Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 03 November, 2021
Accepted date: 20 December, 2021
Published date: 27 December, 2021
Citation: Huang C, Tang X, Liu J, Guo L, Yuan Y et al (2021) Molecular Serotyping and Resistance Profiling of Haemophilus parasuis (Glaesserella) Clinical Strains Isolated from Chinese Pig Farms between 2016 and 2018. J Appl Microb Res. Vol: 4 Issu: 2 (01-10).
Copyright: © 2021 Huang C et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Haemophilus parasuis is the etiological agent of Glässer’s disease and brings great economic losses to the pig industry. In this study, from 2016 to 2018, 8153 Haemophilus parasuis field strains were isolated from 14610 clinical samples of sick pigs with clinical symptoms from 26 provinces and cities of China. Among them, 9.49% (1386/14610) strains were identified as Haemophilus parasuis by PCR. The highest bacterial isolation rate of effusion was 27.27% (3/11), followed by 13.23% (1312/9914) of lung. Besides, the majority of isolate strains were from Guangdong province (15.3%), Zhejiang province (11.82%) and Hunan province (9.38%). The most popular serotypes in China from 2016 to 2018 were serotype 4 (25.31%) and serotype 5/12 (38.44%), followed by serotype 13 (7.81%), serotype 14 (6.56%) and serotype 1 (5.31%).
The susceptibility of 166 Haemophilus parasuis isolates to 18 drugs was determined by disk-diffusion method. The results showed that more than 90% of the isolates were sensitive to polymyxin B (96.99%), cefradine (96.39%), ceftriaxone (92.17%) and florfenicol (91.57%). Besides, about 50% of isolates were resistant to ciprofloxacin (54.82%), streptomycin (51.20%) and ampicillin (48.80%). The 166 Haemophilus parasuis isolates showed 94 drug resistance profiles, 3.01% (5/166) of the isolates were sensitive to all 18 drugs tested, 15.06% (25/166) of the isolates showed resistance to 1 to 2 drugs, 43.37% (72/166) of the isolates showed resistance to 3 to 4 drugs. 38.55% (64/166) of the isolates showed resistance to 5 drugs and so on, as compared to 10 years ago, the resistance of Haemophilus parasuis has become more serious which only 23.6% of the isolates showed resistance to 3 or more drugs and only 1 isolate showed resistance to 7 drugs in 2008. In this study, the prevalence and drug resistance of Haemophilus parasuis in China from 2016 to 2018 were reported for the first time. The data provides theoretical guidance for the prevention and control strategies of Haemophilus parasuis in China.
Keywords
Haemophilus parasuis, Serovars, Drug resistance, China.
Abstract
Haemophilus parasuis is the etiological agent of Glässer’s disease and brings great economic losses to the pig industry. In this study, from 2016 to 2018, 8153 Haemophilus parasuis field strains were isolated from 14610 clinical samples of sick pigs with clinical symptoms from 26 provinces and cities of China. Among them, 9.49% (1386/14610) strains were identified as Haemophilus parasuis by PCR. The highest bacterial isolation rate of effusion was 27.27% (3/11), followed by 13.23% (1312/9914) of lung. Besides, the majority of isolate strains were from Guangdong province (15.3%), Zhejiang province (11.82%) and Hunan province (9.38%). The most popular serotypes in China from 2016 to 2018 were serotype 4 (25.31%) and serotype 5/12 (38.44%), followed by serotype 13 (7.81%), serotype 14 (6.56%) and serotype 1 (5.31%).
The susceptibility of 166 Haemophilus parasuis isolates to 18 drugs was determined by disk-diffusion method. The results showed that more than 90% of the isolates were sensitive to polymyxin B (96.99%), cefradine (96.39%), ceftriaxone (92.17%) and florfenicol (91.57%). Besides, about 50% of isolates were resistant to ciprofloxacin (54.82%), streptomycin (51.20%) and ampicillin (48.80%). The 166 Haemophilus parasuis isolates showed 94 drug resistance profiles, 3.01% (5/166) of the isolates were sensitive to all 18 drugs tested, 15.06% (25/166) of the isolates showed resistance to 1 to 2 drugs, 43.37% (72/166) of the isolates showed resistance to 3 to 4 drugs. 38.55% (64/166) of the isolates showed resistance to 5 drugs and so on, as compared to 10 years ago, the resistance of Haemophilus parasuis has become more serious which only 23.6% of the isolates showed resistance to 3 or more drugs and only 1 isolate showed resistance to 7 drugs in 2008. In this study, the prevalence and drug resistance of Haemophilus parasuis in China from 2016 to 2018 were reported for the first time. The data provides theoretical guidance for the prevention and control strategies of Haemophilus parasuis in China.
Keywords
Haemophilus parasuis, Serovars, Drug resistance, China.
Introduction
Haemophilus parasuis (H. parasuis) is pleomorphic and belongs to Pasteurellaceae family. H. parasuis is a NADdependent, Gram-negative bacterium [1]. H. parasuis can cause Glässer’s disease, pneumonia and septicemia during different breeding periods [2]. H. parasuis has brought serious economic losses to the pig industry [3]. Scientists had studied the epidemiology and pathogenesis of H. parasuis, which contains 15 serovars and a large number of non-typeable (NT) isolates [4]. Because of the diversity of the H. parasuis genotype, the prevention and treatment are particularly difficult, such as the low cross-protection of vaccines and antibiotic resistance [5].
The pathogenesis of H. parasuis is very complex, which is related to virulence genes, serum and biofilm production [6]. There are many Serotyping methods for H. parasuis, including coagglutination test (CA), agar gel diffusion (AGD), indirect hemagglutination (IHA) and a multiplex PCR [7,8]. In 2015, Howell et al. established a multiplex PCR method which could be used to quickly identify serotypes of H. parasuis. The authors designed 14 pairs of primers for typing. Among them, serotype 5 and serotype 12 shared a pair of primers and cannot be distinguished. In addition, one pair of primers were used to identify H. parasuis [8].
