Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 20 October, 2022
Accepted date: 23 December, 2022
Published date: 28 December, 2022
Citation: Mohamed WS, Aldabbagh HA, Aldughmani HA, Abdullah ZF, Kassem SA, Abdel-Almoniem IA, Ibrahim MF (2022) Prevalence of Human Herpesviruses (HHV1-7), and its Clinical Significance in Middle Eastern Pediatric Leukemia Patients Using 2 Independent PCR Assays. J Appl Microb Res. Vol: 5 Issu: 2 (30-38).
Copyright: © 2022 Mohamed WS et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: Human herpes viruses can cause life-threatening diseases in immunocompromised children, especially leukemic patients. Therefore, the aim of this study is to detect the human herpes viruses (HHV1-7) and to investigate its clinical significance in Middle Eastern Pediatric Leukemia Patients by using 2 Independent PCR assays.
Methods: Detection of human herpes virus DNA has been done in blood samples of 200 pediatric leukemia patients in addition to 90 blood donors as a control group using multiplex PCR assays. When a ‘‘positive’’ result was observed, real-time PCR was performed to measure the viral load.
Results: The most frequent herpes virus infection in Middle Eastern Pediatric Leukemia cases was CMV, followed by EBV, then HHV6, VZV, HHV7, HSV1, and HSV2, where they were 92/200 (46%), 76/200 (38%), 72/200 (36%), 48/200 (24%), 12/200 (6%), 8/200 (4%), and 2/200 (1%) respectively. Also, there was a statistically significance difference between leukemic patients and their controls regarding CMV, EBV, HHV6, and VZV (P <0.05). Correlation between percentage of co-infection, and clinical parameters for the 7 herpes viruses has been studied, and there is an increase in absolute neutrophilic count (ANC), total leukocyte count (TLC) and duration of fever and neutropenia in age group 6-11 years for HHV6/CMV, then in age group 12-18 years especially for EBV/CMV and CMV/HHV6. Also, our results show that multiplex PCR assay is close to single PCR assay in relation to specificity and sensitivity which in turn prove its validity for early diagnosis of herpes viral infection.
Conclusions: Adopting multiplex PCR technique is helpful in screening of virus infections. It will save time, effort, cost effective and will assist in rapid diagnosis. However, the clinical relevance of the virus infection needs to be evaluated by quantitative real-time PCR which in turn will help patient’s management by using appropriate antiviral treatment.
Keywords
Multiplex PCR, Real time PCR, Human herpes viruses, Clinical significance, Pediatric Leukemia patients.
Abstract
Objectives: Human herpes viruses can cause life-threatening diseases in immunocompromised children, especially leukemic patients. Therefore, the aim of this study is to detect the human herpes viruses (HHV1-7) and to investigate its clinical significance in Middle Eastern Pediatric Leukemia Patients by using 2 Independent PCR assays.
Methods: Detection of human herpes virus DNA has been done in blood samples of 200 pediatric leukemia patients in addition to 90 blood donors as a control group using multiplex PCR assays. When a ‘‘positive’’ result was observed, real-time PCR was performed to measure the viral load.
Results: The most frequent herpes virus infection in Middle Eastern Pediatric Leukemia cases was CMV, followed by EBV, then HHV6, VZV, HHV7, HSV1, and HSV2, where they were 92/200 (46%), 76/200 (38%), 72/200 (36%), 48/200 (24%), 12/200 (6%), 8/200 (4%), and 2/200 (1%) respectively. Also, there was a statistically significance difference between leukemic patients and their controls regarding CMV, EBV, HHV6, and VZV (P <0.05). Correlation between percentage of co-infection, and clinical parameters for the 7 herpes viruses has been studied, and there is an increase in absolute neutrophilic count (ANC), total leukocyte count (TLC) and duration of fever and neutropenia in age group 6-11 years for HHV6/CMV, then in age group 12-18 years especially for EBV/CMV and CMV/HHV6. Also, our results show that multiplex PCR assay is close to single PCR assay in relation to specificity and sensitivity which in turn prove its validity for early diagnosis of herpes viral infection.
Conclusions: Adopting multiplex PCR technique is helpful in screening of virus infections. It will save time, effort, cost effective and will assist in rapid diagnosis. However, the clinical relevance of the virus infection needs to be evaluated by quantitative real-time PCR which in turn will help patient’s management by using appropriate antiviral treatment.
Keywords
Multiplex PCR, Real time PCR, Human herpes viruses, Clinical significance, Pediatric Leukemia patients.
