Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 19 October, 2018
Accepted date: 01 November, 2018
Published date: 2019-02-01
Citation: Estifanos T, Mesele A, Meseret C (2018) Microbiological Assessment of Gram-negative Bacterial Isolates from Ear, Eye, Nose a Throat among Patients Attending Aresho Advanced Medical Laboratory, Addis Ababa, Ethiopia. J Appl Microb Res. Vol: 1, Issu: 2 (27-32).
Copyright: © 2018 Estifanos T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
ENT samples from patients who referred to Arsho Advanced Medical laboratory were isolated for further microbiological assessment. Ninetyone (91) from both male and female patients comprising 42 ear, 21nasal, 1eye and 27 throat swabs respectively were screened between April -2017 to July-2017 and the samples were analyzed using culture technique, direct microscopy and identification of bacteria (Vtek 2 compact). Finding from the study identified twelve species of bacteria i.e Pseudomonas aeruginosa, Proteus mirabilis, Kelebsiella oxytoca, Entrobacter cloacae, Citrobacter koseri, Citrobacter pauclus, Pseudomonas fulorescens, Klebsiella, pneumoniae, Acinetobacter baumanii, E.coli, Lecletciaa decarboxylate and Proteus vulgaris. Proteus mirabilis recorded the highest rates (30%) followed by Klebsiella pneumoniae (20%) which was isolated in ear, and nose swabs, while the least were recorded on Citrobacter koseri Citrobacter pauclus, Pseudomonas fulorescens, Proteus vulgaris, Acinetobacter baumanii E.coli, Lecletciaa decarboxylate, Klebsiella pneumonia (3.3%).
Keywords
Bacteria, Species; Swab; Nasal.
Abstract
ENT samples from patients who referred to Arsho Advanced Medical laboratory were isolated for further microbiological assessment. Ninetyone (91) from both male and female patients comprising 42 ear, 21nasal, 1eye and 27 throat swabs respectively were screened between April -2017 to July-2017 and the samples were analyzed using culture technique, direct microscopy and identification of bacteria (Vtek 2 compact). Finding from the study identified twelve species of bacteria i.e Pseudomonas aeruginosa, Proteus mirabilis, Kelebsiella oxytoca, Entrobacter cloacae, Citrobacter koseri, Citrobacter pauclus, Pseudomonas fulorescens, Klebsiella, pneumoniae, Acinetobacter baumanii, E.coli, Lecletciaa decarboxylate and Proteus vulgaris. Proteus mirabilis recorded the highest rates (30%) followed by Klebsiella pneumoniae (20%) which was isolated in ear, and nose swabs, while the least were recorded on Citrobacter koseri Citrobacter pauclus, Pseudomonas fulorescens, Proteus vulgaris, Acinetobacter baumanii E.coli, Lecletciaa decarboxylate, Klebsiella pneumonia (3.3%).
Keywords
Bacteria, Species; Swab; Nasal.
Introduction
Ear, Eye, and Nose infection are among the most widespread and serious infections that compel an individual to seek medical attention. It represents some of the most common bacterial disease encountered affecting people of all ages. These infections are one of the leading causes of morbidity and mortality in critically ill patients [1]. The ear, nose, and throat are the frequent sites of infection, because they come in direct contact with the physical environment and are exposed to air borne microorganisms.
Disease of ear and nose affect the functioning of adults as well as children, often with significant impairment of the daily life of affected patients [1]. It has been predicted that with increase in global population, infection remain the most important causes of disease with upper respiratory infections causing hearing loss and learning disability particularly in children [2-4]. Ear infection such as chronic otitis (tinnitus), have serious consequences in developing countries such as retarded language development and progress in school among children [5]. Tinnitus which is now known to be the most common childhood infections, lead annually to the death of over 50,000 children under 5 years [6].
In addition, antimicrobial resistance profile of bacteria varies among population because of the difference in geography, local antimicrobial prescribing practices and prevalence of resistant bacterial strains in a given area [7]. So there should be up to date information on microbial resistance pattern at national and local levels to guide the rational use of the existing antimicrobial drugs.