To date, a total of 15 serotypes of H. parasuis had been identified, including serovars 1 to 15. However, the scientists could not discriminate between serovars 5 and 12 [8]. The serovars 1, 5, 10, 12, 13, and 14 were regarded highly virulent; the serovars 2, 4, 8 and 15 were regarded moderately virulent; the serovars 3, 6, 7, 9 and 11 were considered low virulent [7]. The serovars 5 and 4 of H. parasuis were widely regarded as pathogenic serums and were the most common serovars isolated from clinically sick pigs worldwide [9].
The abuse of antibiotics and the lack of biosafety knowledge hinder the prevention and control of H. parasuis [1]. More and more clinical studies had shown that the protection of inactivated vaccine was mainly against isolates of the same serovars, and its cross-protection was extremely limited [10]. Therefore, up to now, the outbreak of H. parasuis due to vaccination failure is a major concern for researchers and pig farmers. In order to prevent and control H. parasuis safely and effectively, developing an effective vaccine is still the best choice at present [11,12]. However, the key to the development of a vaccine is to find a highly virulent and widespread strain in our country, therefore epidemiological investigation is a necessary process.
With the large-scale and intensive development of the pig industry, the incidence of H. parasuis is increasing year by year, but the cross-protection of H. parasuis vaccines between different serotypes are poor. In order to avoid huge economic losses, pig farms have to rely on antibiotics to control this disease. Therefore, it is necessary to screen drugs based on the results of in-vitro drug sensitivity tests.
In this study, the serotypes of H. parasuis isolated from large-scale pig farms in China from 2016 to 2018 and the drug resistance of 166 H. parasuis to 18 common antibiotics, which played a positive role in the prevention and control of H. parasuis in China.
Materials and Methods
Clinical isolates
From January 2016 to December 2018, a total of 14610 clinical samples included lungs, joints, effusion, spleen, brain, liver, kidneys and heart were collected from 30 to 70 days old pigs suspected of being infected with H. parasuis by veterinarians from many pig farms. The clinical samples covered 26 provinces and municipalities. All clinical samples were stored at −80 ℃. Detailed information was recorded for each clinical sample, such as viscera, time, location and clinical symptoms.
Bacterial isolation and identification
Clinical samples were streaked onto tryptic soy agar (TSA) plates (TSA; Becton, Dickinson and Company, Franklin Lakes NJ, USA) containing 10 μg/ml NAD (NAD; Roche, Basel, Switzerland) and 5% newborn calf serum (Gibco, New York, USA), and then incubated at 37 °C for 36 h. The suspect colonies should be translucent colonies of 1 mm in diameter and be subjected to further identification by Gram staining and PCR [13]. Sequences of primers used in multiplex PCR were showed in table S1.
DNA preparation and Serotyping
For the serotyping of H. parasuis, the bacteria were grown in tryptic soy broth (TSB; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for overnight at 37 °C and bacteria suspension was spinned down at 10,000 × g for 5 min and then resuspended in phosphate buffered saline (pH=7.4). The suspension was heated at 100 °C for 10 min and followed by a centrifugation under 10,000×g for 5 min. The supernatant was collected to a nucleic acidfree tube and stored at −20 °C. The serovars of H. parasuis were determined by multiplex PCR [8]. The genomes of H. parasuis were used as template for multiple PCR, and PCR reaction system was 20 μL, including 1 μL template and 1 μL upstream and downstream primers, respectively, 2×DNA Taq Mix 10 μL and ddH2O up to 20 μL. The PCR amplification program was 95oC for 10 min ; 94oC for 30 s ; 56oC for 30 s ;72oC for 1 min ; total 35 cycles ; then 72oC for 10 min, at last decreased to 4oC. The results were confirmed by two repeated experiments. The PCR products were stained with GelRedTM (Biotium, Fremont, CA, USA) and performed gel electrophoresis in a 1.0 % agarose gel in Tris-acetate-EDTA (TAE) buffer at 120 volts for about 20-30 min.
Drug susceptibility test
A disk diffusion method was used to evaluate the drug resistance profiles of 166 isolates, the drug sensitive disks used in this research were purchased from Hangzhou Tianhe Microbial Reagent Company. The drugs used mainly include Cefradine (CEF), Ceftriaxone (CRO), Amoxicillin (AML), Ampicillin (AMP), Streptomycin (STR), Gentamicin (GEN), Spectinomycin (SPE), Kanamycin (KAN), Azithromycin (AZM), Levofloxacin (LEV), Ciprofloxacin (CIP), Enrofloxacin (ENO), Polymycin B (PB), Cefotaxime (CAZ), Cefotaxime (CTX), Amikacin (AMI), Norfloxacin (NOR) and Florfenicol (FLO).
Table 1: Purification Table for Pectinase from Bacillus subtilis.