Background
Human herpes viruses can cause life-threatening diseases in immunocompromised children, especially leukemic patients, and those undergoing hematopoietic stem cell transplantation [1,2]. Since these viruses have similar clinical manifestations but different management and prognosis therefore, laboratory diagnosis is essential to identify those herpes viruses to ensure optimal patient management and reducing severe complications as these viruses can be treated with antiviral drugs [2,3].Human herpesviruses, particularly herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), cytomegalovirus (CMV), Epstein-Barr virus (EBV), varicella-zoster virus (VZV), human herpesvirus 6 (HHV-6), and human herpesvirus 7 (HHV-7) are the major cause of morbidity and mortality in childhood. Common identification methods like antibody detection and virus isolation in cell culture have a lot of limitations. Other diagnostic tools are required for rapid and reliable detection of these viruses to overcome these limitations. Accordingly, there is an increasing need for the accurate and timely diagnosis of herpes infections for these powerful anti-viral drugs to be used effectively [4]. Polymerase chain reaction (PCR) is a useful tool for detecting or monitoring viral genomes. However, a conventional viral PCR assay detects only a single virus. Therefore, we recently developed a qualitative multiplex PCR assay to detect 7 kinds of viral DNA genomes quickly and simultaneously from blood samples [3- 5]. The qualitative multiplex and quantitative real-time PCR procedures take only 3 hours to complete. With this assay system, we can identify viremia at the early stage and thereby prevent it from progressing to overt and symptomatic viral infection in immunocompromised patients. So, rapid diagnosis will help in a quick cancer patients’ management. In the current study, multiplex PCR and real-time PCR assays have been used for the analysis of human herpes virus family genomic DNA of the human herpes viruses (HHV1- 7) in blood samples of pediatric leukemia patients and their controls. When a ‘‘positive’’ result was observed, real-time PCR was performed to measure the viral load. When more than 50 copies/tube (5x103 copies /ml) were observed, the value was considered to be significant.
Methods
Patients
This study was done on the blood specimens of 200 pediatric leukemic Saudi patients [160 acute lymphocytic leukemia (ALL), and 40 acute myeloid leukemia (AML)], and 90 apparently healthy normal individuals as matched controls. These leukemia patients were diagnosed at Al Qurayyat General Hospital, Al Qurayyat, Saudi Arabia, through the period from January 2019 to December 2021. All experiments were performed in compliance with relevant laws and institutional guideline and in accordance with the ethical standards of the Declaration of Helsinki. The Institutional Review board (IRB) of the Qurayyat Health Affairs approved the protocol (Reg NO: H-13-S-071). Informed written consent form was obtained from all patients and individuals enrolled in the study. Inclusion criteria were pediatric ALL or AML patients until 18 years old with no previous treatment nor antiviral treatment. All patients were subjected to pretreatment assessment including full history and physical examination as well as routine baseline investigations required for diagnosis and staging according to each disease category using WHO criteria for hematological malignancies.
Specimen collection and Nucleic acid extraction
Whole Blood specimens were collected and processed from each patient and control. DNA was extracted using QIA amp viral RNA extraction kit (Qiagen, Valencia, USA). The extraction was done according to the manufacturer’s instructions. The amount of viral DNA was measured by spectrophotometry using a Nano-Drop 2000 spectrophotometer (Thermo Scientific/US, Canada) and 100ng of DNA template was used in the PCR assays. DNA extracts were placed on ice and were used immediately for PCR, then stored at -80oC until further analysis. Multiplex PCR and realtime PCR assays have been used for the analysis of human herpes virus family genomic DNA in blood samples of leukemia patients. When a ‘‘positive’’ result was observed, real-time PCR was performed to measure the viral load. When more than 50 copies/tube (5x103 copies /ml) were observed, the value was considered to be significant.
Polymerase chain reaction
Human herpes virus DNA was measured using 2 independent PCR assays: multiplex qualitative PCR, and real-time quantitative PCR techniques.
Herpesviruses DNA detection by multiplex PCR
Multiplex PCR was designed to qualitatively measure genomic DNA of seven human herpes viruses, that is, Herpes implex virus type 1 (HSV-1), type 2 (HSV-2), Varicella-zoster virus (VZV), Epstein– Barr virus (EBV), Cytomegalovirus (CMV), Human herpes virus type 6 (HHV6), and type 7 (HHV7). The multiplex PCR was performed as described previously by Tanaka et al. [3] (Table1).
Comparison of sensitivity and specificity of multiplex PCR with Conventional PCR
For 50 blood positive samples that were screened by the multiplex PCR assay, the conventional PCR method was also applied using individual primer sets for each virus to detect human herpes virus DNA, and to prove the validity of multiplex PCR technique.
These sets of primers used optimize PCR conditions for each virus separately as previously described [3,6-10].
Quantitative real-time PCR
Real-time PCR was performed only for the human herpes viruses that found to be positive by multiplex PCR. The realtime PCR was performed using Amplitaq Gold and the Real- Time PCR 7300 system (ABI, Foster City, CA). The primer sequences for the 7 herpes viruses, and probes are shown in table 2. The primers and the PCR conditions have been described in previous reports [11,12].