The human ear, nose and throat (ENT) are closely related and inter connected parts of the body. Infections, diseases and health problems related to the ENT are therefore jointly studied and managed like the most other part of the human body. The ear, nose and throat were found to be colonized by a wide range of microorganisms some of which are more or less harmless under normal condition [8].
In Ethiopia particularly in the study area, there is no such type of recent data that shows the magnitude of the problem. Therefore, the aim of this study was to determine the gramnegative bacterial isolates and their drug susceptibility patterns from patients who gave ear and nose swab samples at Arsho Advanced medical Laboratory.
Objectives
General objectives
To determine the frequency of gram-negative bacterial outline and antibiotic susceptibility pattern isolated from ENT at Arsho advanced medical laboratory.
Specific objectives: To assess the gram-negative pathogens responsible for the ENT infection
To verify the antimicrobial resistance and sensitivity pattern of commonly isolated gram-negative bacteria from ENT patients
Methods
Study area and period
A study was conducted from, April-2017 to July-2017 at Arsho Advanced Medical Laboratory, Addis Ababa, Ethiopia.
Samples collections
The throat swab, nasal (nose) swab, eye swab and aural (ear) swab were collected by using a sterile cotton swab, and then the swabs were transported with Amies transport media to the micro biology laboratory.
Culture technique
Throat swab were inoculated on blood agar plates, were as nasal (nose), eye and ear swab were inoculated on chocolate, blood and MacConkey agar plates. All the plates were incubated for 48 hours 5% CO2 an aerobically with the exception of MacConkey agar plate that was inculcated in aerobic. The plates were examined for the growth of gram-negative bacteria and the pathogenic colonies were identified by VITEK 2 compact.
Bacteria identification test
Pure colonies of gram-negative colonies which were grown on macConkey Agar, chocolate Agar and Blood Agar were used for identification in VITEK 2 compact.
Suspension preparations for ID and AST card
Suspension preparation for ID card: First Transfer 3ml of 0.45% saline into a test tube. Select an isolated colony and dissolve it. Mix well and check the density within dens check. Inoculum density for gram positive and gram-negative colonies should be between 0.5-0.63MCF. Then place the identification (ID) card into the test tube and then transfer the test tube into the Cassette.
Suspension preparation for AST card: First Transfer 3ml of 0.45% saline into a Test Tube. Transfer 145 micro liter for gram negative of the ID suspension into the saline test tube. Then place the AST card into the test tube and then transfer the test tube into the cassette.
Filling and loading the card into VITEK 2 system
Put all the Test Tubes containing Cards and suspension a Cassette. From computer work station print Cassette worksheet and record job ID and bar code for each card. When instrument status is OK, then press start fill button. Remove the Cassette from the loading station when the machine indicates.
Antibiotics used
The antibiotics used in this investigation were Ampicillin, Amoxicillin/ClavulanicAcid, piperacillin/Tazobactam, Cefalotin,Cefazolin, cefuroximeAxil, Cefoxitin, Cefpodoxime, Ceftazidime, Ceftriaxone, Cefepime, Gentamicin, Toberamycin, Ciprofloxacin, Levofloxacin, Teteracyclin, Nitrofurantoin and Trimethoprim/sulfamethoxazole for gram negative. The control strains were run simultaneously with the test organisms.
Data Analysis
Data were checked for completeness, manually, entered, and analyzed using SPSS version 20 statistical software and Excel.
Result
A total number of 42 ear swabs, 21 nasal swabs, 1 eye and 27 throat swabs were collected from the patients attending at Aresho Advanced Medical Laboratory. Out of these, ear swabs were positive in 20 (22 %), 10 (11 %) in nose swabs.
The ear swabs was positive in 9 (9.9 %) male and 11(12.1 %) in female, nose swabs was positive 4 (4.4 %) was positive in male and 5 (5.5 %) in female (Table 1). The findings in this study show that twelve genera of gram negative bacteria which are P. mirabilis, K. pneumoniae, E. coli, P. vulgaris, P.aeruginosa, P.fulorescens, K.oxytoca, E.cloacea, C.koseri, C.pauclus.Leclerciaa decarboxylata, and A.baumanii were involved in various ear, and nose amongst patients examined at the study area. Out of all the organisms isolated 20 (66.67 %) occurred in ear swabs followed by nasal 10 (33.3 %) while there is no growth in eye and throat.