| Province | Number of samples | Number of positive samples | Isolation rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | Total | 2016 | 2017 | 2018 | Total | 2016 | 2017 | 2018 | Total | |
| Hu Bei | 1941 | 1829 | 1682 | 5452 | 176 | 161 | 91 | 428 | 9.07 | 8.80 | 5.41 | 7.85 |
| GuangDong | 889 | 1043 | 839 | 2771 | 146 | 168 | 110 | 424 | 16.42 | 16.11 | 13.11 | 15.30 |
| He Nan | 324 | 921 | 990 | 2235 | 28 | 79 | 75 | 182 | 8.64 | 8.58 | 7.58 | 8.14 |
| Hu Nan | 298 | 291 | 168 | 757 | 24 | 35 | 12 | 71 | 8.05 | 12.03 | 7.14 | 9.38 |
| Si Chuan | 220 | 216 | 71 | 507 | 14 | 16 | 5 | 35 | 6.36 | 7.41 | 7.04 | 6.90 |
| Fu Jian | 78 | 198 | 173 | 449 | 8 | 12 | 19 | 39 | 10.26 | 6.06 | 10.98 | 8.69 |
| Shan Dong | 40 | 184 | 116 | 340 | 2 | 23 | 6 | 31 | 5.00 | 12.50 | 5.17 | 9.12 |
| An Hui | 97 | 139 | 94 | 330 | 7 | 7 | 12 | 26 | 7.22 | 5.04 | 12.77 | 7.88 |
| Guang Xi | 57 | 101 | 133 | 291 | 2 | 12 | 12 | 26 | 3.51 | 11.88 | 9.02 | 8.93 |
| Jiang Su | 63 | 122 | 92 | 277 | 4 | 5 | 10 | 19 | 6.35 | 4.10 | 10.87 | 6.86 |
| Zhe Jiang | 103 | 58 | 59 | 220 | 8 | 6 | 12 | 26 | 7.77 | 10.34 | 20.34 | 11.82 |
| Jiang Xi | 85 | 58 | 45 | 188 | 7 | 3 | 5 | 15 | 8.24 | 5.17 | 11.11 | 7.98 |
| He Bei | 20 | 77 | 90 | 187 | 2 | 9 | 8 | 19 | 10.00 | 11.69 | 8.89 | 10.16 |
| Shan Xi | 73 | 51 | 15 | 139 | 10 | 2 | 1 | 13 | 13.70 | 3.92 | 6.67 | 9.35 |
| San Xi | 54 | 71 | 0 | 125 | 4 | 3 | 0 | 7 | 7.41 | 4.23 | 0 | 5.60 |
| Gui Zhou | 2 | 39 | 18 | 59 | 0 | 4 | 0 | 4 | 0 | 10.26 | 0 | 6.78 |
| Xin Jiang | 2 | 35 | 22 | 59 | 0 | 5 | 0 | 5 | 0 | 14.29 | 0 | 8.47 |
| Nei Meng | 20 | 24 | 11 | 55 | 0 | 5 | 0 | 5 | 0 | 20.83 | 0 | 9.09 |
| Liao Ning | 16 | 17 | 6 | 39 | 1 | 3 | 0 | 4 | 6.25 | 17.65 | 0 | 10.26 |
| Hai Nan | 10 | 13 | 12 | 35 | 0 | 2 | 0 | 2 | 0 | 15.38 | 0 | 5.71 |
| Chong Qing | 9 | 20 | 0 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shang Hai | 6 | 12 | 6 | 24 | 1 | 1 | 1 | 3 | 16.67 | 8.33 | 16.67 | 12.50 |
| Gan Su | 20 | 3 | 0 | 23 | 2 | 0 | 0 | 2 | 10.00 | 0 | 0 | 8.70 |
| Bei Jing | 0 | 7 | 2 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tian Jin | 8 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ji Lin | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
This study refers to the standard CLSI M2 A12 Ed. 12 (2015)《Performance Standards for Antimicrobial Disk Susceptibility Tests; approved standard - twelfth edition》 for drug susceptibility testing. The specific method was picking a purified single colony in the ultra-clean workbench and inoculated in TSB medium and cultured in a shaker at 37 ℃ for about 12h forming bacterial suspension, then dipped a sterile cotton swab into the bacterial suspension and spread on a TSA agar plate containing 10 μL/mL NAD and 5% (v/v) inactivated bovine serum, after the plate dry for 3 min, using sterilized tweezers to pick up the drug sensitive disk and place it on the dried plate. There are no more than 6 drug sensitive disk on each plate. At last, placing the plate at 37°C for 24 to 36 h, and measuring the diameter of the inhibition zone.
Data analysis
Data from all samples were analyzed as descriptive statistics. All data were statistically analyzed by GraphPad software (GraphPad software®, La Jolla, CA, USA).
Results
Prevalence of H. parasuis
From January 2016 to December 2018, 8153 strains were isolated from 14,610 samples in 26 provinces from 30 to 70 days old pigs suspected of being infected with H. parasuis. Through morphological observation, Gram stain (Figure1A) and PCR identification (Figure 1B), 1386 strains of H. parasuis were finally confirmed, and the isolation rate was 9.49%.
Table 2: Drug sensitivity and resistance rates of H. parasuis isolates in China.
| Antibiotics category | Drug sensitive slips | Sensitive rates | Resistance rates (%) |
|---|---|---|---|
| β-lactams | Amoxicillin | 79.52 | 9.64 |
| Ampicillin | 43.98 | 48.80 | |
| Cefradine | 96.39 | 3.01 | |
| Ceftriaxone | 92.17 | 7.83 | |
| Cefotaxime | 84.34 | 12.05 | |
| Cefotaxime | 82.53 | 8.43 | |
| Aminoglycosides | Amikacin | 50.60 | 35.54 |
| Kanamycin | 45.18 | 29.52 | |
| Streptomycin | 36.75 | 51.20 | |
| Gentamicin | 71.69 | 21.08 | |
| Spectinomycin | 60.84 | 29.52 | |
| Macrolides | Azithromycin | 80.72 | 13.86 |
| Polypeptides | Polymyxin B | 96.99 | 3.01 |
| Quinolones | Enrofloxacin | 78.31 | 21.08 |
| Ciprofloxacin | 44.58 | 54.82 | |
| Norfloxacin | 59.04 | 36.14 | |
| Levofloxacin | 63.86 | 33.73 | |
| Chloramphenicol | Florfenicol | 91.57 | 8.43 |
Figure 1: Prevalence of H. parasuis. The H. parasuis was identified by Gram stain (A) and PCR (B).
The site and geographical distribution of H. parasuis isolation
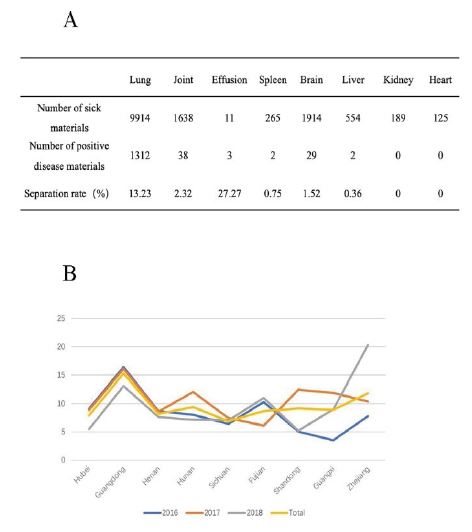
Between 2016 and 2018, the separation rate of samples from different tissues was analyzed statistically in order to find out the isolation of H. parasuis from different tissues. The result shows that the highest bacterial isolation rate of effusion was 27.27% (3/11), followed by 13.23% (1312/9914) of lung. A small amount of H. parasuis could also be isolated from joints, spleen, brain and liver, while it could not be isolated from kidney and heart (Figure 2A).