Table 1:Sequences of primers used for multiplex PCR.
| Herpes virus | GenBank Accession number | Sequence (5'-3') | 5' Position | Predicted Product Size (bp) |
|---|---|---|---|---|
| HSV-1/2 | HSV1:M10792 HSV-2: M16321 | F: GCCAAGAAAAAGTACATCGGCGTCATC R: TGAGGACAAAGTCCTGGATGTCCCTCT | HSV-1: 3389 HSV-2: 3058 HSV-1: 3680 HSV-2: 3349 | 292 bp |
| VZV | X04370 | F: TCCGACATGCAGTCAATTTCAACGTC R: GGTCGGGTAGACGCTACCACTCGTTT | (49651) (49811) | 161 bp |
| EBV | NC_007605 | F: CTTAGAATGGTGGCCGGGCTGTAAAAT R: ATCCAGTACGTCTTTGTGGAGCCCAAG | (153240) (153468) | 229 bp |
| CMV | NC_001347 | F: GCGCGTACCGTTGAAAGAAAAGCATAA R: TGGGCACTCGGGTCTTCATCTCTTTAC | (80362) (80492) | 131 bp |
| HHV6A/B | 6A:NC_001664 6B: AB021506 | F: ATGCGCCATCATAATGCTCGGATACA R: CCCTGCATTCTTACGGAAGCAAAACG | (57837 to 58019) (58791 to 58973) | 183 bp |
| HHV7 | (NC_001716) | F: GCCCGTTTTCGGAAATATTGGAGAGAT R: ACGCACGAGACGCACTTTTCTTAAACA | (55671) (56017) | 347 bp |
| *Note: Sequences of primers used for multiplex PCR are cited from Tanaka et al., [3] | ||||
Table 2:Sequence for primers and probes in human herpes viruses using real-time PCR.
| Herpes virus | Sequence for primers and probes | Amplification |
|---|---|---|
| HSV1/2 | HSV-F:CGCATCAAGACCACCTCCTC HSV-R: GCTCGCACCACGCGA HSV1-P:JOE-TGGCAACGCGGCCCAAC-TAMRA HSV2-P:FAM-CGGCGATGCGCCCCAG-TAMRA | gB |
| VZV | VZV-F: AACTTTTACATCCAGCCTGGCG VZV-R: GAAAACCCAAACCGTTCTCGAG VZV-P:FAM-TGTCTTTCACGGAGGCAAACACGT-TAMRA | ORF29 |
| EBV | EBV-F: CGGAAGCCCTCTGGACTTC EBV-R: CCCTGTTTATCCGATGGAATG EBV-P: FAM-TGTACACGCACGAGAAATGCGCC-TAMRA | BALF5 |
| CMV | CMV-F: CATGAAGGTCTTTGCCCAGTAC CMV-R: GGCCAAAGTGTAGGCTACAATAG CMV-P:FAM-GGCCCGTAGGTCATCCACACTAGG-TAMRA | IE-1 |
| HHV6 | HHV6-F: GACAATCACATGCCTGGATAATG HHV6-R:TGTAAGCGTGTGGTAATGTACTAA HHV6-P:FAMAGCAGCTGGCGAAAAGTGCTGTGC-TAMRA | U65-U66 |
| HHV7 | HHV7-F: CGGAAGTCACTGGAGTAATGACAA HHV7-R: CCAATCCTTCCGAAACCGAT HHV7-P: FAM-CTCGCAGATTGCTTGTTGGCCATG-TAMRA | U37 |
| The real-time herpes simplex virus (HSV) PCR is a multiplexing PCR that can detect both HSV1 and HSV2 DNA in the same reaction. The optimised gB primer pairs amplify both HSV1 and 2 with equal efficiency, with the two type-specific probes labelled with different fluorescent dyes. HSV1 probe is labelled with JOE at the 5’-end and with TAMRA at the 3’-end. HSV2 probe is labelled with FAM at the 5’-end and with TAMRA at the 3’-end. | ||
The value of viral copy number in the sample was considered to be significant, when more than 50 copies/tube (5x103 copies /ml) were observed.
Statistical methods
Data was analyzed using IBM SPSS advanced statistics version 22 (SPSS Inc., Chicago, IL). Numerical data were expressed as median and range. Qualitative data were expressed as frequency and percentage. Chi-square test or Fisher’s exact test was used to examine the relation between qualitative variables. For the quantitative data that are not normally distributed, comparison between two groups was done using Mann-Whitney test (non-parametric t-test). All tests were two-tailed. A p-value < 0.05 was considered significant.
Results
Clinical Features of Pediatric Leukemic Patients
The current study was done on 200 pediatric leukemic patients where 176 (88%) patients were at the induction phase of therapy, 12 were at maintenance phase of therapy, and 12 received salvage treatment for relapsing disease (Table 3).
