Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 15 January, 2021
Accepted date: 17 February, 2021
Published date: 24 February, 2021
Citation: Coulibaly S, Hien SA, Somé AF, Bamogo R, et al. (2021) Molecular Diagnostic of Wuchereria bancrofti Infection in Vector Populations from Endemic Regions of Burkina Faso: Preliminary Results. J Appl Microb Res. Vol: 4 Issu: 1 (25-33).
Copyright: © 2021 Coulibaly S et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Since 2001, Burkina Faso had adopted a community-based treatment of lymphatic filariasis (LF) with annual mass drug administration (MDA) of Ivermectin-Abendazole. it remained some hotspots with active transmission. Therefore, in the elimination perspectives toward 2030, it is crucial to validate a diagnostic approach to assess the prevalence of Wuchereria bancrofti especially in vector populations for surveillance prospects. For the validation of diagnostic tool, we used samples from previous parasitological and entomological surveys conducted in August 2014 and September 2015 in hotspots where the transmission was still active. Mosquitoes were collected by human landing catches and indoor spraying but no diagnostic tool was used to assess W. bancrofti prevalence. Based on molecular technique using DNA of pooled mosquitoes successfully tested in other countries, we tried to validate such protocols from specimens collected during the previous surveys. The results confirmed the W. bancrofti infection in human populations as revealed by parasitological tests and also in vector populations tested for the first time by this molecular diagnostic. Within the An. gambiae s.l. populations, only An. coluzzii was found infected by W. bancrofti in the Centre-East and East regions, whereas An. nili was the most infected in the South-West region. Some specimens of An. funestus were tested positive to W. bancrofti with the LAMP PCR but failed to be confirmed by the conventional technique. Our preliminary data confirmed the validity of molecular technique to detect W. bancrofti infection within vector populations even additional calibration is still needed. In the South West, surprisingly An. nili was found positive to W. bancrofti infection by the two molecular techniques extending the list of LF potential vectors in the country. It raised the evidence that diagnostic tool is crucial to better define endpoints for the national LF elimination programme.
Keywords
Wuchereria bancrofti, Prevalence, Mosquitoes, Burkina Faso.
Abstract
Since 2001, Burkina Faso had adopted a community-based treatment of lymphatic filariasis (LF) with annual mass drug administration (MDA) of Ivermectin-Abendazole. it remained some hotspots with active transmission. Therefore, in the elimination perspectives toward 2030, it is crucial to validate a diagnostic approach to assess the prevalence of Wuchereria bancrofti especially in vector populations for surveillance prospects. For the validation of diagnostic tool, we used samples from previous parasitological and entomological surveys conducted in August 2014 and September 2015 in hotspots where the transmission was still active. Mosquitoes were collected by human landing catches and indoor spraying but no diagnostic tool was used to assess W. bancrofti prevalence. Based on molecular technique using DNA of pooled mosquitoes successfully tested in other countries, we tried to validate such protocols from specimens collected during the previous surveys. The results confirmed the W. bancrofti infection in human populations as revealed by parasitological tests and also in vector populations tested for the first time by this molecular diagnostic. Within the An. gambiae s.l. populations, only An. coluzzii was found infected by W. bancrofti in the Centre-East and East regions, whereas An. nili was the most infected in the South-West region. Some specimens of An. funestus were tested positive to W. bancrofti with the LAMP PCR but failed to be confirmed by the conventional technique. Our preliminary data confirmed the validity of molecular technique to detect W. bancrofti infection within vector populations even additional calibration is still needed. In the South West, surprisingly An. nili was found positive to W. bancrofti infection by the two molecular techniques extending the list of LF potential vectors in the country. It raised the evidence that diagnostic tool is crucial to better define endpoints for the national LF elimination programme.
Keywords
Wuchereria bancrofti, Prevalence, Mosquitoes, Burkina Faso.