In order to find out the status of H. parasuis infection in different areas of China in recent three years, the isolation rate of samples in various provinces were counted in detail, the results showed that the majority isolate strains were from Guangdong province (15.3%), Zhejiang province (11.82%) and Hunan province (9.38%) (Figure 2B).
Figure 2: The site and geographical distribution of H. parasuis isolation. The H. parasuis was separated from different organs, such lung, joint, effusion, spleen, brain, liver, kidney and heart (A). The nine provinces in China where H. parasuis are most commonly distributed (B).
The separation rates of different H. parasuis serotypes
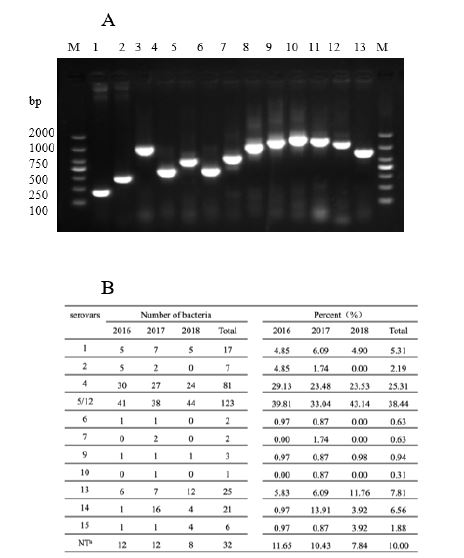
From 2016 to 2018, a total of 1386 strains of H. parasuis were identified, including 446 strains in 2016, 561 strains in 2017 years, and 379 strains in 2018 years. The serotypes of H. parasuis isolates were identified by PCR (Figure 3A, from left to right: serotype 1, 2, 3, 4, 5/12, 6, 7, 9, 10, 11, 13, 14 and 15. Serotype 3, serotype 8 and serotype 11 strains were not identified in this study, and serotype 3 and serotype 11 positive controls were laboratory preserved positive strains). The results showed that the serotypes of the isolates were different in different years. In 2016, H. parasuis serotype 4 was the most common isolate (29.13%), followed by serotype 5/12 (39.81% in total) (Figure 3B). In 2017, the highest prevalence was serotype 4 (23.48%), followed by serotype 5/12 (33.04% in total) and serotype 14 (13.91%) (Figure 3B). In 2018, the most common serotypes were serotype 5/12 (43.14%) and serotype 4 (23.53%), followed by serotype 13 (11.76%) (Figure 3B). In general, the most popular serotypes in China from 2016 to 2018 were serotype 4 (25.31%) and serotype 5/12 (38.44%), followed by serotype 13 (7.81%), serotype 14 (6.56%) and serotype 1 (5.31%).
Figure 3: The separation rates of different isolates of H. parasuis serotypes. The H. parasuis serovars were identified by PCR (A). Distribution of 13 H. parasuis serovars in China from 2016 to 2018 (B).
Geographical distribution of different H. parasuis serotypes
In order to understand the infection of H. parasuis in different regions of China in recent years, the separation rate of samples sent by provinces and municipalities from 2016 to 2018 were analyzed, the results were shown in table 1.
Among all provinces with no less than 100 samples submitted for detection in 2016, Guangdong Province accounted for the highest separation rate (16.42%, 146/1941), followed by Hubei Province (9.07%, 176/1941) and Henan Province (8.64%, 28/ 324), In addition, the separation rates of Hunan Province (8.05%, 24/298), Zhejiang Province (7.77%, 8/103), and Sichuan Province (6.36%, 14/220) were successively reduced. In 2017, Guangdong Province accounted for the highest separation rate(16.11%, 168/1043), followed by Shandong Province (12.5%, 23/184) and Hunan Province (12.03%, 35/291), the separation rate of Guangxi Province (11.88%, 12/101), Hubei Province (8.8%, 161/1829), Henan Province (8.58%, 79/921), Sichuan Province (7.41%, 16/216), Fujian Province (6.06, 12/198), Anhui Province (5.04%, 7/139), Shaanxi Province (4.23%, 3/71), Jiangsu Province (4.1%, 5/122) and Shanxi Province (3.92%, 2/51) decreased in turn. In 2018, Zhejiang Province accounted for the highest separation rate (20.34%, 12/59), followed by Guangdong Province (13.11%, 110/839), besides, the separation rate of Fujian Province (10.98%, 19/173), Guangxi Province (9.02%, 12/133), Henan Province (7.58%, 75/990), Hunan Province (7.14%, 12/168), Hubei Province (5.41%, 91/1682) and Shandong Province (5.17%, 6/116) decreased in turn.
Among all provinces with no less than 200 samples submitted for detection in three years, Guangdong Province accounted for the highest separation rate (16.42%, 146/1941), followed by Zhejiang Province (11.82%, 26/220), besides, Hunan Province (9.38%, 71/757), Shandong Province (9.12%, 31/340), Guangxi Province (8.93%, 26/291), Fujian Province ( 8.69%, 39/449), Henan Province (8.14%, 182/2235), Anhui Province (7.88%, 26/330), Hubei Province (7.85%, 428/5452), Sichuan Province (6.9%, 35/507 ) and Jiangsu Province (6.86%, 19/277) decreased in turn.
The antibiotic susceptibility Testing Results of H. parasuis
The antibiotic susceptibility testing results of H. parasuis were shown in table 2, as seen from this table, H. parasuis was more sensitivity to macrolide antibiotics, polypeptide antibiotics, chloromycetin, and β-lactam antibiotics (except ampicillin), among the 18 selected drugs tested, H. parasuis showed the highest sensitivity to polymyxin B (96.99%, 161/166) and cefradine (96.39%, 160/166), followed by ceftriaxone (92.17%, 153/166), florfenicol (91.57%, 152/166), cefotaxime (84.34%, 140/166), ceftazidime (82.53%, 137/166) and azithromycin (80.72%, 134/166). At the same time, H. parasuis was resistant to ciprofloxacin (54.82%, 91/166), streptomycin (51.20%, 85/166), ampicillin (48.80%, 81/166), norfloxacin (36.14%, 60/166), amikacin (35.54%, 59/166) and levofloxacin (33.73%, 56/166).
