively for total RNA extraction and samples were chill in liquid nitrogen and stored at -80oC other use.
RNA extraction and gene expression analyses
Total RNA extraction both treatment and control tomato sample using EasyPure Plant RNA Kit, TransScript One- Step gDNA Removal and cDNA Synthesis SuperMix, and TransScript Top Green qPCR SuperMix (TransGen Biotech, Beijing, China) conducted an experiment according to the manufacturer’s guidelines. Relative quantitative real-time PCR analyses were performed to measure transcript levels of SlPR1, SlPR10, SlPR-Leaf 4, SlNP24, SlPRS TH-2, SlEndo chitinase EP3, SlPeroxidase, SlPeroxidase 12, and SlACC1, after Hrip1 treatment and buffer as control. Tomato (Solanum lycopersicum) leaves was infiltrated with 50μM of Hrip1 and buffer as control. Systemic leaves of untreated higher were harvested samples from 6h, 18h, 24h, 48h, 72h, and 96h after post-injection. The reverse transcription reaction was performed as mentioned above and the PCR with a proper program was performed using the reverse transcription product as template, PCR condition for cDNA synthesis incubate at 42oC for 15min then incubate at 85oC for 5s to activate enzymes. A primer used in this experiment was shown in table 1. A 20μl of qPCR reaction volume containing cDNA produced approximately a 500ng of total RNA. The PCR was processed on the following program such as one cycle of 94oC for the 30s then 40 cycles of 94oC for 5s, 58oC for 15s and 72oC for 10s. Three technical replicates of each reaction were performed and SlActin as a reference gene for constitutively expressed genes. Threshold cycle values were used for further analysis. Quantification of the relative changes in the gene transcript level was performed using the 2-ΔΔCt method. The relative expression levels of target genes were shown as fold changes in expression level.
Table 1: Primer sequence used for real-time quantitative PCR (RT-qPCR) of gene expression in tomato.
| Gene | Forward primer (5->3) | Reverse primer (5->3) |
|---|---|---|
| SlPR1 | ATCATTTGTTTCCTTACCTTTG | ACTCCAACTTGTCTACGA |
| SlPR10 | TTACAAGACAACAACTGAGTAT | AGCGTAGACAGAAGGATT |
| SlPR-Leaf 4 | GACTATCTTGCGGTTCAC | GCTCTTGAGTTGGCATAG |
| SlNP24 | TTGTTCTCTTCTTCCTTCTT | GGTGTATGGACAGTTGTT |
| SlPRSTH-2 | TGTGTTGAAGGATGAAGAA | TAAGCGTAGACAGAAGGA |
| SlEndo chitinase EP3 | TGTTGGTTCTACTGATGAT | GGTAATCTGTGTTGTTCTC |
| SlPeroxidase | ACTTCTCGTGCTAATAACAAT | CAGTAGTTGAGTCTCTTCTTC |
| SlPeroxidase 12 | GGCTTACTTCGTCTTCATT | GACACAACTTGACCACAT |
| SlACC1 | GTAATGGACACAGTAGAGA | GAGATATTAGAAGTAGGAAGATG |
| SlActin | GGTGTGATGGTGGGTATGG | GCTGACAATTCCGTGCTC |
Detection of ROS accumulation
Hydrogen peroxide production in Tomato (Solanum lycopersicum) leaves was treated with recombinant protein Hrip1 solution (50μM) and buffer as control. Tomato leaves were injected at 3-4 leaf stage of full expanding leaves, tissues harvested at 24h after protein treatment and control. Detached leaf sections infiltrate with a solution of DAB and vacuum 2-3min for several times, incubate at room temperature for 8-12h prior to sampling in darkness. The leaves are then put into boiling ethanol (95%) solution for 20 min to eliminate the chlorophyll (green). ROS was detected as dark-brown deposits at 24h in leaf tissues using the DAB uptake method. The ROS was not detected in leaves agroinfiltrated with a buffer and photographed with a digital camera.