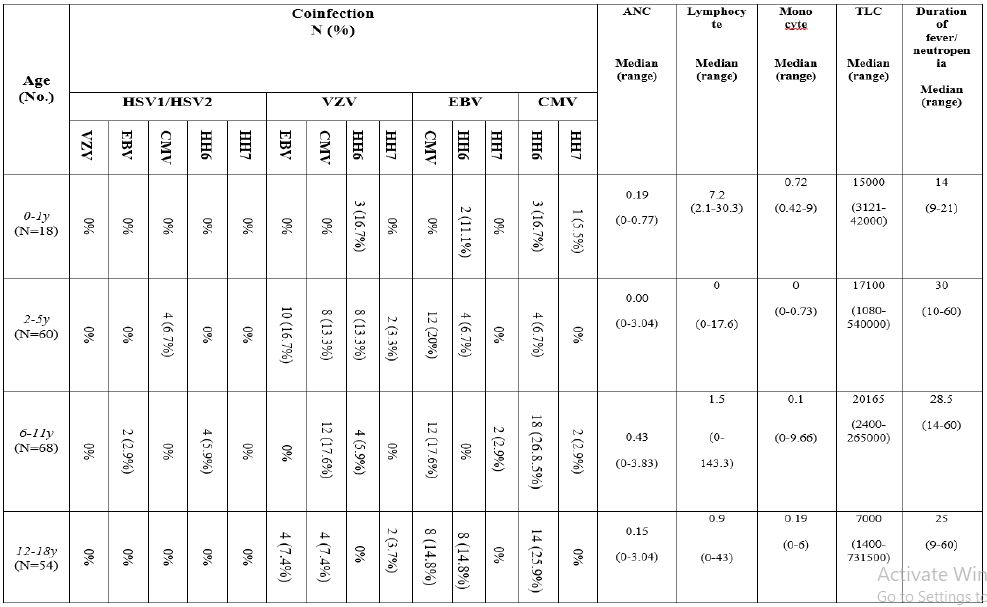
Correlation between percentage of co-infection and clinical data among seven herpes viruses in pediatric leukemia cases
Pediatric leukemic patients have been divided into four age groups;1st group ranges from 0-1y, 2nd group ranges from 2-5y, 3rd group ranges from 6-11y and 4th group ranges from 12-18y. Numbers of patients in every single group were 18, 60, 68 and 54 respectively. Correlation between percentage of co-infection, and clinical parameters for the 7 herpes viruses has been studied. There is an increase in absolute neutrophilic count (ANC), total leukocyte count (TLC) and duration of fever and neutropenia in age group 6-11 years for HHV6/CMV, then in age group 12-18 years especially for EBV/CMV and CMV/HHV6 (Table 4).
Table 3:Clinical and laboratory findings for leukemia patients.
| Parameters | Leukemia (N=200) | Control (N=90) | P value | |
|---|---|---|---|---|
| Age | Median (range) | 7(0.8-24)yrs | 11(4-18)yrs | 0.04* |
| Sex | Male:Female M:F ratio | 104:96 1.08:1 | 60:30 2:1 | 0.0215* |
| Clinical Symptoms | Fever, n(%) Mucositis,Otitis media,or others n(%) CSF (not free), n(%) Echo (abnormal, n(%) Organomegaly,n(%) Lymphadenopathy, n(%) Chest infection n(%) | 200(100%) 20(10%) 44(22%) 28(14%) 140(70%) 28(14%) 200(100%) | 0(0%) 0(0%) 0(0%) 0(0%) 0(0%) 0(0%) 0(0%) | 0.001* 0.056 0.03* 0.12 0.001* 0.12 0.001* |
| Phase of therapy | Group 1 (Induction) n=44 Group 2(Maintenance), n=3 Group 3(relapse), n=3 | 176 (88%) 12 (6%) 12 (6%) | 0(0%) 0(0%) 0(0%) | 0.001* 0.3 0.3 |
| Duration of FN | 26.5(9-60) day | N/A | N/A | |
| LFT | ALT (IU/L) AST (IU/L) T.Bil (mg/dl) | 20.1(1-317) 23.50(9-273) 0.45(0.1-1.7) | 31(0-50) 30.4(0-60) 0.9(0-1.4) | 0.47 0.491 0.37 |
| KFT | Creatinin (mg/dl) Uric Acid (mg/dl) | 0.4(0.1-2.2) 3.4(1-24) | 0.4 (Under .5) 3 (2-6) | 0.97 0.67 |
| CBC | Hb (g/dl) TLC (x109/l) Plt (x109/l) ANC (x109/l) Mono (x109/l) LDH (IU/L) | 7.4(3.9-14.1) 11950(1080-731500) 39.5(7-694) 0.13 (0-3.83) 0.11(0-9.66) 907(11-9635) | 12.4(10-13) 5.60 (4.00-11.0) 191.6 (170-380) 4.7 (2.5-7.5) 0.43 (0.2-0.8) 263.12 (180–360) | 0.38 0.001* 0.001* 0.021* 0.04* 0.001* |
| *P-value < 0.05 is statistically significant. CSF: Cerebrospinal fluid, ESR1: Erythrocyte sedimentation rate 1st hour, ESR2: Erythrocyte sedimentation rate 2nd hour, FN: Febrile neutropenia. LFT: Liver function test, ALT: Alanin aminotransferase, AST: Aspartate aminotransferase,T.Bil: Total bilirubin KFT: Kidney function test, CBC: Complete blood picture, Hb: Hemoglobin concentration, TLC: Total leukocytic count Plt: Platelet count, ANC: Absolute neutrophilic count, Mono: monocyte, LDH: Lactate dehydrogenase. | ||||
Table 4:Coinfection between 7 herpes viruses in relation to clinical data among pediatric leukemia cases using both multiplex PCR and real-time PCR.