Drug resistance profiles of H. parasuis isolates
The drug sensitivity analysis of 166 H. parasuis isolates found that 18 tested drugs included a total of 94 drug resistance profiles. As shown in table S2, only 5 isolates were sensitive to all tested drugs, 10 isolates were resistant to 1 tested drug, 15 isolates were resistant to 2 tested drugs, 33 isolates were resistant to 3 tested drugs, and 39 isolates were resistant to 4 tested drugs resistance, 21 isolates were resistant to 5 tested drugs, 16 isolates were resistant to 6 tested drugs, 13 isolates were resistant to 7 tested drugs, 10 The isolates were resistant to 8 tested drugs, the tested isolates were mainly resistant to 3-5 tested drugs.
Discussion
H. parasuis is a Gram-negative, nicotinamide adenine dinucleotide dependent bacterium, which can cause Glässer’s disease in pigs [14]. H. parasuis usually appear in swine respiratory tract, causes systemic infections, pneumonia, fibrin polyserositis, polyarthritis and meningitis [15]. This bacterial infectious disease can infect pigs of any age, which brings serious influences to the pig breeding industry. Although the mortality rate of pigs is relatively low, it will seriously affect the disease resistance of the pig herd, resulting in decreased immune function and easy to be infected with a variety of infectious diseases, following with complex clinical symptoms, making disease diagnosis difficult. At present, prevention and control of H. parasuis are difficult, the trend of large-scale and intensive development of the pig industry in our country are becoming more and more obvious recent years, and the prevalence of H. parasuis diseases is becoming diversified and complicated, which often presents as a secondary or mixed infection, and brings great difficulties to the diagnosis and treatment of diseases. In order to reduce the economic damage caused by Glässer’s disease, a kind of inactivated whole cell vaccine is widely used in the world [16]. While inactivated whole cell vaccine does not produce local immunity and cell-mediated immunity ability is weak, so the immunity is slow, and good immunity is usually obtained 2 weeks after vaccination. Now, subunit vaccine is currently the best research direction for researchers [14].
This research on H. parasuis serotypes isolated from large-scale pig farms in China during 2016-2018 provides guidance for the direction of vaccine research and development, which is of great significance for the study of H. parasuis serotypes in China.
In this study, 8153 strains of H. parasuis from 14610 disease materials derived from 26 provinces and cities of China were identified, the sample size and typing method were larger and more representative. 320 H. parasuis strains were selected for serotyping by multiplex PCR. Molecular typing was an excellent alternative test compared to regular serotyping (gel immunodiffusion, Kielstein and Rapp- Gabrielson scheme), it was very cumbersome to perform because of the necessity of producing specific antisera [16]. The Kielstein-Rapp-Gabrielson agar diffusion method for serotyping of H. parasuis was classic serological typing method, which could identify 15 serotypes, but 15 high immune serums were required, and about 20% of strains could not be typed. The multiple PCR molecular serotyping method was established by Howell et al in 2015 to serotype 320 H. parasuis isolates. The results showed that only 10% of the isolates could not be typed. The molecular method was easy to operate and spend less time, but it was worth noting that this method could not distinguish between serotype 5 and serotype 12. Besides, a multiplex PCR assay and a specific PCR reaction for the H. parasuis serotyping were more precise [8].
According to the research on the serotype of H. parasuis, the most prevalent serotypes were serotype 5, followed by serotype 2 and serotype 4 in Quang Binh and Thua Thien Hue provinces in Central Vietnam [17]. One study about the prevalence and characteristics of H. parasuis from healthy pigs in China from 2016 to 2017 showed that the most prevalent serovars were 7, followed by 3, 2, 11, 5/12 and 4 [18]. Besides, there were other research about the most prevalent serotypes of H. parasuis, 4, 5, 12, 13, NT (nontypeable isolates), and 2 were the most prevalent strains in southern China [19]. One research in Sichuan province of China showed that Serovars 5 (25.98%) and 4 (23.62%) were the most prevalent Serotypes [3]. The results of this study indicate that serotype 4 (25.31%) and serotype 5/12 (38.44%) were the most popular strains in China, followed by serotype 13 (7.81%) and serotype 14 (6.56%). Only a few strains of types 1, 2, 6, 7, 9, 10, and 15 had been identified. Serotypes 3, 8, and 11 were not identified in this study. This might be because the collected disease materials mainly come from sick pigs with suspected symptoms of H. parasuis. In the research of Cai Xuwang, KPG agar diffusion test identified the existence of H. parasuis serotype 11 isolates in China from 2002 to 2004. Zhou Xueli used the same method identified the existence of H. parasuis serotype 3 and serotype 8 in China from 2007 to 2008. Up to now, 15 serotypes of H. parasuis were distributed in China. Most of the isolates used for serotyping in this study were from Guangdong, Hubei and Henan provinces, while the number of samples submitted for inspection in other provinces was relatively small and not representative. In these three provinces, serotype 4 and serotype 5/12 were the most prevalent. In addition, serotypes 1, 13, and 14 were prevalent in Guangdong and Henan provinces; serotypes 1, 13, 14, and 15 were prevalent in Hubei province. From the statistical results, the main serotypes in Henan, Hubei provinces and Guangdong Province were roughly same, and the percentages of each serotype were slightly different, but the most prevalent serotypes in China were types 4 and 5 [20]. While one previous study also studied the isolation of H. parasuis in China from September 2016 to October 2017, they obtained 244 isolates from 1675 nasal samples from 6 provinces, H. parasuis isolation was more successful in weaner pigs (22.6%, 192/849), followed by finisher pigs (9.3%, 43/463), and sows (2.5%, 9/363). The most prevalent serovar was type 7 (20.1%, 49/244), followed by type 3 (14.8%, 36/244), type 2 (14.3%, 35/244), type 11 (12.7%, 31/244), type 5/12 (5.7%, 14/244) and type 4 (2.5%, 6/244).
Combined with data analysis over the past three years, the incidence of H. parasuis in coastal areas of high temperature and humidity such as Guangdong, Fujian, Zhejiang and Guangxi were relatively high. This might be due to the local climates were suitable for bacterial growth and reproduction, because climates were very important factors affecting the growth of bacteria [21].