Hrip1-Induced disease resistance in tomato plant
Tomato (Solanum lycopersicum) plant leaves at 3-4 week-old or 3 leaf state in plant growth chamber condition were sprayed with a 50μM of recombinant Hrip1 protein solution as treatment and buffer as control. Afterward, 3 days post-spraying of Hrip1 and buffer were inoculated with Agrobacterium tumefaciens contain TYLCV infectious clone was kindly provided by Bio-pesticide Engineering Laboratory, Institute of Plant Protection, Graduate School of Chinese Academy of Agricultural Sciences, Beijing, China. Agrobacterium tumefaciens strain was streaked freshly in LB solid agar plate supplement with the appropriate antibiotic of 50μg/ml kanamycin and 100μg/ml rifampicin in 100ml of LB solid agar media and incubates for 48h at 28oC before inoculation of the tomato plant. The further a monoclone of Agrobacterium tumefaciens strain was selected from LB solid agar plate and grown in the 50ml of LB broth medium for 24h at 28oC including appropriate antibiotic of 25μg/ml kanamycin and 50μg/ml rifampicin. The pellet of bacterial cells was collected using a centrifuge at 4000rpm for 5min at room temperature and resuspended with 10mM MgCl2, 10mM MES (pH5.6), 200μM Acetosyringone to a final OD600 was 1.5 and incubation for 3-4h at dark room temperature. The tomato plant stem (phloem) from the soil surface to inoculated area about 10cm high was inoculated infectious TYLCV clone with a 1ml syringe using a needle. The level of the resistance induced in tomato against the TYLCV was evaluated at 0, 5, 10, 15, 20, 25 and 30 days by using a 0-5 arbitrary scale. Ratings were as follows condition: 0 for no leaves showing yellowing (normal plants), 1 for slight yellow leaves in 1%-9% of leaves with yellowing, 2 for 10%-24% of leaves with yellowing and reduced in size, 3 for sectored yellowing in 25%-49% of leaves showing yellow associated with rolled upwards, 4 for pronounced leaves curling in 50%-75% of leaves showing curling and bent downwards, 5 for systemic leaves show interveinal chlorosis and are stunted or dwarfed, wrinkled. Mean severity of disease index as percent (%) was calculated from each treatment by summing the score of 45 tomato plants. Three replicates of 15 plant plants for each treatment, and expressing the value as a percentage. The tomato plans as treatment and control were an observed diary of the TYLCV symptom development.
Analysis of Tomato TYLCV Concentration
Total DNA extraction using EasyPure Plant Genomic DNA Kit (TransGen Biotech, Beijing, China) conducted an experiment according to the manufacturer’s guidelines. Tomato leaf samples (100mg) of upper leaves were collected at 0, 5, 10, 15, 20, 25, and 30 days after inoculation of TVLCV infectious clone for DNA extraction. The tomato leaves tissue respectively grind in liquid nitrogen with pestle and mortar as a good powder. Primer sequences used for PCR in this experiment were forward primer 5’-ATGTCGAAGCGACCAGGCGATATAAT-3’ and reverse primer 5’-TTAATTTGATATTGAATCATAGAAAT-3’ for the TYLCV gene. PCR was processed on the following program such as one cycle of 94oC for 10min then 30 cycles of 94oC for 35s, 50oC for 45s and 72oC for 1min. PCR reaction was run electrophoresis on 1% agarose gel in staining gold view nucleotide to check TYLCV presence. The viral TVLCV DNA band was exposed by UV light excitation to visualize obviously band. On the other hand, the qualitative analysis of TYLCV gene expression was performed a real-time qualitative polymerase chain reaction (RT-qPCR) showed there was reduced in TYLCV concentration after Hrip1 treatment compares with a buffer as control.