Multiplex PCR and real-time PCR assays have been used for the analysis of human herpes virus family genomic DNA in blood samples of leukemia patients. When a ‘‘positive’’ result was observed, real-time PCR was performed to measure the viral load. When more than 50 copies/tube (5x103/ ml) were observed, the value was considered to be significant. HHV6-DNA was detected by multiplex PCR in 72 patients, and real-time PCR found that all 72 of these patients also had a high HHV6 viral load. Also, HSV1-DNA was detected by multiplex PCR in 8 patients, and real-time PCR found that all 8 of these patients also had a high HSV1 viral load. In addition, HSV2-DNA was detected in 2 patients, with all of these patients having a high viral load, as shown in Table 6. On the other hand, CMV-DNA was detected by multiplex PCR in 92 patients, but only 61 out of the 92 cases were positive (66%) by real time PCR. EBV-DNA was detected by multiplex PCR in 76 patients, with 54 out of the 76 cases (71%) found to be positive by the real-time PCR. VZV-DNA was detected by multiplex PCR in 48 patients, with 36 out of the 48 cases (75%) found to be positive by the real-time PCR. Also, HHV7- DNA was detected by multiplex PCR in 12 patients, with 9 out of the 12 cases (75%) found to be positive by the realtime PCR.
Detection of the 7 herpes viruses by Multiplex PCR technique
Presence of 7 herpes virus genomes were tested by Qualitative multiplex PCR assay in both leukemia and normal control groups. The most frequent herpes virus infection in Middle Eastern Pediatric Leukemia cases was CMV, followed by EBV, then HHV6, VZV, HHV7, HSV1, and HSV2, where they were 92/200 (46%), 76/200 (38%), 72/200 (36%), 48/200 (24%), 12/200 (6%), 8/200 (4%), and 2/200 (1%) respectively. Also, there was a statistically significance difference between leukemic patients and their controls regarding CMV, EBV, HHV6, and VZV (P <0.05) (Table 5).
Table 5:Prevalence of 7 human herpes viruses using multiplex PCR assay.
| Herpes virus | Leukemia Patients (N=200) | Blood donors (Controls; N=90) | P Value |
|---|---|---|---|
| HSV1 | 8 (4%) | 3 (3.3%) | 0.86 |
| HSV2 | 2 (1%) | 0 (0%) | 0.97 |
| VZV | 48 (24%) | 3 (3.3%) | 0.03* |
| EBV | 76 (38%) | 10 (11%) | 0.01* |
| CMV | 92 (46%) | 22 (24%) | 0.04* |
| HHV6 | 72 (36%) | 19 (21%) | 0.0136* |
| HHV7 | 12 (6%) | 1 (1.1%) | 0.45 |
| Herpes co-infection status | No virus=64(32%) | No virus=42(47%) | 0.006* |
| Single virus=68(34%) | Single virus=45(50%) | ||
| 2 or more virus=68(34%) | 2 or more virus=3(3%) | ||
| *P-value < 0.05 is statistically significant. | |||
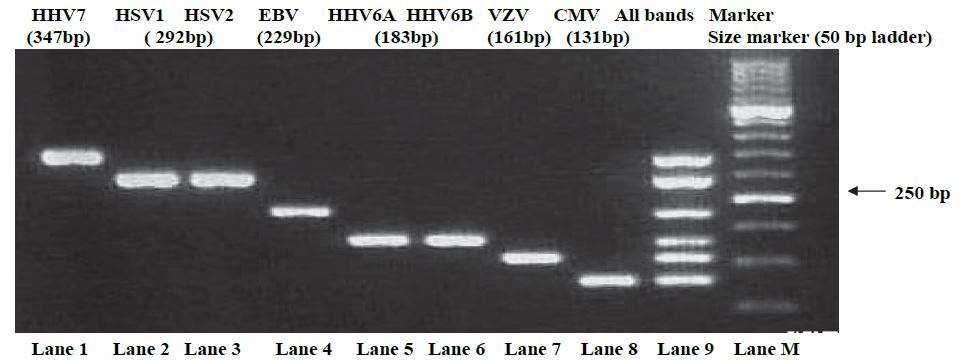
On the other hand, detection of the multiplex PCR products with UV showed that the amplified fragments of the expected size have been obtained when using the primer sets specific to the 7 types of human herpes virus (Figure 1).
Comparison of multiplex PCR assay with Single PCR assay
For the validity of multiplex technique, 50 DNA positive samples were further analysis by the conventional PCR method using individual primer sets for each virus. Our results have shown that multiplex PCR assay is close to single PCR assay in relation to specificity and sensitivity which in turn prove its validity for early diagnosis of herpes viral infection (Table 6).
Discussion
Herpesviruses infections are important causes of morbidity and mortality for patients with a hematological malignancy. Approximately, 50% of the children younger than 15 who suffer from cancer have leukemia or lymphoma, and ALL is the most common one [13-16]. Common identification methods of herpes virus infections such as antibody detection and virus isolation in cell culture have a lot of limitations. Other diagnostic tools are required for rapid and reliable detection of these viruses to overcome these limitations. Therefore, there is an increasing need for the accurate and timely diagnosis of herpes infections for these powerful anti-viral drugs to be used effectively [4].