Table 1: Sex Wise Distribution and Culture Result of ENT Patients diagnosed from April -2017 to July-2017 in Arsho Advanced Medical Laboratory.
| Sample | Sex | Negative | Positive | Total examined |
|---|---|---|---|---|
| Ear | M | 10 | 9 | 19 |
| F | 12 | 11 | 23 | |
| Nose | M | 3 | 4 | 7 |
| F | 7 | 7 | 14 | |
| Throat | M | 13 | 0 | 13 |
| F | 14 | 0 | 14 | |
| Eye | M | 1 | 0 | 1 |
| F | 0 | 0 | 0 |
Generally, the most prevalent organisms were Proteus mirabilis (30 %) while the least were Citrobacter kuseri, citrobacter pauclus, Pseudomonasfulorescens, Acinetobacter baumanii and Leclerciaa decarboxylate with (3.33 %). Proteus mirabilis, K. pneumoniae, and K.oxytoca were isolated in both ear and nose swabs, E. coli, P.aeruginosa, P.fuloresce, E.cloacea, C.koseri, C.pauclus and A.baumanii were isolated in ear only while L.decarboxylata and P.vulgaris were isolated from only nasal swabs (Table 2).
Table 2: Gram negative Bacteria Associated with ENT Infection in Arsho Advanced Medical Laboratory.
| Isolates | Ear | Nose | Throat | Eye | Total (%) |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 3 | - | - | - | 3 (10 %) |
| Proteus mirabilis | 7 | 2 | - | - | 9 (30 %) |
| Kelebsiella oxytoca | 2 | 1 | - | - | 3 (10 %) |
| Entrobacter cloacae | 2 | - | - | - | 2 (6.67 %) |
| Citrobacter koseri | 1 | - | - | - | 1 (3.33 %) |
| Citrobacter pauclus | 1 | - | - | - | 1 (3.33 %) |
| Pseudomonas fulorescens | 1 | - | - | - | 1(3.33 %) |
| Klebsiella pneumoniae | 1 | 5 | - | 6 (20 %) | |
| Acinetobacter baumanii | 1 | - | - | - | 1(3.33 %) |
| E.coli | 1 | - | - | - | 1(3.33 %) |
| Lecletciaa decarboxylate | - | 1 | - | - | 1(3.33 %) |
| Proteus vulgaris | - | 1 | - | - | 1(3.33 %) |
The age-related prevalence of the ENT infection in the study area showed that the highest prevalence of the bacterial infection (40 %) was amongst those aged 15-24 years, (26.67 %) 25-44 years, (23.33 %) 1-14 years and (10 %) 45-64 while no growth observed amongst <1 and >65 years aged as shown in (Table 3).
Table 3: Age Related Prevalence of gram negative bacterial Isolates in ENT Infection in Arsho Advanced Medical Laboratory.
| Isolates | Age | |||||
|---|---|---|---|---|---|---|
| <1 | 1-14 | 15-24 | 25-44 | 45-64 | >65 | |
| Pseudomonas aeruginosa | - | 2 | 1 | - | - | |
| Proteus mirabilis | - | 4 | 3 | 1 | 1 | - |
| Kelebsiella oxytoca | - | - | 2 | 1 | - | - |
| Entrobacter cloacae | - | - | - | 2 | - | - |
| Citrobacter koseri | - | - | - | - | 1 | - |
| Citrobacter pauclus | - | - | 1 | - | - | - |
| Pseudomonas fulorescens | - | - | 1 | - | - | - |
| Klebsiella pneumoniae | - | - | 2 | 3 | 1 | - |
| Acinetobacter baumanii | - | 1 | - | - | - | - |
| E.coli | - | 1 | - | - | - | - |
| Lecletciaa decarboxylate | - | 1 | - | - | - | - |
| Proteus vulgaris | - | - | 1 | - | - | - |
| Total | 0 | 7 | 12 | 8 | 3 | 0 |