Introduction
Lymphatic filariasis (LF) is the second parasitic disease after malaria, persisting in tropical and subtropical countries worldwide and recognized by World Health Organisation (WHO) as a Neglected Tropical Disease (NTD) [1,2]. It is caused mainly by Wuchereria bancrofti in Africa, transmitted by mosquitoes belonging to the genus Anopheles. In 1999 the Global Programme to Eliminate Lymphatic Filariasis (GPELF) had been initiated by the WHO with the overall objective to interrupt transmission by 2020 using mass drug administration (MDA) of Ivermectin in combination with Abendazole at least in 53 LF endemic countries worldwide [3]. In 2001, Burkina Faso implemented it’s National LF Programme committed to manage the elimination by 2015 in all health districts [4]. The first round of MDA occurring in 2001 was followed by several other rounds. By 2014 out of 70 health districts receiving >10 MDAs, only 39 health districts presented their respective microfilariae prevalence low than 10% reaching the interruption threshold [5]. In the other remaining districts, the microfilariae were still upper than 1% and did not match the interruption level. Specifically, the health districts in Centre-East, East and South-West parts of the country showed high and persistent microfilariae rates implying to pursue MDA until today in such districts which become the hotspots of LF in Burkina Faso [4,5]. Hence, in these areas many efforts are still making to cut down the prevalence until elimination threshold. Unfortunately, the monitoring and evaluation scheme to assess the impact of MDA in Burkina Faso is only focusing on antigen tests and also parasitological tests with microfilariae assays in the human populations which did not include yet the detection of microfilaria in vectors [5-7]. As in Ghana molecular approach permitted to detect Wuchereriainfected An. gambiae s.l. [8,9], integrating this technique can be a complementary way for the monitoring and the evaluation of post TAS (Transmission Assessment Surveys) validation in Burkina Faso. The objective of the current study is to validate a molecular diagnostic tools to assess the prevalence of W. bancrofti in mosquitoes. If successful, this molecular approach will benefit to the MDA programme for the future monitoring and evaluation of post LF validation in Burkina Faso as experimented in Ghana and Togo [10,11]. This molecular tool will complement parasitological investigations in human populations. It will serve as an important tool for the MDA programme for LF elimination towards 2030. For that we needed to exploit field samples already collected from areas where LF transmission was still active that explaining the use of collections of 2014-2015 from the hotspot areas where the infections were confirmed by parasitological data.
Material and Methods
Study sites
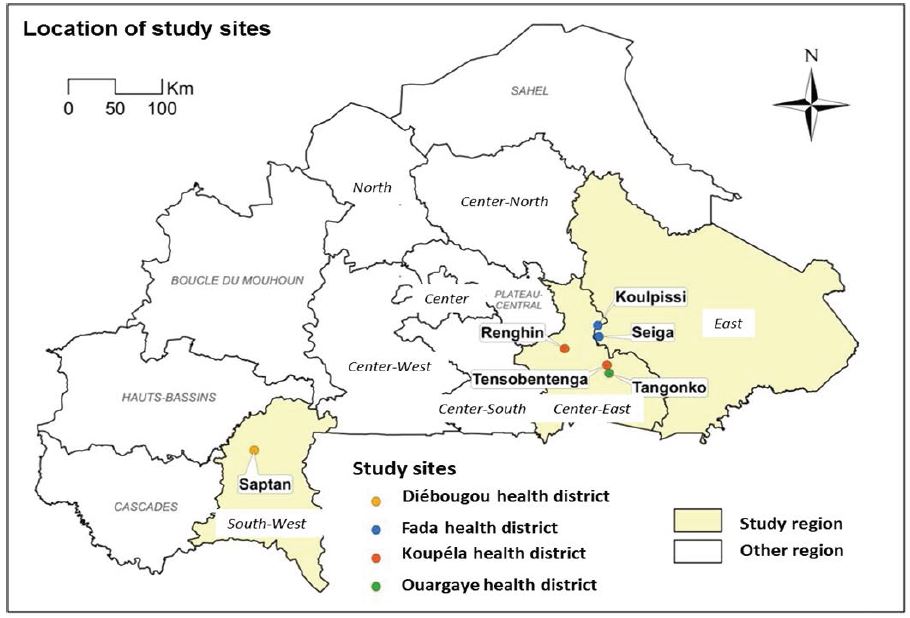
We worked on mosquitoes’ specimens subsampled from entomological and parasitological collections realised in August 2014 and in September 2015 across six sites where LF was still actively recorded in human populations in Burkina Faso: Seiga (11°57’55.998’’N;0°51’34.956’’W) and Koulpissy (12°4’41.228’’N; 0°5’52.706’’W) in the East region; Renghin (11°50’46.057’’N; 0°25’0.661’’W) and Tensobtenga (11°40’53.72’’N; 0°0’33.509’’W) Tangonko (11°35’59.388’’N; 0°0’41.166’’W) in the Centre-East and Saptan (12°4’41.228’’N; 0°5’52.706’’W) in the South West (Figure 1).
Blood sample collection for parasitological diagnostic
Blood samples were nightly collected in August 2014 and September 2015 between 10 pm and 2 am from 100 individuals randomly selected per village and per month between 5 - 65 years old. A thick blood smear was performed from single finger-prick that allowed collecting 60 - 100 μL blood sample staggered in 3 drops. The thick blood films were air-dried and fixed in ethanol, then stained with 10% Giemsa (Sigma) in phosphate buffer (pH 7.0) for 30 minutes. Microscopic examination was then performed by two independent readers, using oil immersion magnification (100 x), for the presence of W. bancrofti.