Our current study has aimed at performing multiplex PCR technique and evaluating its efficacy for 7 herpes viruses’ detection. This will save time, effort, be cost effective and helping in rapid diagnosis of these herpes viruses and guiding cancer patient’s management. A qualitative multiplex PCR is useful in the screening of herpes viral infections. However, the clinical relevance of the virus infection needs to be evaluated by quantitative real-time PCR.
Multiplex PCR was applied to detect HSV-1, -2, VZV, CMV, EBV, HHV6, and HHV7 among pediatric cancer patients. It was found that 8 cases of 200 patients (4%) had HSV1 infection, and 2 cases of 200 patients (1%) had HSV2 infection. In the control group, HSV1 and HSV2 infections were detected in 3/90 (3.3%), and 0/90 (0%) respectively (p>0.05). Our results agreed with the study done by who observed that only (2%) of 51 pediatric cancer patients were positive for HSV1/2 [17]. While in another study done by HSV-1 was detected in 8 cases (25%) and it was absent in controls (p=0.009). HSV-2 was not detected in any case or control by qualitative PCR [18].
Table 6:Comparison of sensitivity and specificity of multiplex PCR with conventional PCR.
| Multiplex PCR Assay | Conventional PCR Assay (N=200) | Sensitivity | Specificity | |
|---|---|---|---|---|
| Positive | Negative | |||
| CMV No. Positive No. Negative | 18 2 | 2 28 | 90% | 93.3% |
| EBV No. Positive No. Negative | 18 4 | 2 26 | 81.9% | 92.9% |
| HHV6 No. Positive No. Negative | 8 2 | 2 38 | 80% | 95% |
| VZV No. Positive No. Negative | 10 2 | 2 36 | 83.3% | 94.8% |
| HHV7 No. Positive No. Negative | 8 2 | 0 40 | 80% | 100% |
| HSV2 No. Positive No. Negative | 6 2 | 0 42 | 75% | 100% |
Figure 1:Electrophoresis of PCR products was done in 3% agarose gel with a molecular size marker. Lane 1, HHV-7; lane 2, HSV-1; lane 3, HSV-2; lane4, EBV; lane 5, HHV-6A; lane 6, HHV-6B; lane 7, VZV; lane 8, CMV; lane 9, HHV-7, HSV-1, EBV, HHV-6B, VZV and CMV; lane M, size marker (50bp ladder).
For Varicella Zoster Virus infection, 48/200 of the pediatric cases (24%) were positive while only 3 cases (3.3%) were positive in the controls which was statistically significant (p=0.03). Similar observations were made by who stated that the incidence of VZV in renal transplant recipients was approximately 4 to 12% [19]. These results differ from those obtained by who studied 30 cases of immunocompromised patients and they found 21 (70%) cases were positive [20].
Regarding EBV infection, there was a statistically significant difference (p=0.01) between the patients and its controls where it was detected in 76/200 (38%) of the cases and in 10/90 (11%) of the control group. Similar findings were found by [21,22]. On the other hand, different results were stated by who found that EBV-DNA was positive in 3 (8.1%) of 37 children with oncological diseases [23]. Also, Cukuranovic et al. established the incidence of EBV in renal transplant recipients and it was approximately 1 to 3% EBV infection [19].
Infection with cytomegalovirus (CMV) has always been extremely widespread with high infection rates occurring in the third world countries which is (up to 100%) than in western world (50-80%) [22]. CMV infections are very common among children that why blood tests show that 60 to 90% of adults have had a CMV infection at some time. From our results, CMV infection was detected in 92/200 (46%) cases and in 22/90 (24%) controls (p=0.04).
Similar observations were found by who detected CMVDNA in 98/711 (13.8%) of the whole blood samples and in 168/711 (23.6%) of plasma samples among Hematopoietic Stem Cell Transplant Recipients [24]. While other studies showed that CMV viral loads in whole blood tend to be higher than those in serum, as whole blood contains free and cell-associated virions, while serum contains only cellfree viruses; Lisboa et al. stated 154 of 219 (70.3%) patients with the whole blood versus 105 of 219 (52.1%; P<0.001) patients with the plasma assay. Also, Wada et al found CMV in whole blood 34/303 (11.2%) higher than in plasma 16/303 (5.9%) [25,26].
Regarding human herpes virus 6 (HHV-6) infection, the present work focused on diagnosis both HHV-6A and HHV-6B because HHV-6B causes the childhood illness roseola infantum while HHV-6A has been isolated mainly in immunocompromised hosts; however, both HHV-6A and HHV-6B may be pathogenic in the settings of transplantation and AIDS. As HHV-6 is now recognized as lymphotropic virus; a T-cell lymphotropic virus with high affinity for CD4 lymphocytes [27]. Our results have showed that there is a statistically significant difference (p=0.048) between the cases and controls where HHV6 was positive in 72/200 (36%) patients and in 19/90 (21%) of the controls. Similar observations were confirmed by who detected HHV6 DNA in 16/50 cases (32%) and none of their healthy volunteers showed HHV6 infection. Also, related observations were affirmed by [9,28]. These results were different from those obtained by who detected only 6 (9%) ALL pediatric cases in bone marrow samples and were also different from who detected HHV6 in (19.6%) of 51 pediatric cancer patients [17].