| Percent (%) | 0 | 23.33 | 40 | 26.67 | 10 | 0 |
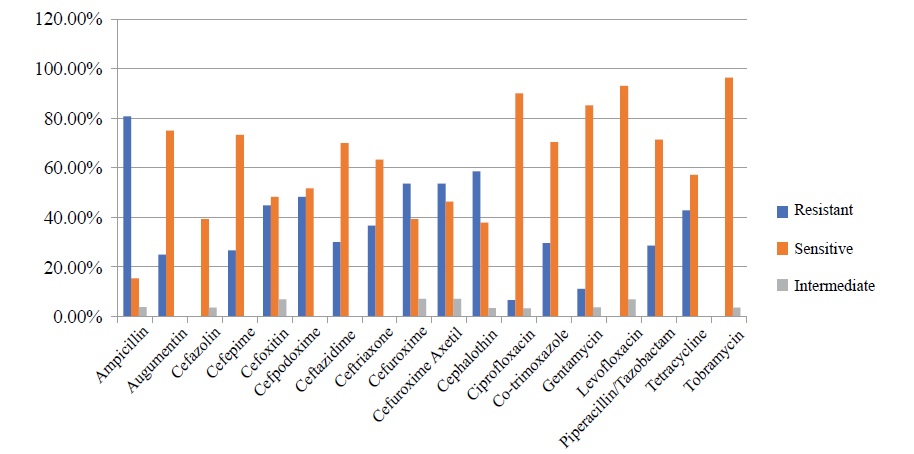
During the study period 18 different antimicrobial agents were used to test the antibiotic susceptibility patterns of the pathogenic gram-negative bacteria isolated from ENT patients. The general susceptibility profiles of bacterial isolates are shown in table 4. Out of the total antibiotics examined during the study period, Ampicillin had the highest overall resistance rate (80.8 %) followed by Cephalothin (58.6.6 %), Cefazolin (57.14 %), Cefuroxime and Cefuroxime Axetil(53.6 %) and Cefpodoxime (48.27 %). on the other hand, majority of bacterial isolates were susceptible to Levofloxacin, Tobramycin, Ciprofloxacin and Gentamycin with overall resistance rates of 0 %, 0 %, 6.6 %, and 11.11 %, respectively (Figure 1).
Figure 1: Antibiotic susceptibility profiles of isolated bacteria from ENT.
Table 4: Overall antibiotic susceptibility profiles of isolated bacteria from ear discharge.
| Type of antibiotics used | Frequency of Each antibiotic tested | Susceptibility patterns | ||
|---|---|---|---|---|
| Resistant | Sensitive | Intermediate | ||
| Ampicillin | 26 | 80.8 % (21) | 15.4 % (4) | 3.8 % (1) |
| Augumentin | 28 | 25 % (7) | 75 % (21) | - |
| Cefazolin | 28 | 57.14 % (16) | 39.3 % (11) | 3.6 % (1) |
| Cefepime | 30 | 26.7 % (8) | 73.3 % (22) | - |
| Cefoxitin | 29 | 44.83 % (13) | 48.27 % (14) | 6.9 % (2) |
| Cefpodoxime | 29 | 48.27 % (14) | 51.73 % (15) | - |
| Ceftazidime | 30 | 30 % (9) | 70 % (21) | - |
| Ceftriaxone | 30 | 36.67 % (11) | 63.33 % (19) | - |
| Cefuroxime | 28 | 53.6 % (15) | 39.3 % (11) | 7.14 % (2) |
| Cefuroxime Axetil | 28 | 53.6 % (15) | 46.4 % (13) | 7.14 % (2) |
| Cephalothin | 29 | 58.6 % (17) | 37.9 % (11) | 3.4 % (1) |
| Ciprofloxacin | 30 | 6.67 % (2) | 90 % (27) | 3.33 % (1) |
| Co-trimoxazole | 27 | 29.63 % (8) | 70.37 % (19) | - |
| Gentamycin | 27 | 11.11 % (3) | 23 (85.2 %) | 3.7 % (1) |
| Levofloxacin | 29 | - | 93.1 % (27) | 6.9 % (2) |
| Piperacillin/Tazobactam | 28 | 28.6 % (8) | 71.4 % (20) | - |
| Tetracycline | 28 | 42.9 % (12) | 57.1 % (16) | - |
| Tobramycin | 28 | - | 96.4 % (27) | 3.6 % (1) |
86.67% of the isolated bacteria were found to be resistant to one and more of the commonly used antibiotics listed in table-5. Among the total Klebsiella spp. Isolated all (100 %) of the isolates have developed resistant to one or more antibiotics used, among the total Proteus spp. isolated, the majority (77.8 %) of the isolates have developed resistance to one and more antibiotics used. Similarly, about threefourth of (75 %) Pseudomonas spp. isolates were able to resist one and more antibiotics commonly used to treat them. All other bacterial isolates have also showed overall antibiotic resistance to one and more antibiotics used except L.decarboxylata and C.koseri which shows 100% sensitivity.