Mosquito collections
The vectors were sampled consecutively during two years in August 2014 and September 2015 by human landing catches (HLC) as well as indoors and outdoors per village from 6:00pm to 6:00am. After collections, mosquitoes were identified using the key of Gillies and Coetzee [12]. The indoor resting mosquitoes were also collected by Pyrethrum Spray Catch (PSC) performed the next morning following each HLC into 10 houses around HLC houses between 6 - 9am. All mosquitoes collected were identified morphologically and stored into tubes for further laboratory analysis for species identification and the prevalence of W. bancrofti infections in the anopheline vector populations.
Laboratory analysis
The laboratory works were performed recently on July- September 2019 consisting to validate molecular diagnostic with mosquitoes collected in the field and kept in Eppendorf tubes on silica gel. They were identified by site, species, and collection date before being stored at -20°C together with thick smears samples.
Species identification within An. gambiae complex
Legs and abdomens of An. gambiae s.l. females from indoor and outdoor collections identified morphologically were analysed to species level using routine diagnostic PCR [13]. The DNA from heads and thoraces were kept for further analysis for the presence of L3 of W. bancrofti. As only An. funestus s.s. was reported in Burkina Faso as malaria and potentially LF vector, no PCR identification was needed for species identification [14,15].
Molecular detection of Wuchereria bancrofti from Anopheles mosquitoes
The detection of Wuchereria bancrofti L3 within mosquitoes was realized by conventional PCR using DNA from heads and thoraces of mosquitoes grouped by pool before PCR processes [8,16,17]. This technique permitted to analyze the An. gambiae s.l. collected in the Centre-Eastern and Eastern sites namely Seiga, Renghin, Tensobtenga and Tangonko. Furthermore, during this process, we received the primers for the LAMP-PCR that we applied for the detection of W. bancrofti from Saptan mosquitoes which presented a diversity of species such as An. gambiae s.l., An. funestus s.l. and An. nili. This technique was performed according to the Loop-mediated isothermal method (LAMP-PCR) which final PCR products were revealed by electrophoresis gel [18]. Then all positive pools for the LAMP-PCR were systematically analyzed with the conventional PCR technique as described above just to confirm the results in accordance with those tested for the other five sites (Koulpissy, Seiga, Renghin, Tangonko and Tensobtenga).
Figure 1: Study sites’ location in Burkina Faso.
Data analysis
Statistical analysis was performed with the software R version 4Ri386. The “Pool Screen® 2.0” software using a statistical model to estimate vector’s infection from the number of positive pools with 95% CI served to determine the W. bancrofti infection rates. The microfilaria vectors infections rates (expressed over about 1000 mosquitoes) have been estimated by the algorithm of Katholi et al. [19].
Result
Prevalence of microfilariae in human populations
Out of 1985 thick blood smears analyzed, 652 were from August 2014 and 1333 from September 2015 including all the six study sites (Table 1). In 2014, W. bancrofti was detected in four villages (Seiga, Koulpissy, Renghin, Tensobtenga and Saptan) revealing that its prevalence was still relatively higher in human populations in such sites. The related microfilariae prevalences were higher in Koulpissy (Dmf=350mf/mL), Seiga (Dmf=2330mf/mL and Renghin (Dmf=183mf/mL) and relatively low in Saptan (Dmf=50mf/ mL). In 2015, W. bancrofti was detected only in two villages namely Seiga and Tensobtenga with low microfilariae prevalence compared to 2014 reaching mean infection of 17mf/mL in each site but no more in Saptan and Koulpissy. Additionally to W. bancrofti, Mansonella perstans was found in 2014 in Saptan with a mean prevalence of 1.64 % with 50 mf/mL.
Mosquito abundance and species composition
A total of 29,415 Culicidae were sampled by the two collection methods with 9,272 in August 2014 and 20,143 in September 2015 throughout the six sites both indoors (11,924 mosquitoes) and outdoors (10,243 mosquitoes). In addition, An. gambiae s.l. was the predominant species found in both round of surveys (87.25%) followed by An. nili (5.72%) and An. funestus s.l. (1.24%) in Saptan. Aedes (0.82%), Culex sp (5.06%) and Mansonia (0.005%) were found in relatively low proportions (Table 2).
Anopheles gambiae s.l. species composition.
An. coluzzii was the predominant species in Koulpissy, Seiga and Renghin whatever the year. However, An. gambiae represented more than 80 % of the complex species in Tangonko and Tensobtenga in August 2014 but not in September 2015 where An. coluzzii were found predominant in these localities (Figure 2-4). An. arabiensis was retrieved in low frequencies in all sites except in Tensobtenga in 2014 but not occurring in 2015 collections. An. gambiae was the main species of the complex in Saptan whatever the year.