Protein assay
The concentration of protein fraction was detected by Promega enzyme labeled instrument (Glomax Multi Detection System) to measure at all purification steps using the Easy II Protein Quantitative Kit (BCA) (TransGen Biotech, Beijing, China) referred to the manufacturer’s instruction for the method of operation. Bovine serum albumin (BSA) standard (0-500μg/ml) was used as a standard protein and moreover run within test samples and the protein concentration was calculated from the BSA standard curve.
Data analysis
All data provided in this study were from at least three independent replicates. Significant differences between treatments and control were determined with analysis of variance using Microsoft Excel 2010 submit in a one-way analysis of variance (ANOVA). Significant differences were evaluated at the 5 % level.
Results
Purification of recombinant protein and induced HR
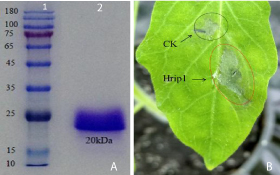
The protein supernatant was collected from a broth culture medium after resuspended with a BMMY liquid medium at 3 days by a centrifuge at 4oC. The supernatant was filtered with a syringe filter through a 0.22μm membrane and 25mm diameter to remove impurities (Millipore, Corp., Billerica, MA, USA) and purification using His column chromatography containing His-Tag resin (Trans Gen Biotech, Beijing), loading buffer with elution buffer B to remove possible residual impurities or unbound proteins and binding buffer A for balances the column. The protein fraction was centrifuged using a desalting tube, Millipore column (10000MWCO). The recombinant Hrip1 protein was apparent a single band protein on an SDS-PAGE gel (Figure 1A) with a molecular weight approximately 20kDa, which was agreed with the calculated protein concentration as determined using a BCA Protein Assay Kit (TransGen Biotech, Beijing, China). The fraction of recombinant Hrip1 was agro-infiltrated into behind surface leaves of Tomato (Solanum lycopersicum) via the stoma leaf cell. There were clearly defined HR necrotic areas at the agroinfiltration site at 72h post-agroinfiltration. The corresponding buffer had also induced HR slightly on the infiltrated leaf site (Figure 1B).
Figure 1: Confirmed by SDS-PAGE assay of recombinant Hrip1 protein. The expression of protein was purified by a His column chromatography containing His-Tag resin and elution buffer B (50mM Tris HCl, 200mM NaCl, 500 mM Imidazole, pH=8.0). The purified protein exhibited a single band on a SDS-PAGE was stained with Coomassie Brilliant Blue R-250. Panel (A1) Molecular weight marker 10-180kDa; (A2) protein molecular mass 20kDa. Panel (B) Hrip1-induced HR in plant leaves. HR in tomato leaves was observed at 72h post-agroinfiltration with Hrip1 and buffer. Red circle was treated with 50μl of Hrip1 protein solution (50μM) and exhibited clearly HR in area, black circle with buffer (50mM Tris-HCl, pH=8.0) as a control also induced HR slightly and immediately photographed with a digital camera.
Accumulation of ROS
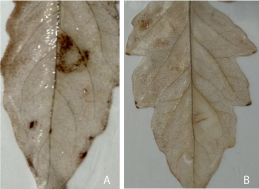
Reactive oxygen species (ROS) have offered as a significant component of plant adaptation to all conditions as both biotic and abiotic pressures. In such situations, ROS may play two very various roles exacerbating damage or signaling the activation of plant defense responses to attack micro pathogens and environmental condition [21]. In other circumstances, plants appear to purposefully engender ROS as signaling molecules to inspect different processions including pathogen defense, programmed cell death (PCD), and stomatal behavior [22]. To inspect of the recombinant Hrip1 function activated HR biochemical responses, analyzed the accumulation of ROS in tomato leaves was treated. Tomato leaves were injected at 3-4 leaf stage of full expanding leaves, tissue harvest at 24h after treatment and control. Detached leaf sections infiltrate with a solution of 3,3′-diaminobenzidine (DAB). Significant brown DABstained precipitates were easily and clearly observed in the recombinant Hrip1-treated site (Figure 2).