Table 5: Antibiotic susceptibility test of pathogenic bacteria isolated from ENT patients.
| Organisms | AMP | AMC | TZP | CFA | CFZ | CFU | CFXA | FOX | CPD | CAZ | CRO | CFP | GM | TBM | CIP | LEV | TEC | SXT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.mirabilis | - | S | S | - | S | S | S | S | S | S | S | S | S | S | ||||
| P.mirabilis | R | S | R | R | R | R | R | R | R | R | R | R | S | S | S | S | R | R |
| P.mirabilis | R | S | R | R | R | R | R | R | R | R | R | R | R | S | R | S | R | R |
| P.mirabilis | R | S | R | R | R | R | R | R | R | R | R | R | I | S | S | S | R | R |
| P.mirabilis | - | - | - | - | S | - | S | S | S | S | - | |||||||
| P.mirabilis | S | S | S | R | S | S | S | S | S | S | S | S | S | S | S | S | R | S |
| P.mirabilis | R | S | R | R | R | R | R | S | R | R | R | R | R | S | S | S | R | R |
| P.mirabilis | R | S | R | R | R | R | R | I | R | R | R | R | S | S | S | S | R | R |
| P.vulgaris | R | S | R | R | R | R | R | R | R | R | R | R | S | S | S | S | R | S |
| P.aeruginosa | R | R | R | R | R | R | R | R | R | R | R | S | S | S | S | S | R | R |
| P.aeruginosa | R | R | S | R | R | R | R | R | R | S | R | S | S | S | I | I | R | R |
| P.aeruginosa | R | R | S | R | R | R | R | R | R | S | R | S | S | S | S | S | R | R |
| P. fluorescens | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.oxytoca | R | S | S | I | I | R | R | S | S | S | S | S | S | S | S | S | S | S |
| K.oxytoca | R | R | S | S | R | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.oxytoca | R | S | S | S | S | R | S | S | S | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | R | S | R | S | R | R | S | S | S | S | S | S | S | S | S |
| K.pneumoneae | R | S | S | R | R | S | I | R | S | S | S | S | S | S | S | S | S | S |
| E.cloacae | R | R | S | R | R | I | R | R | R | S | S | S | S | S | S | S | S | S |
| E.cloacae | R | S | R | R | I | R | R | R | S | S | S | S | S | S | S | R | S | |
| C.koseri | S | S | S | S | S | I | S | S | S | S | S | S | S | S | S | S | S | |
| C.pauclus | S | S | R | R | S | S | S | S | S | S | S | S | S | |||||
| A.baumanii | I | S | S | S | S | R | R | R | S | S | S | S | S | S | S | S | S | S |
| E.coli | R | R | S | R | R | R | R | I | R | R | R | R | R | I | R | I | S | S |
| L. adecarboxylata | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
Discussion
ENT infection is a more frequent treatable health care problem worldwide, yet if left untreated; it can cause a serious complication such as a speech disorder, pain in patients and their family quality of life, and economic burden on the health care system [9]. The burden and prevalence of ENT infection are more intense in developing countries due to the poor living standard and hygienic conditions along with lack of proper nutrition [10]. Kumar [11] reported that from gram negative bacteria spp., Proteus spp. where responsible for most cases of ENT infections, Whereas Edwin [9] find out that Pseudomonas spp., Proteus spp. and Klebsiella spp., are the common bacteria that cause ENT infection in Japura India, similarly Argaw-Denboba [10] find out that P. aeruginosa, Klebsiella spp. E. coli are the bacteria associated with ENT infection and Pseudomonas aeruginosa was the most prevalent aetoliogic agent of ENT infection in Benin city.