Table 1: Prevalence of Wuchereria bancrofti in human populations from the study sites (LF: lymphatic filariasis).
| August 2014 | ||||||
|---|---|---|---|---|---|---|
| Site | Number persons examined | Nb of MDA | LF positive cases | Total number of Microfilaria (in 60μl) | Microfilarial density (Dmf) | Prevalence of Wb infection (%) |
| Seiga | 140 | 12 | 1 | 14 | 233mf/mL | 0.71 |
| Koulpissy | 106 | 12 | 3 | 21 | 350 mf/mL | 2.83 |
| Renghin | 81 | 12 | 1 | 11 | 183mf/mL | 1.23 |
| Tangonko | 93 | 12 | 0 | 0 | 0 | 0 |
| Tensobtenga | 110 | 12 | 0 | 0 | 0 | 0 |
| Saptan | 122 | 17 | 1 | 3 | 50mf/mL | 1.64 |
| Total | 652 | - | 6 | 49 | 92mf/ML | 0.92 |
| September 2015 | ||||||
| Seiga | 296 | 13 | 1 | 1 | 17mf/mL | 0.34 |
| Koulpissy | 282 | 13 | 0 | 0 | 0 | 0 |
| Renghin | 226 | 13 | 0 | 0 | 0 | 0 |
| Tangonko | 87 | 13 | 0 | 0 | 0 | 0 |
| Tensobtenga | 233 | 13 | 1 | 1 | 17mf/mL | 0.43 |
| Saptan | 209 | 19 | 0 | 0 | 0 | 0 |
| Total | 1333 | - | 2 | 2 | 6mf/mL | 0.15 |
Prevalence of Wuchereria bancrofti in mosquito populations
The infection of W. bancrofti within An. gambiae s.l. populations were revealed by conventional PCR in two samples sets from Koulpissy in 2014 with an infection rate of 4.5% (1.17-11.4) and Tensobtenga in 2015 reaching an infection rate of 1.04% [0.03-5.2] (Table 3). The species PCR of each positive pool revealed that all positive pools were identified as An. coluzzii as well as in Koulpissy and Tensobtenga. Furthermore, the pools of Saptan composed by An. gambiale s.l., An. funestus s.l. and An. nili collected both in 2014 and 2015 were tested first using LAMP technique and secondly with conventional PCR. The results obtained with LAMP-PCR technique showed that both An. funestus s.l. and An. nili were infected by W. bancrofti respectively in 2014 and 2015 with related infection rates of 0.5% (0.05-2.9) and 0.06% [0.002-0.3] respectively (Table 4). However, these results were checked by conventional PCR that confirmed only one pool of An. nili as effectively positive to W. bancrofti reaching an infection rate of 0.8% (0.3-1.4). The other pools of An. funestus failed to be confirmed by conventional PCR. No An. gambiae s.l. pool was tested positive to W. bancrofti neither by LAMP none by conventional PCRs in Saptan.
Discussion
This study was one of the first which attempted to validate a molecular diagnostic tool to investigate infection status of Wuchereria bancrofti especially within vector populations in Burkina. The results were consistent both in human and vector populations being in reduced trend from one year to another as well as by the intensity of the infection in human populations as revealed by the microfilariae density and by the infection rate within vector populations. These results were confirmed two years after in the same areas by Kima et al. [5] who had also notified many cases of W. bancrofti in human populations confirming thus the hotspot status of this transmission belt [5]. Compared to Ghana, a neighboring country experiencing the elimination of LF, the infection of W. bancrofti in human population was rare or absent after 11 to 16 MDAs that lead to conduct post TAS evaluation studies [8]. Here in Burkina Faso many investigations are still needed before decision making to stop the MDA. Nevertheless, when comparing the prevalence in human populations from 2014 to 2015, it can be noted an important reduction of W. bancrofti either in of microfilariae or in the number of villages with positive cases. This situation can be explained as a progress resulted by integrated vector control measures implemented with the high coverage of LLINs in nationwide but also by the continuing MDA realized in these sites [20]. Analyzing the prevalence of W. bancrofti in vectors populations, it can be noted that the main and potential vectors of LF in Burkina Faso were assumed to be anopheline mosquitoes as early reported by very old studies [21]. Globally the biting dynamic in these sites was dominated by An. gambiae s.l. but mostly identified as An. coluzzii and An. gambiae, the third species of the complex, An. arabiensis being very rare in the collections. It is fair to assume that in our studies the results were considered as preliminary as we did not dissect any mosquitoes for microscopic identification of microfilaria (L3) nor testing PSC collected mosquitoes by PCR for W. bancrofti infection. So no detail can be documented like those realized in Ghana [22]. Nevertheless the molecular detection indicated that belonging the An. gambiae complex represented only by two species, all the pools tested positive were composed by An. coluzzii that suggested that in the Centre-East and East parts, this species should be the most involved in W. bancrofti transmission. Furthermore, the vector bionomics in the West and specifically in the South-
Table 2: Total vector collected by Hunan Landing Catches (HLC) method.