Figure 2: Induction of ROS in tomato leaves by Hrip1 and buffer. Panel (A) H2O2 accumulation in tomato (Solanum lycopersicum) leaves was treated with recombinant protein Hrip1 solution (50μM) at 3-4 leaf stage of full expanding leaf, the tissues harvest at 24h and buffer as control; (B) Filtrated areas were stained brown compared with buffer treatment as a control and immediately photographed with a digital camera.
Hrip1 caused expression of defence-related gene
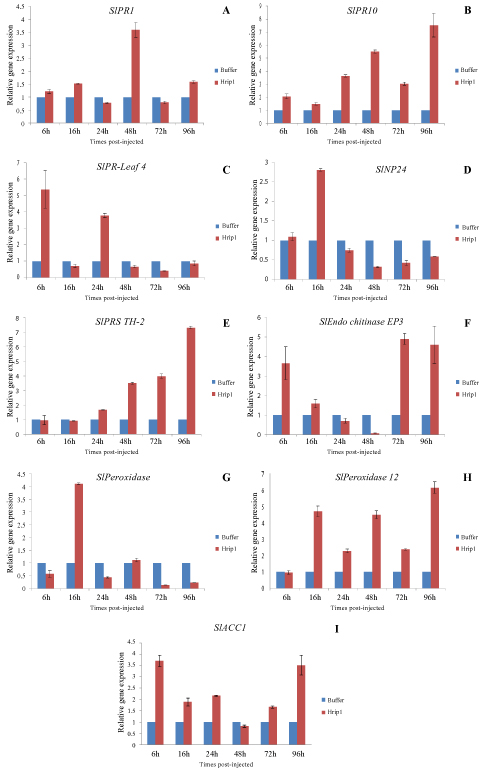
To further identify was examined the molecular mechanism related with gene induced by recombinant Hrip1 treatment to resistance in tomato and to assess gene expression pattern changes, we analyzed the expression pattern of the related gene, plants were injected leaves of 3-4 leaves stage. Relative expression of these genes induced by recombinant Hrip1 treatment and buffer as the control in tomato leaves at 6, 16, 24, 48, 72 and 96 hours after injection is shown in (Figure 3). The expression of these genes was swiftly induced in protein elicitor-treated plants. The relative expression levels of the SlPR1 gene was significantly up-regulated at 48h of time post-injection and the maximum level of the gene increased by 3.6-fold, the level then decreased but was also up-regulated compared with buffer as control (Figure 3A). The SlPR10 gene was significantly up-regulated at 96h post-injection and the maximum level of the gene increased by 7.53-fold, the level then decreased but was also up-regulated compared with control (Figure 3B). The expression of the SlPR-Leaf 4 gene continuously increased by 3.77 to 5.36-fold at 6h of time post-injection (Figure 3C). While recombination Hrip1 treatment triggered the expression of SlNP24 gene was up-regulated at 1.1 to 2.8- fold at 6 to 16h (Figure 3D). The SlPRS TH-2 gene continuously increased by 1.67 to 7.34-fold at 96h of time post-injection (Figure 3E). The enhanced expression of the SlEndo chitinase EP3 genes at 72 h after treatment, expression levels of this gene reached 4.91-fold at 72h after Hrip1 treatment (Figure 3F). The Hrip1-induced SlPeroxidase and SlPeroxidase 12 gene expression level reached its highest point at 16-96h post-inoculation, expression levels of these genes reached 4.11-fold of SlPeroxidase gene (Figure 3G) and 6.15-fold of SlPeroxidase 12 gene (Figure 3H). The SlACC1 gene was significantly up-regulated at 6h of time post-injection and level of the gene increased by 3.70-fold (Figure 3I).