For human herpes virus 7 (HHV-7) infection, it was positive in 12/200 (6%) cases compared to 1/90 (1.1%) controls (p=0.56). Previous study done by Zheng et al on 119 blood samples (74 from blood donors and 45 from Chinese patients suspected of pityriasis rosea infection) found that 40%, and 73.3% of cases were positive for HHV-6 and HHV- 7 infections compared to 16.2% and 55% of HHV-6 and HHV-7 infections in the control group respectively [29]. On the other hand, prior work done by Sugita et al on 111 ocular fluid samples of patients with uveitis for detection of human herpes virus genome (HHV1-8) using multiplex PCR and real-time PCR, they declared that HHV7- and HHV8- DNA were absent [12]. Other earlier study performed by Wada et al on 105 cerebrospinal fluid samples and 46 Sera samples for detection and quantification of HSV, HHV-6, and HHV-7 DNA using multiplex real-time PCR, they found that HHV7DNA was present in 2/105 (1.9%) of CSF samples and 0/46 (0%) of sera samples [30]. In a previous report done by Handous et al, they found HHV7 infection in 4/95 (4.2%) of leukemia patients which was close to our results [31].
There have been considerable developments in the field of viral diagnosis, which have been brought about by the introduction of the rapidly advancing PCR methods that enable the diagnosis of specific viral infections. Since herpes viral infection often induce similar clinical symptoms, it’s very important for the rapid and accurate diagnosis and effective treatment of herpes viral infections. Therefore, it has been developed a multiplex PCR technique to simultaneously detect and identify DNA from 7 types of herpes viruses (HSV-1/2, VZV, EBV, CMV, HHV-6, and HHV- 7) which was the main target of our study.
Correlation between percentage of co-infection, and clinical parameters for the 7 herpes viruses has been studied. It has been observed that there is an increase in absolute neutrophilic count (ANC), total leukocyte count (TLC) and duration of fever and neutropenia in age group 6-11 years for HHV6/CMV, then in age group 12-18 years especially for EBV/CMV and CMV/HHV6. This may be attributed to immunosuppression from leukemia disease and its treatment may predispose patients to higher risk of coinfection. These results were in agreement with those done by previous report [9]. Handous et al performed study on co-infections of human herpesviruses (CMV, HHV-6, HHV-7 and EBV) in non-transplant acute leukemia patients undergoing chemotherapy. they found that CMV/HHV-6 was the most frequent co-infection which is similar to our findings [31].
Regarding the sensitivity and the specificity of the multiplex method, 50 DNA samples were evaluated. In comparison to the results of the single assay, the sensitivity of the multiplex assay was 80%, 75%, 83.3%, 81.9%, 90%, 80%, and 80% for HSV1, HSV2, VZV, EBV, CMV, HHV6, and HHV7 respectively. On the other hand, the specificity of the multiplex assay was 100%, 100%, 94.8%, 92.9%, 93.3%, 95%, and 95% for HSV1, HSV2, VZV, EBV, CMV, HHV6, and HHV7 respectively. Based on our results, multiplex PCR assay is close to single PCR assay in relation to specificity and sensitivity which in turn prove its validity for early diagnosis of herpes viral infection. Therefore, this enabled us to detect and identify the 7 types of human herpes virus using multiplex PCR methods which help patient’s management by using appropriate antiviral treatment easily and rapidly. Moreover, because multiplex PCR is a qualitative method of measurement, a human herpes virus, regardless of the stage of infection (i.e., even at a latent stage), may be detected in some samples obtained from healthy individuals; therefore, selection the appropriate sample source is essential. Close findings were reported by Wada et al. who compared to the results of the single assay where the sensitivity and specificity values of the multiplex assay were 96.0 and 100% for EBV, 94.7 and 95.7% for CMV, and 89.2 and 94.6% for HHV-6, respectively. Also, similar findings were done by another author [26,3].
Conclusions
Adopting qualitative multiplex PCR technique in routine diagnosis is helpful in the screening of viral infections. It will save time, effort, cost effective and will assist in rapid diagnosis of 7 major herpes viruses within few hours. Also, quantitative real-time PCR should be performed in order to evaluate the clinical relevance of these 7 herpes virus infections. This is in turn will help patient’s management by using appropriate antiviral treatment.
To our knowledge, this is the first study that has been performed in the middle east region using multiplex PCR and real time PCR assays for the analysis of human herpes virus family genomic DNA in blood samples of Saudi pediatric leukemia patients and applying bioinformatics tool for primer design. Also, Correlation between percentage of coinfection, and clinical parameters for these 7 herpes viruses has been investigated.
Declarations
Ethics approval and consent to participate.
This study was performed in compliance with relevant laws and institutional guideline and in accordance with the ethical standards of the Declaration of Helsinki. The Institutional Review board (IRB) of the Qurayyat Health Affairs approved the protocol (Reg NO: H-13-S-071). Informed written consent form was obtained from all patients and individuals enrolled in the study.
Consent for publication
Not applicable
Availability of data and material
Datasets that have been analyzed during the current study available from the corresponding author on reasonable request.
Competing interest
The authors declare that they have no competing interests.
Funding
The current research is funded by College of Applied Medical sciences, Jouf University, KSA.