| August 2014 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Collection site | Anophelinae | Culicinae | Total | |||||||
| An. gambiae Total s.l. | An. funestus s.l. | An. nili | An. pharoensis | Anopheles sp. | Aedes s.p. | Culex sp. | Mansonia s.p. | ||||
| Seiga | Indoor | 634 | 1 | 0 | 1 | 0 | 7 | 1 | 1 | 645 | 1155 |
| Outdoor | 494 | 0 | 0 | 0 | 0 | 15 | 1 | 0 | 510 | ||
| Koulpissy | Indoor | 639 | 0 | 0 | 0 | 0 | 5 | 0 | 7 | 651 | 1325 |
| Outdoor | 658 | 0 | 0 | 0 | 0 | 12 | 0 | 4 | 674 | ||
| Renghin | Indoor | 496 | 0 | 0 | 0 | 0 | 1 | 30 | 0 | 527 | 959 |
| Outdoor | 369 | 4 | 0 | 3 | 1 | 13 | 38 | 4 | 432 | ||
| Tensobtenga | Indoor | 633 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 635 | 1352 |
| Outdoor | 699 | 0 | 0 | 0 | 0 | 7 | 8 | 3 | 717 | ||
| Tangonko | Indoor | 517 | 0 | 0 | 0 | 0 | 2 | 66 | 0 | 585 | 1198 |
| Outdoor | 504 | 0 | 0 | 0 | 0 | 7 | 102 | 0 | 613 | ||
| Saptan | Indoor | 198 | 206 | 120 | 0 | 4 | 19 | 2 | 48 | 597 | 1038 |
| Outdoor | 143 | 74 | 127 | 0 | 9 | 45 | 0 | 43 | 441 | ||
| September 2015 | |||||||||||
| Seiga | Indoor | 2500 | 0 | 0 | 0 | 0 | 9 | 2 | 0 | 2511 | 4832 |
| Outdoor | 2292 | 0 | 0 | 0 | 0 | 26 | 3 | 0 | 2321 | ||
| Koulpissy | Indoor | 1286 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 1293 | 2310 |
| Outdoor | 997 | 0 | 0 | 0 | 0 | 15 | 0 | 5 | 1017 | ||
| Renghin | Indoor | 2058 | 0 | 0 | 0 | 0 | 7 | 13 | 0 | 2078 | 3546 |
| Outdoor | 1441 | 0 | 0 | 4 | 0 | 11 | 12 | 0 | 432 | ||
| Tensobtenga | Indoor | 1021 | 0 | 0 | 0 | 0 | 3 | 5 | 2 | 1031 | 1602 |
| Outdoor | 549 | 0 | 0 | 1 | 0 | 4 | 5 | 12 | 571 | ||
| Tangonko | Indoor | 445 | 0 | 0 | 1 | 1 | 4 | 92 | 0 | 543 | 1005 |
| Outdoor | 382 | 1 | 0 | 0 | 2 | 9 | 65 | 3 | 462 | ||
| Saptan | Indoor | 214 | 22 | 570 | 1 | 8 | 3 | 7 | 3 | 828 | 1845 |
| Outdoor | 105 | 8 | 851 | 0 | 16 | 1 | 26 | 10 | 1017 | ||
Table 3: Infections rates of Wuchereria bancrofti in An. gambiae s.l. populations from 5 study sites assessed by conventional PCR.
| Anopheles gambiae s.l. | |||||
|---|---|---|---|---|---|
| Localities | Date of collection | Nb pools tested | Nb positive pool | Nb mosquitoes tested | Infection rates |
| Seiga | Aug 2014 | 13 | 0 | 100 | 0 |
| Sept 2015 | 10 | 0 | 100 | 0 | |
| Koulpissy | Aug 2014 | 15 | 4 | 100 | 4.5 (1.17-11.4) |
| Sept 2015 | 10 | 0 | 100 | 0 | |
| Renghin | Aug 2014 | 14 | 0 | 100 | 0 |
| Sept 2015 | 10 | 0 | 100 | 0 | |
| Tensobtenga | Aug 2014 | 13 | 0 | 100 | 0 |
| Sept 2015 | 10 | 1 | 100 | 1.04 (0.03-5.2) | |
| Tangoko | Aug 2014 | 13 | 0 | 100 | 0 |
| Sept 2015 | 10 | 1 | 100 | 1.04 (0.03-5.2) | |
Table 4: Infection rates of Wuchereria bancrofti in An. gambiae s.l., An. funestus and An. nili by populations compared between LAMP and conventional PCRs at Saptan in the South West region.