Figure 3: Expression analysis of defence-related genes in tomato plant after recombinant Hrip1 treatment and using buffer as control. The tomato leaves were collected from systemic leaves at the indicated times from 6 to 96h, and RT-qPCR was performed to investigate the relative expression levels of the SlPR1, SlPR10, SlPR-Leaf 4, SlNP24, SlPRS TH-2, SlEndo chitinase EP3, SlPeroxidase, SlPeroxidase 12, and SlACC1genes. The samples were normalized against SlActin gene as inference gene, and expression levels are represented as fold changes in relation to the control.
Detection of tomato-infected TYLCV
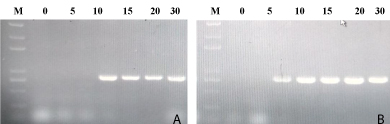
Tomato plant was challenged with TYLCV inoculum at 3-4 leaf stage after recombinant Hrip1 treatment and buffer as control. In the course of inoculation of Agrobacterium tumefacien containing an infectious clone of TYLCV was used to infect tomato plant. Tomato plant was treated recombinant Hrip1 infected TYLCV disease from 15 to 30 day, whereas tomato plant was used a buffer as control infected TYLCV disease from 10 to 30 day. The tomato leaf sample (100mg) of upper leaves was collected at 0th, 5th, 10th, 15th, 20th, 25th and 30th after TVLCV infection for total DNA extraction. The tomato leaves tissue respectively was ground in liquid nitrogen with pestle and mortar as a good powder. PCR reaction was run electrophoresis on 1% agarose gel in staining gold view nucleotide (Figure 4).
Figure 4: Detection of presented TYLCV DNA in tomato plants after inoculation with the infectious TYLCV clone. Agarose gel electrophoresis of amplification products from polymerase chain reactions conducted using primers TYLCV-5DT forward and TYLCV-3DT reverse primers. The detection of presented TYLCV DNA after tomato plants inoculation at 0, 5, 10, 15, 20, 25 and 30 days. (A) The tomato plant was infiltrated recombinant Hrip1 treatment at lane 0-10 day uninfected TYLCV of tomato plants; (B) for a buffer as control at lane 10-30 day tomato infected TYLCV. (M) Trans2K® Plus DNA marker.
Hrip1-induced disease resistance in tomato
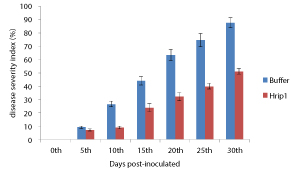
Resistance induced tomato plant by Hrip1 treatment, initial disease symptoms appeared on treatment and control plants no signaling leaves at 0 days. The mean dpi in these plants was 0%. The progress of the disease in control plants increased with time and by 5 dpi, most of the plant leaves developed very slightly severe yellowing. The mean dpi in these plants reached 87.60%. After treatment of Hrip1 protein, there was a reduction in dpi of TYLCV (Figure 5). The time between initial treatment with Hrip1 and subsequent inoculation with TYLCV significantly affected the efficacy of induced resistance. Although all interval times significantly reduced the dpi, the greatest disease suppression was caused by recombinant Hrip1-treated 3 days before inoculation. The resistance was induced by the Hrip1 treatment was already evident 5 dpi and lasted for the entire experimental period until 30 dpi. The disease index was reduced by 76.07% in Hrip1-treated tomato at 15 dpi, and this was maintained at the same level until 30 dpi. After DPI of control plants was 87.60% whereas those of Hrip1-treated tomatoes were only 51.07% at 30 dpi. Since the lowest disease ratings were recorded at a time interval of 5 days, this interval was taken into consideration in order to determine the level of TYLCV concentration. Table 3.