Most Clinical studies had shown that the protection of inactivated vaccines were mainly against isolates of the same serovars, but its cross-protection was extremely limited, for this reason, choosing inactivated vaccines of local epidemic serotypes was more beneficial to defend against H. parasuis [10]. Different regions and combination of protective antigens might be able to provide effective protection against multiple H. parasuis serovars [22]. But for most farmers, the treatment of H. parasuis still mainly relies on antibiotic therapy. The rational application of antibiotics can effectively inhibit pathogenic microorganisms and reduce the occurrence of animal diseases, but the widespread abuse of antibiotics will also bring great problems, such as the increase of bacterial resistance and drug resistance mechanism, so the rational use of antibiotics is particularly important, and the development of new vaccines is also a way to prevent and treat diseases.
The susceptibility testing results in this study showed that H. parasuis was more sensitivity to macrolide antibiotics, polypeptide antibiotics, chloromycetin, and β-lactam antibiotics (except ampicillin) among all 18 selected drugs tested, H. parasuis showed the highest sensitivity to polymyxin B and cefradine, followed by ceftriaxone, florfenicol, cefotaxime, ceftazidime and azithromycin. At the same time, H. parasuis was resistant to ciprofloxacin, streptomycin, ampicillin, norfloxacin, amikacin and levofloxacin. The drug sensitivity analysis of 166 H. parasuis isolates found that 18 drugs tested included a total of 94 drug resistance profiles. Only 5 isolates were sensitive to all tested drugs. The number of resistant strains increased significantly and a wider spectrum of resistan compared to previous studies, which warns us to use antibiotics prudently and standardized.
Although, H. parasuis was very sensitive to the peptide antibiotic polymyxin B, but peptide antibiotics was regarded as the last line of defense against bacteria in clinical, despite the bacteriostatic effect was very remarkable, they should be used carefully.
Conclusion
Haemophilus parasuis (H. parasuis), the causative agent of Glässer’s disease, which seriously affected the global pig breeding industry. This research showed a national trend of H. parasuis in China, this study was carried on from 2016 to 2018 and 8153 of H. parasuis field strains were isolated from 14610 clinical samples collected from sick pigs with clinical symptoms from 26 provinces and cities of China, among them, 1386 strains were identified as H. parasuis by PCR, and the isolation rate was 9.49%.
320 of H. parasuis strains were serotyped by multiplex PCR, and the results showed that type 5/12 and type 4 strains occupied the highest proportion respectively were 38.44% and 25.31%, followed by type 13 and type 14 strains respectively were 7.81% and 6.56%, besides, 10% of isolates cannot be typed by this method.
Drug susceptibility test in this study showed that H. parasuis was very sensitivity to polymyxin B and cefradine, then ceftriaxone, florfenicol, cefotaxime, ceftazidime and azithromycin. At the same time, H. parasuis was resistant to ciprofloxacin, streptomycin, ampicillin, norfloxacin, amikacin and levofloxacin.
In general, the results of this study revealed the diversity and distribution of different serotypes of H. parasuis across the country and the resistance characteristics of isolates, which were essential for the prevention and treatments of H. parasuis in China.
Acknowledgement
The author would like to thank the diagnostic Center of Wuhan Keqian Biology Co., Ltd for providing the strain.
Ethics Statement
All animal experimental procedures were performed in accordance with the Hubei Regulations for the Administration of Affairs Concerning Experimental Animals. Animal experiments in this study were subject to approval by the Hubei Province Science and Technology Department, concerning experimental animal ethics. The experiments were carried out under the supervision and inspection of the Scientific Ethical Committee for Experimental Animals of Huazhong Agricultural University, Wuhan, China.
Additional Information and Declarations
Funding
This research was partially funded by Wuhan Keqian Biology Co., Ltd.
Author Contributions
Jinjin Liu designed and performed the experiments, analyzed the data, and wrote the manuscript. Long Guo, Yi Yuan, Wenbo Song, Qianqian Li, Ying Huang, Yunzhi Long, Liu Yang, Daobing Yu and Gong Liang performed the experiments. Chao Huang and Xibiao Tang conceived the project, analyzed the data, and wrote the manuscript.
Oliveira S, Pijoan C (2004) Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet Microbiol 99: 1-12. [ Ref ]
Oliveira S, Galina L, Pijoan C (2001) Development of a PCR test to diagnose Haemophilus parasuis infections. J Vet Diagn Invest 13: 495-501. [ Ref ]
Wang Z, Zhao Q, Wei H, Wen X, Cao S, et al. (2017) Prevalence and seroepidemiology of Haemophilus parasuis in Sichuan province, China. Peer J 5: e3379. [ Ref ]
Zhang J, Xu C, Guo L, Ke B, Ke C, et al. (2011) A rapid pulsed-field gel electrophoresis method of genotyping Haemophilus parasuis isolates. Letters in applied microbiology 52: 589-595. [ Ref ]