| Type of PCR | 2014 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|
| Nb specimens | Nb pools tested | Nb positive pool | Infection rates | Nb specimens | Nb pools tested | Nb positive pool | Infection rates | |
| An. funestus | ||||||||
| LAMP PCR | 385 | 6 | 1 | 0.5 (0.05-2.9) | 33 | 2 | 0 | - |
| Conventional PCR | 385 | 9 | 0 | - | 33 | 2 | 0 | - |
| An. nili | ||||||||
| LAMP PCR | 256 | 8 | 1 | 0.012 (0.1-1.2) | 1423 | 65 | 10 | 0.71 (0.3-1.4) |
| Conventional PCR | 256 | 8 | 0 | - | 1423 | 65 | 1 | 0.06 (0.002-0.3) |
| An. gambiae s.l. | ||||||||
| LAMP PCR | 186 | 6 | 0 | - | 206 | 9 | 0 | - |
| Conventional PCR | 186 | 6 | 0 | - | 206 | 9 | 0 | - |
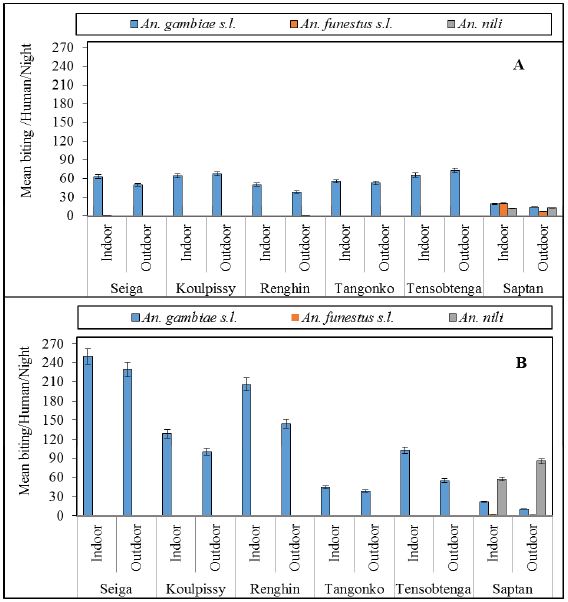
Figure 2: Mean bites per person per night from indoor HLC in the study sites. (A) August 2014 and (B) September 2015 throughout study sites.
West region of Burkina Faso revealed that An. funestus s.l. and An. nili were very dynamic and even playing locally an important role in malaria transmission. It was naturally that we should assume that An. funestus s.l. was tested positive to W. bancrofti infection that was also validated in Ghana and in old reports documenting filariasis transmission pattern in the West region of the country [8,21,23]. It was surprising that these results were not validated at the same time by the two techniques such as LAMP-PCR and conventional PCR as showed by our results. These results could be explained by the fact that the LAMP-PCR should be less specific than the conventional PCR regarding to W. bancrofti. Nevertheless, as this species is abundant and playing a key role in malaria transmission it is fair to consider that it should be a potential vector of W. bancrofti in South-West health region. Then surprisingly An. nili the third vector after the An. gambiae s.l. and An. funestus s.l. was tested positive to W. bancrofti for around 10 pools with LAMP-PCR and only one positive pool with the conventional PCR. Nevertheless, this result confirmed that An. nili was implicated in W. bancrofti as well as malaria transmission in this area biting indoors and outdours being more exophilic (PMI report) So its implication in W. bancrofti transmission remained to be explored as it was the first time this species was found positive. Then knowing that in the field An. nili females were found in relatively high abundance and then no longer infected by W. bancrofti, it is not clear if it was only some specimens infected or if this taxon developed competiveness to transmit LF parasites. Further investigations were recommended in this hotspot area where An. nili is very prolific to better clarify the role of this species in LF transmission. As this species is biting more specifically in some villages outdoor, it can sustain outdoor residual transmission of W. bancrofti where conventional control tool like LLINs cannot be deployed.