Table 3: Severity of TYLCV disease in tomato leaves of Hrip1 treatment and control in tomato plants. Data represent three replicates and 45 plants per replicate and values represent the mean ± SD. Tomato seedlings were inoculation with TYLCV infectious clone. The recording disease scores of the tomato seedlings were evaluated on a scale of 0–5 from 0 to 30 days post-inoculation.
| Time (days) after TYLCV inoculation | The disease severity index (%) of TYLCV score | |
|---|---|---|
| Hrip1 | Control | |
| 0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 5 | 7.07 ± 0.80 | 9.13 ± 0.83 |
| 10 | 9.07 ± 1.10 | 26.60 ± 2.06 |
| 15 | 23.93 ± 2.99 | 44.07 ± 3.17 |
| 20 | 32.13 ± 3.00 | 63.53 ± 4.12 |
| 25 | 39.93 ± 2.19 | 74.60 ± 4.87 |
| 30 | 51.07 ± 2.09 | 87.60 ± 3.76 |
Figure 5: Effect of Hrip1 treatment showed in symptoms of disease severity caused by TYLCV infection. After treatment with Hrip1 and buffer as control, tomatoes were inoculated with TYLCV infectious clone. Inoculated stem were scored at 0, 5, 10, 15, 20, 25 and 30 days post-inoculated using the 0-5 scale as described above. A mean of disease severity index (%) were calculated from each treatment by summing the score of the 45 plants (three replicates of 15 plants per treatment), and expressing the value as a percentage. Experiment repeated twice was very similar and so the results from one representative experiment are given. Data are the mean of three replicate, and bars indicate standard deviation of the means.
Hrip1-Reduced Level of Concentration of TYLCVInfected Tomato
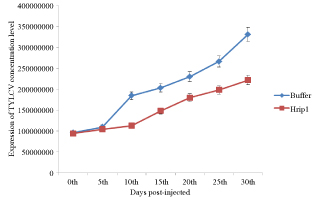
We tested the capacity of recombinant Hrip1 to induced cell death to mitigate TYLCV in tomato plants were injected with recombinant Hrip1. The recombinant Hrip1 protein treatment significantly mitigated the level of TYLCV concentration inoculated tomato plant from 0th to 30th post infection. To inspect of recombinant Hrip1 protein mitigated to TYLCV, Tomato (Solanum lycopersicum) leaves were inoculated with Agrobacterium tumefacien containing an infectious clone of TYLCV. By 10 day post-injection, plant leaves which were a test with recombinant Hrip1 showed that tomato leaves were not apparent of TVLCY symptoms, but tomato leaves were apparent slightly of TYLCV symptom after 10th with a buffer as control. Both the TYLCV concentration levels of the recombinant Hrip1 treatment plant were development TYLCV symptom slightly and low concentration of TYLCV than those of the buffer as control tomato plant (Figure 6).
Figure 6: Effect of Hrip1 treatment on the Agrobacterium tumefaciens in tomato leaves treated with Hrip1 and buffer as control. After treatment with recombinant Hrip1 and buffer, tomatoes were inoculated 3 days later with the Agrobacterium tumefaciens. Data are the mean of two independent experiments, and bars represent standard deviation of the means.
Discussion
In this study, we report a new great protein candidate Hrip1 protein elicitor from the broth culture medium of the pathogenic, Alternaria tenuissima fungus that induced leaf tissue hypersensitive response (HR) activity in tomato showed a single band protein on SDS–PAGE with a relatively obvious molecular weight of 20kDa (Figure 1A). Commonly, HR is part of the plant innate immunity and induces a signaling cascade that triggers a force in plant defense responses, leading to systemic resistance to plant pathogens [23]. Plant recognizes attacking pathogens, one of the first induced reactions is to swiftly produce superoxide (O-2) or hydrogen peroxide (H2O2) to strengthen the plant cell wall. This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction. The stress factor responses produced ROS is strongly influenced by stress factor responses in plants, these factors that increase ROS production include, drought, salinity, chilling, nutrient deficiency [24]. In previous, many researchers study on isolation of protein elicitors both bacteria and fungus pathogens such as PeBA1, BcGs1 and PeBL1 induced plant tissue HR and accumulation of reactive oxygen species (ROS) that ROS production is an important role function in the whole plant defense system and frequently appear in host or non-host plants after treatment with protein elicitor, these elicitors improved plant disease resistance in the tobacco [25,26] and tomato plant [20]. The recombinant Hrip1 protein elicitor induced ROS accumulation of early signaling in tomato cells and enhances the resistance of tomato against plant pathogens infection. In comparison with a known elicitor used a buffer as control and Hrip1-treated in tomato suspension cells showed similar ROS production patterns, indicating that Hrip1 performs similarly to this well-known elicitor. Therefore, Hrip1 is a secreted protein elicitor that can cause the accumulation of ROS which represent the significant types of early signaling molecules in plants.