del Río ML, Martín CB, Navas J, Gutiérrez-Muñiz B, Rodríguez-Barbosa JI, et al. (2006) aroA gene PCR-RFLP diversity patterns in Haemophilus parasuis and Actinobacillus species. Res Vet Sci 80: 55-61. [ Ref ]
de la Fuente AJ, Tucker AW, Navas J, Blanco M, Morris SJ, et al. (2007) Antimicrobial susceptibility patterns of Haemophilus parasuis from pigs in the United Kingdom and Spain. Vet Microbiol 120: 184-191. [ Ref ]
Kielstein P, Rapp-Gabrielson VJ (1992) Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol 30: 862-865. [ Ref ]
Howell KJ, Peters SE, Wang J, Hernandez-Garcia J, Weinert LA, et al. (2015) Development of a Multiplex PCR Assay for Rapid Molecular Serotyping of Haemophilus parasuis. J Clin Microbiol 53: 3812-3821. [ Ref ]
Guizzo JA, Chaudhuri S, Prigol SR, Yu RH, Dazzi CC, et al. (2018) The amino acid selected for generating mutant TbpB antigens defective in binding transferrin can compromise the in vivo protective capacity. Sci Rep 8: 7372. [ Ref ]
Bak H, Riising HJ (2002) Protection of vaccinated pigs against experimental infections with homologous and heterologous Haemophilus parasuis. Vet Rec 151: 502-505. [ Ref ]
Miniats OP, Smart NL, Rosendal S (1991) Cross protection among Haemophilus parasuis strains in immunized gnotobiotic pigs. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 55: 37-41. [ Ref ]
Smart NL, Miniats OP (1989) Preliminary assessment of a Haemophilus parasuis bacterin for use in specific pathogen free swine. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 53: 390-393. [ Ref ]
Howell KJ, Weinert LA, Chaudhuri RR, Luan SL, Peters SE, et al. (2014) The use of genome wide association methods to investigate pathogenicity, population structure and serovar in Haemophilus parasuis. BMC Genomics 15: 1179. [ Ref ]
Liu H, Xue Q, Zeng Q, Zhao Z (2016) Haemophilus parasuis vaccines. Vet Immunol Immunopathol 180: 53-58. [ Ref ]
Cai X, Chen H, Blackall PJ, Yin Z, Wang L, et al. (2005) Serological characterization of Haemophilus parasuis isolates from China. Vet. Microbiol 111: 231-236. [ Ref ]
Pires Espindola J, Balbinott N, Trevisan Gressler L, Machado G, Silene Klein C, et al. (2019) Molecular serotyping of clinical strains of Haemophilus (Glaesserella) parasuis brings new insights regarding Glasser’s disease outbreaks in Brazil. PeerJ 7: e6817. [ Ref ]
Van CN, Thanh TVT, Zou G, Jia M, Wang Q, et al. (2019) Characterization of serotypes and virulence genes of Haemophilus parasuis isolates from Central Vietnam. Vet Microbiol 230: 117-122. [ Ref ]
Zhang P, Zhang C, Aragon V, Zhou X, Zou M, et al. (2019) Investigation of Haemophilus parasuis from healthy pigs in China. Vet Microbiol 231: 40-44. [ Ref ]
Jia A, Zhou R, Fan H, Yang K, Zhang J, et al. (2017) Development of Serotype-Specific PCR Assays for Typing of Haemophilus parasuis Isolates Circulating in Southern China. J Clin Microbiol 55: 3249-3257. [ Ref ]
Zhou X, Xu X, Zhao Y, Chen P, Zhang X, et al. (2010) Distribution of antimicrobial resistance among different serovars of Haemophilus parasuis isolates. Veterinary microbiology 141: 168-173. [ Ref ]
Liu C, Hofstra N, Franz E (2016) Impacts of Climate and Management Variables on the Contamination of Preharvest Leafy Greens with Escherichia coli. Journal of food protection 79: 17-29. [ Ref ]
Li M, Cai RJ, Song S, Jiang ZY, Li Y, et al. (2017) Evaluation of immunogenicity and protective efficacy of recombinant outer membrane proteins of Haemophilus parasuis serovar 5 in a murine model. PLoS One 12: e0176537. [ Ref ]
Table s1: Sequences of primers used in multiplex PCR.
| Primers | Primer sequence (5'→3') | PCR product / bp |
|---|---|---|
| HPS-1-F | CTGTGTATAATCTATCCCCGATCATCAGC | 180 |
| HPS-1-R | GTCCAACAGAATTTGGACCAATTCCTG | |
| HPS-2-F | CTAACAAGTTAGGTATGGAGGGTTTTGGTG | 295 |
| HPS-2-R | GGCACTGAATAAGGGATAATTGTACTG | |
| HPS-3-F | CATGGTGTTTATCCTGACTTGGCTGT | 650 |
| HPS-3-R | TCCACATGAGGCCGCTTCTAATATACT | |
| HPS-4-F | GGTTAAGAGGTAGAGCTAAGAATAGAGG | 320 |
| HPS-4-R | CTTTCCACAACAGCTCTAGAAACC | |
| HPS-5-F | CCACTGGATAGAGAGTGGCAGG | 450 |
| HPS-5-R | CCATACATCTGAATTCCTAAGC | |
| HPS-6-F | GATTCTGATGATTTTTGGCTGACGGAACG | 360 |
| HPS-6-R | CCTATTCTGTCTATAAGCATAGACAGGAC | |
| HPS-7-F | CTCCGATTTCATCTTTTCTATGTGG | 490 |
| HPS-7-R | CGATAAACCATAACAATTCCTGGCAC | |
| HPS-8-F | GGAAGGGGATTACTACTACCTGAAAG | 650 |
| HPS-8-R | CTCCATAGAACCTGCTGCTTGAG | |
| HPS-9-F | AGCCACATCAATTTTAGCCTCATCA | 710 |
| HPS-9-R | CCTTAAATAGCCTATGTCTGTACC | |
| HPS-10-F | GGTGACATTTATGGGCGAGTAAGTC | 790 |
| HPS-10-R | GCACTGTCATCAATAACAATCTTAAGACG | |
| HPS-11-F | CCATCTCTTTAACTAATGGGACTG | 890 |
| HPS-11-R | GGACGCCAAGGAGTATTATCAAATG | |
| HPS-12-F | CCACTGGATAGAGAGTGGCAGG | 450 |
| HPS-12-R | CCATACATCTGAATTCCTAAGC | |
| HPS-13-F | GCTGGAGGAGTTGAAAGAGTTGTTAC | 840 |
| HPS-13-R | CCATACATCTGAATTCCTAAGC | |
| HPS-14-F | GCTGGTTATGACTATTTCTTTCGCG | 730 |
| HPS-14-R | GCTCCCAAGATTAAACCACAAGCAAG | |
| HPS-15-F | CAAGTTCGGATTGGGAGCATATATC | 550 |
| HPS-15-R | CCTATATCATTTGTTGGATGTACG | |
| HPS-F | ACAACCTGCAAGTACTTATCGGGAT | 275 |
| HPS-R | TAGCCTCCTGTCTGATATTCCCACG |
Table s2: Antimicrobial resistance profiles of H. parasuis isolates (2016-2018).