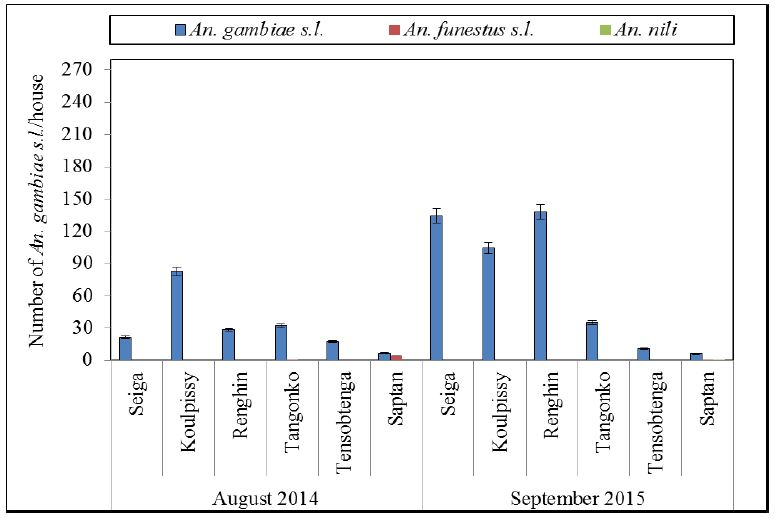
Figure 3:Mean number of mosquitoes collected from indoor PSC.
In other hands, the accuracy of the LAMP technique which had been validated in many studies as the xenomonitoring tool remained to be recalibrated in our context as the results were early step data and need to be extended to a large sampling size and regions [24,25]. Doing so we should perform the two techniques rigorously in the same series of samples to calculate how strongest the margin of error between the two is. Of cause, if xenomonitoring is essential tool for many countries aiming to perform TAS validation studies before the LF elimination beyond the horizon 2030, its validity and accuracy need to be exempt of critics [26].
Nevertheless, and with regard to the prevalence of LF and the infection rates within human in these sites we can state that our results were consistent, following the same trends when one may consider vector or human populations. It is evident that the incidence of LF in these areas is still persisting. Integrated and innovative actions are needed for its elimination by 2030 [26]. These actions can be focused on integrated vector control as luckily LF and malaria share the same vectors in Burkina Faso. In addition it is crucial to implement community-based actions leading to make drugs available for anyone without any discrimination and social inequity during the MDA. But to achieve the success socioanthropological surveys may be implemented in association with entomological investigations to better understand the bottleneck that block the elimination of such long time neglected disease.
Conclusion
The study has documented the persistence of W. bancrofti infection in human and vector populations after more than 11 rounds of mass drug administration in Burkina Faso. Entomological assessment showed that An. gambiae, An. coluzzii, An. funestus and An. nili were the Anopheles species largely dominating in all sites during the collection periods. An. coluzzii was detected positive to W. bancrofti being the potential vector of this parasite in the Centre and East region whereas in the South West, surprisingly An. nili was confirmed by the two molecular techniques positive to W. bancrofti and so considered as potential vector of LF for the first time in Burkina Faso. However, as all these data remain preliminary, further additional surveys and investigations are needed to better calibrate the two molecular techniques. Nevertheless, it raises evidence that the use of molecular diagnostic tool is crucial to better define endpoints for LF elimination.
Figure 4:Anopheles gambiae s.l. species composition assessed per study sites (n=30 per site).
Declarations
Ethics approval
The works performed in this study did not need consent form which was obtained during the previous surveys.
Consent for publication
Not applicable
Availability of data and materials
All data are included in the manuscript
Competing interests
Authors declared that there is no competing interest
Funding
This study was financially supported by the Liverpool School for Tropical Medicine
Authors’ contribution
RKD and KB conceived and designed the study. SC and ASH performed the field study, analyzed the data, and drafted the manuscript. RB assured the laboratory analyses. FAS, AD, FF, RWB, IS, and AGO reviewed the first draft of the article. RKD is guarantor of the study. All authors contributed to the drafts and read and approved the final manuscript.
Acknowledgement
The authors express their gratitude to community health workers for their availability during the study periods. They thank warmly the inhabitants of the study sites for having accepted the implementation of the study in their villages and to have gone with. They thank likewise the national program to fight against neglected tropical diseases for his collaboration.
Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T (2010) Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infection, Genetics and Evolution 10: 159-177. [ Ref ]
Mitra AK, Mawson AR (2017) Neglected tropical diseases: epidemiology and global burden. Tropical medicine and infectious disease 2: 36. [ Ref ]
WHO (2014) Global programme to eliminate lymphatic filariasis: progress report. [ Ref ]
Ouedraogo AN, Somda EB, Traoré F, Ouédraogo MS, Tapsoba GP, et al. (2016) Impact du traitement de masse de la filariose lymphatique par l’albendazole-ivermectine en zone de savane : cas de la région de l’Est du Burkina. Health Sciences and Disease. [ Ref ]
Kima A, Guiguemde KT, Meda ZC, Bougma R, Serme M, et al. (2019) Évaluation de l’impact du traitement médicamenteux de masse contre la filariose lymphatique dans 3 districts sanitaires et implication en santé publique: à propos de 12 sites de surveillance épidémiologique au Burkina Faso. Médecine et Santé Tropicales 29: 55-60. [ Ref ]
Samadoulougou S, Pearcy M, Yé Y, Kirakoya-Samadoulougou F (2017) Progress in coverage of bed net ownership and use in Burkina Faso 2003–2014: evidence from population-based surveys. Malaria journal. BioMed Central 16: 1-12. [ Ref ]
Stanton MC, Molyneux DH, Kyelem D, Bougma RW, Koudou BG, et al. (2013) Baseline drivers of lymphatic filariasis in Burkina Faso. Geospat Health 8: 159-73. [ Ref ]
Pi-Bansa S, Osei JHN, Kartey-Attipoe WD, Elhassan E, Agyemang D, et al. (2019) Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana. Trop Med Infect Dis 4: 49. [ Ref ]
Mehlotra RK, Gray LR, Blood-Zikursh MJ, Kloos Z, Henry-Halldin CN, et al. (2010) Molecular-based assay for simultaneous detection of four Plasmodium spp. and Wuchereria bancrofti infections. The American journal of tropical medicine and hygiene. ASTMH 82: 1030-1033. [ Ref ]
Pi-Bansa S, Osei JHN, Joannides J, Woode ME, Agyemang D, et al. (2018) Implementing a community vector collection strategy using xenomonitoring for the endgame of lymphatic filariasis elimination. Parasites & Vectors. [ Ref ]
Dorkenoo MA, de Souza DK, Apetogbo Y, Oboussoumi K, Yehadji D, et al. (2018) Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: no evidence for active transmission. Parasit Vectors 11: 52. [ Ref ]
Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Publications of the South African Institute for Medical Research 54: 1-343. [ Ref ]
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, et al. (2008) Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria journal 7: 163. [ Ref ]
Costantini C, Sagnon NF, Ilboudo-Sanogo E, Coluzzi M, Boccolini D (1999) Chromosomal and bionomic heterogeneities suggest incipient speciation in Anopheles funestus from Burkina Faso. Parassitologia 41: 595-612. [ Ref ]
Dabiré KR, Baldet T, Diabaté A, Dia I, Costantini C, et al. (2007) Anopheles funestus (Diptera: Culicidae) in a humid savannah area of western Burkina Faso: bionomics, insecticide resistance status, and role in malaria transmission. Journal of medical entomology 44: 990- 997. [ Ref ]
Ramzy RM, Farid HA, Kamal IH, Ibrahim GH, Morsy ZS, et al. (1997) A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans R Soc Trop Med Hyg 91: 156-160. [ Ref ]
Pam DD, de Souza DK, D’Souza S, Opoku M, Sanda S, et al. (2017) Is mass drug administration against lymphatic filariasis required in urban settings? The experience in Kano, Nigeria. PLoS Negl Trop Dis 11: e0006004. [ Ref ]
Takagi H, Itoh M, Kasai S, Yahathugoda TC, Weerasooriya MV, et al. (2011) Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitology international. Elsevier 60: 493-497. [ Ref ]
Katholi CR, Toé L, Merriweather A, Unnasch TR (1995) Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis 172: 1414-1417. [ Ref ]
Kelly-Hope LA, Molyneux DH, Bockarie MJ (2013) Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasit Vectors 6: 39. [ Ref ]
Brengues J, Subra R, Mouchet J, Nelson GS (1968) La transmission de Wuchereria bancrofti Cobbold en Afrique occidentale. Etude préliminaire d’un foyer de savane nord-guinéenne. Bulletin of the World Health Organization 38: 595-608. [ Ref ]
Aboagye-Antwi F, Kwansa-Bentum B, Dadzie SK, Ahorlu CK, Appawu MA, et al. (2015) Transmission indices and microfilariae prevalence in human population prior to mass drug administration with ivermectin and albendazole in the Gomoa District of Ghana. Parasit Vectors 8: 562. [ Ref ]
de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, et al. (2012) Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors 5: 259. [ Ref ]
Okorie PN, de Souza DK (2016) Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 110: 90-97. [ Ref ]
Jones C, Ngasala B, Derua YA, Tarimo D, Reimer L, et al. (2018) Lymphatic filariasis transmission in Rufiji District, southeastern Tanzania: infection status of the human population and mosquito vectors after twelve rounds of mass drug administration. Parasites vectors 11: 588. [ Ref ]
Group NMCLF (2019) The roadmap towards elimination of lymphatic filariasis by 2030: insights from quantitative and mathematical modelling. Gates open research 3: 1538. [ Ref ]