The other protein elicitors from various microbe pathogens-derived proteins with the capacity to induce plant immunity responses, signal transduction and induced resistance to plants have been recognized and show great potential in progress of environment-friendly for biological control [27,28]. Oligosaccharide (OGs) are endogenous elicitors, host plant elicitors of defense responses released after partial degradation of pectin in the plant cell wall and increase resistance to the necrotrophic fungal pathogen Botrytis cinerea independently of signaling pathways mediated by jasmonate, salicylic acid, and ethylene [29]. OGs induce typical PTI responses, such as oxidative burst and the role of reactive oxygen species in elicitor mediated defense [30,31]. The early signaling events in plant defense responses, Hrip1 is a new great protein candidate isolated from necrotrophic fungus, A. tenuissima, represents a powerful tool, expression of defence-related genes and their transduction pathways involved in induced disease resistance in necrotrophic fungi in tobacco [32], and induced expression of elicitor gene enhances stress tolerance as a significantly higher effect on plant height, silique length, plant dry weight, root length, seed germination, under salt and drought in Arabidopsis [33]. To elucidate downstream signaling pathways, we used RT-qPCR performance of defense responses induced by Hrip1 in tomato. We found that the relative expression levels of these defense-related genes were differentially upregulated after infiltration with recombinant Hrip1-treated plant (Figure 3).
Our current results illustrated that concentration of 50μM recombinant Hrip1 protein elicitor was adequate to induce leaf tissue HR in tomato to display a great activity. Nevertheless, subsequent experiments that observed the induction of resistance in tomato caused by Hrip1 protein elicitor indicated that plant resistance against pathogenic infection was conferred on the plant, suggesting that Hrip1 induces confident plant defense signaling molecules that confer on plant immune system against plant micro pathogens. Hrip1 can also induce the transient expression of PR genes in tomato, confer on activities of resistance-related enzymes and increase tomato plant resistance to TYLCV disease. These results indicate that Hrip1 is a wide-ranging inducer of the plant immune system against plant pathogens and potential programs for plant protection. To determine the activation of the defense system in tomato can confer on resistance to pathogens, the experiment was inoculated TYLCV infectious clone, disease presence and concentration of tomato TYLCV-infected were reduced infection compared to control plants.
Conclusion
We report Hrip1 is a new great protein candidate derived from the, A. tenuissma. The recombinant Hrip1 protein could elicit the HR in tomato leaves and induce the production of signaling molecules of ROS accumulation as well as the expression defense related genes to enhance the tomato systemic resistance against plant pathogens. The recombinant protein proved to be an efficient activator of several plant defense mechanisms that induce resistance against TYLCV infection in the tomato plant. Regarding that the protein dependent resistance does not appear to be due to an anti-microbial effect of the compound, Hrip1 seems to be a useful tool for induced resistance in tomato as observed in other plant species. Protein elicitor is the conventional biopesticide agent in a good choice to reduce the application of chemical pesticides for environment-friendly, healthy plants and human health. However, the Hrip1 may become a vital tool candidate in a plant protection program.
Acknowledgment
This research was supported by the State Key Laboratory for Biology of Plant Diseases and Insect Pest, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China. We also highly appreciate all authors for good comments and revise on the manuscript.
There are no references