| Number of isolates | Number of antimicrobial agents | Resistance phenotype |
|---|---|---|
| 0 | No antimicrobial resistance | 5 |
| 1 | AMI | 1 |
| 1 | AMP | 2 |
| 1 | KAN | 2 |
| 1 | STR | 3 |
| 1 | NOR | 1 |
| 1 | CRO | 1 |
| 2 | ENO+LEV | 1 |
| 2 | CIP+STR | 1 |
| 2 | KAN+AMP | 1 |
| 2 | STR+AMI | 1 |
| 2 | GEN+AMI | 2 |
| 2 | GEN+SPE | 1 |
| 2 | CRO+AMP | 1 |
| 2 | CAZ+STR | 1 |
| 2 | LEV+NOR | 5 |
| 2 | SPE+GEN | 1 |
| 3 | AMP+CIP+STR | 2 |
| 3 | AMP+NOR+AZM | 1 |
| 3 | ENO+CIP+STR | 1 |
| 3 | ENO+CIP+NOR | 4 |
| 3 | CIP+STR+AMI | 1 |
| 3 | KAN+AMP+AML | 2 |
| 3 | KAN+AMP+STR | 1 |
| 3 | KAN+CIP+STR | 1 |
| 3 | KAN+GEN+AZM | 1 |
| 3 | KAN+GEN+STR | 2 |
| 3 | STR+AZM+AMI | 1 |
| 3 | LEV+AMP+CIP | 5 |
| 3 | LEV+AMP+NOR | 1 |
| 3 | SPE+AMP+AMI | 1 |
| 3 | SPE+AMP+CIP | 3 |
| 3 | SPE+AMP+STR | 6 |
| 4 | AMP+CIP+NOR+AZM | 2 |
| 4 | AMP+SPE+CAZ+NOR | 1 |
| 4 | ENO+LEV+AMP+AMI | 1 |
| 4 | FLO+STR+AZM+AMI | 4 |
| 4 | KAN+CIP+AMP+STR | 1 |
| 4 | KAN+STR+AZM+AMI | 1 |
| 4 | KAN+GEN+STR+AMI | 4 |
| 4 | KAN+LEV+AMP+CIP | 1 |
| 4 | CRO+CIP+AMI+AML | 1 |
| 4 | CRO+LEV+AMP+CIP | 1 |
| 4 | CTX+SPE+CRO+AMP | 1 |
| 4 | LEV+AMP+CIP+STR | 1 |
| 4 | LEV+CIP+STR+AMI | 2 |
| 4 | SPE+AMP+CIP+STR | 10 |
| 4 | SPE+GEN+LEV+CIP | 1 |
| 5 | AMP+CIP+NOR+AZM+AML | 1 |
| 5 | ENO+LEV+AMP+CIP+AMI | 2 |
| 5 | ENO+LEV+CIP+NOR+AMI | 4 |
| 5 | KAN+AMI+NOR+LEV+CIP | 1 |
| 5 | KAN+FLO+STR+AZM+AMI | 3 |
| 5 | KAN+GEN+CIP+STR+AMI | 1 |
| 5 | KAN+GEN+CRO+STR+AML | 1 |
| 5 | KAN+LEV+CIP+NOR+AMI | 3 |
| 5 | KAN+SPE+LEV+AMP+STR | 1 |
| 5 | GEN+ENO+AMP+CIP+AMI | 1 |
| 5 | CTX+FLO+CIP+NOR+AMI | 1 |
| 5 | LEV+CIP+NOR+AZM+AMI | 1 |
| 5 | SPE+ENO+FLO+NOR+AMI | 1 |
| 6 | AMP+SPE+KAN+CTX+NOR+CIP | 1 |
| 6 | KAN+CEF+AMP+CIP+STR+AML | 1 |
| 6 | KAN+GEN+LEV+STR+AZM+AMI | 1 |
| 6 | KAN+CTX+SPE+AMP+CIP+NOR | 3 |
| 6 | KAN+SPE+PB+AMP+STR+AMI | 1 |
| 6 | KAN+SPE+GEN+NOR+STR+AMI | 2 |
| 6 | GEN+ENO+LEV+CIP+STR+AMI | 1 |
| 6 | CTX+ENO+LEV+AMP+CIP+NOR | 1 |
| 6 | CAZ+KAN+GEN+LEV+AMP+STR | 2 |
| 6 | SPE+ENO+AMP+CIP+NOR+STR | 2 |
| 6 | SPE+ENO+CRO+AMP+STR+AMI | 1 |
| 7 | KAN+CEF+AMP+CIP+NOR+STR+AMI | 1 |
| 7 | KAN+GEN+ENO+LEV+AMP+CIP+STR | 1 |
| 7 | GEN+ENO+LEV+CIP+NOR+STR+AMI | 4 |
| 7 | CAZ+KAN+GEN+CRO+LEV+AMP+STR | 1 |
| 7 | CAZ+KAN+SPE+CRO+AMP+NOR+STR | 1 |
| 7 | CAZ+CTX+SPE+GEN+AMP+CIP+NOR | 1 |
| 7 | CAZ+CTX+SPE+LEV+AMP+CIP+AML | 2 |
| 7 | CAZ+KAN+SPE+LEV+AMP+STR+AML | 1 |
| 7 | SPE+GEN+ENO+CIP+NOR+STR+AMI | 1 |
| 8 | KAN+PB+CEF+CRO+AMP+NOR+STR+AMI | 1 |
| 8 | KAN+GEN+ENO+LEV+AMP+CIP+STR+AML | 2 |
| 8 | KAN+SPE+ENO+CRO+AMP+CIP+NOR+AML | 1 |
| 8 | CTX+PB+ENO+FLO+CIP+NOR+STR+AMI | 3 |
| 8 | CTX+SPE+GEN+AMP+CIP+NOR+STR+AZM | 2 |
| 8 | CAZ+CTX+SPE+CEF+AMP+CIP+NOR+AML | 1 |
| 9 | KAN+CTX+GEN+ENO+AMP+CIP+NOR+STR+AML | 1 |
| 10 | CAZ+CTX+GEN+ENO+LEV+AMP+CIP+NOR+STR+AMI | 1 |
| 11 | CAZ+CTX+SPE+ENO+CEF+CRO+FLO+AMP+NOR+STR+AML | 1 |
| 13 | CAZ+KAN+CTX+SPE+CRO+- FLO+LEV+AMP+CIP+STR+AMI+AZM+AML | 1 |