In this study, the main pathogenic bacterium associated with ENT infection was Proteus mirabilis. Followed by Klebsiella pneumonia, Kelebsiella oxytoca and Pseudomonas aeruginosa. respectively. Similarly, previously published articles from our study area and other parts of Ethiopia also reported Proteus spp. were the foremost bacteria associated with middle ear infection [9,10]. Although it needs a further nationwide study, taking into account our study finding and others, it seems that Proteus Mirabilis re the leading bacterial isolates associated with ENT infection in Ethiopia. Conversely, several other published data pieces from Africa and elsewhere in the world reported Pseudomonas spp., mainly P. aeruginosa, is the primary pathogenic bacteria associated with middle ear infection [12]. One possible explanation for this difference might be due to climate, poor living standard, hygienic condition and geographical variations between Ethiopia and other countries.
In general, Ampicillin, Cephalothin, Cefazolin, Cefuroxime and Cefuroxime had shown the highest antibiotic resistance rates to all bacterial pathogens isolated from ENT discharge, respectively. Correspondingly, Tetracycline, were the most clinically used antibiotics that showed a higher resistance rate to Proteus spp., which is in line with earlier reports from Ethiopia, Nigeria, and Egypt [13]. Among the total Klebsiella spp. and Proteus spp. isolated during the study period about 100% and 77.8% of the isolated bacteria have developed resistance to one and more antibiotics that were once in clinical use, respectively. Overall, more than 60% of the bacterial isolates of this study were characterized as multi-antibiotic-resistant pathogenic bacteria. The reason for this high degree of multiantibiotic resistance might be connected to inappropriate use of antibiotics, including incomplete dose, self-medication, and poor infection prevention and control practices as indicated by the recent WHO antimicrobial resistance report. An Interestingly, our study revealed almost all the isolated pathogenic bacteria were considerably susceptible to Levofloxacillin, Toberomycin, Ciprofloxacin and Gentamicin. Particularly, Levofloxacillin, and Toberomycin were shown to be highly effective for pathogenic bacteria associated with ENT infection in this study. Likewise, with a slight variation several other authors have shown a similar high efficiency of ciprofloxacin against these bacterial species. Thus, we propose that Levofloxacillin, and Toberomycin can be taken as a first-line optional treatment for ENT infection attending in Arsho Advanced medical Laboratory, Addis Ababa, Ethiopia. This suggestion holds true, since the current firstline treatment for both acute and chronic otitis media in Ethiopia is Ampicillin and Augmentin which already showed a high resistance rate (80.8 % and 25 % respectively) for the majority of the bacterial isolates in this study.
Conclusion and Recommendation
This study showed that the risk of acquiring particularly ear infection is strongly associated in Proteus mirabilis, Pseudomonas aeruginosa and Klebsiella oxytoca were the three predominant bacteria isolates from patient ear discharges suspected of otitis media whereas,in nose infection Klebsiella pneumonia was the most predominant isolated organism. Almost all the isolated bacteria showed a considerable level of resistance to one antibiotic that are commonly used in health institutes; particularly, majority of isolated bacteria were found to be highly resistant to Ampicillin Cephalothin, Cefazolin, Cefuroxime and Cefuroxime Axetil and Cefpodoxime treatments. Among the total Klebsiella spp. Isolated all (100 %) of the isolates have developed resistant to one or more antibiotics used, among the total Proteus spp. isolated, the majority (77.8 %) of the isolates have developed resistance to one and more antibiotics used. This study also indicated Levofloxacillin, and Toberomycin are effective against all the bacterial isolates and most was highly sensitive to Toberomycin. In general, the result of this study revealed that antibioticresistant bacteria are alarmingly increasing in Aresho Advanced Medical Laboratory study area, Addis Ababa, Ethiopia, and becoming a major public health problem in patients with ENT infection. Therefore, we recommend nationwide antimicrobial inspection to make the right recommendation of alternative antibiotics along with strict devotion to antibiotic policy to reduce the spread of drug resistant microbes in the country.
Acknowledgment
We would like to thank all staff members of Arsho Advanced Medical Laboratory, microbiology department.
There is no references