Authors’ contribution
WS designed the current study, supervised, performed the statistical analysis of the results, edited, and revised manuscript. HA supervised the clinical part and participated in editing the clinical part of the study, HAA collected samples and performed the practical part. ZF carried out and supervised the practical part. SK performed the practical part. IA revised and edited the manuscript. MF evaluated the results and participated in editing clinical part of the study. All authors read and approved the final manuscript.
Acknowledgement
We are grateful for all members at the Internal Medicine, and Clinical Laboratory Departments of Qurayyat General Hospital, Ministry of Health, KSA, for supporting us in the practical part.
Chang Ho, Shin et al (2003) Detection and Typing of HSV-1, HSV-2, CMV and EBV by Quadruplex PCR. Yonsei Medical Journal 44: 1001-1007. [ Ref ]
Schmutz hard J, Merete Riedel H, Zweygberg Wirgart B, Grillner L (2004) Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. Journal of Clinical Virology 29: 120-126. [ Ref ]
Tanaka T, Kazuhiro K, Hidenori S, Shigeaki N, Kenichi F, et al. (2009) Rapid and simultaneous detection of 6 types of human herpes virus (herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, human herpes virus 6A/B, and human herpes virus 7) by multiplex PCR assay. Biomedical research 30: 279-285. [ Ref ]
Durzyn´ ska J, Pacholska-Bogalska J, Kaczmarek M, Hanc´ T, Durda M, et al. (2011) Multiplex PCR for Identification of Herpes Virus Infections in Adolescents. Journal of Medical Virology 83: 267-271. [ Ref ]
Inazawa N, Hori T, Hatakeyama N (2015) Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol 87: 1427-1435. [ Ref ]
Mitchell PS, Espy MJ, Smith TF, Toal DR, Rys PN, et al. (1997) Laboratory Diagnosis of Central Nervous System Infections with Herpes Simplex Virus by PCR Performed with Cerebrospinal Fluid Specimens. Journal of Clinical Microbiology 35: 2873-2877. [ Ref ]
Hashimoto YE (1995) Investigation of EB virus and cytomegalovirus in rapidly progressive interstetial pneumonitis in polymyositis/ dermatomyositis by in situ hyberidization and polymerase chain reaction. Clin Immunol immuno Pathol 77: 298-306. [ Ref ]
Madi N, Al-Nakib W, Pacsa A (2011) Does cytomegalovirus develop resistance following antiviral prophylaxis and treatment in renal transplant patients in Kuwait? Adv Virol 2011: 260561. [ Ref ]
Loutfy SA, Fawzy M, El-Wakil M, Moneer MM (2010) Presence of human herpes virus 6 (HHV6) in pediatric lymphomas: impact on clinical course and association with cytomegalovirus infection. Virol J 7: 287. [ Ref ]
Morales-Sánchez A, Pompa-Mera EN, Fajardo-Gutiérrez A, Alvarez- Rodríguez FJ, Bekker-Méndez VC, et al. (2014) EBV, HCMV, HHV6, and HHV7 Screening in Bone Marrow Samples from Children with Acute Lymphoblastic Leukemia. Biomed Res Int 2014: 548097. [ Ref ]
Hara S, Kimura H, Hoshino Y (2002) Detection of herpesvirus DNA in the serum of immunocompetent children. Microbiol Immnol 46: 177-180. [ Ref ]
S Sugita, N Shimizu, K Watanabe, M Mizukami, T Morio, et al. (2007) Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol 92: 928- 932. [ Ref ]
World Health Organization (2014) Health Statistics and Information Systems: WHO Mortality Database. [ Ref ]
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108. [ Ref ]
Magel D, Tyring S (2012) Herpesviridae – A Look into This Unique Family of Viruses. Human Herpesviruses in Hematologic Diseases. InTech. [ Ref ]
Wade JC (2006) Viral infections in patients with hematological malignancies. Hematology Am Soc Hematol Educ Program 2006: 368-374. [ Ref ]
Selim MA, Parra V, Sangueza OP, Requena L, Sangueza MA (2015) Infectious Diseases and Infestations of the Vulva. Vulvar pathology. Springer, New York, NY. [ Ref ]
Aggarwal R, Bansal D, Naru J, Salaria M, Rana A, et al. (2014) HSV- 1 as well as HSV-2 is frequent in oral mucosal lesions of children on chemotherapy. Support Care Cancer 22: 1773-1779. [ Ref ]
Cukuranovic J, Ugrenovic S, Jovanovic I, Visnjic M, Stefanovic V (2012) Viral Infection in Renal Transplant Recipients. The Scientific World Journal 2012: 820621. [ Ref ]
Tabrizi R, Dehghani A, Nazhvani, Vahedi A, Gholami M, et al. (2014) Herpes Zoster Induced Osteomyelitis in the Immunocompromised Patients: A 10-year Multicenter Study. J Dent (Shiraz) 15: 112-116. [ Ref ]
Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, et al. (2016) The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood: 127:2007-2017 09-672030. [ Ref ]
Loutfy SA, Abo-Shadi MA, Fawzy M, El-Wakil M, Metwally SA, et al. (2017) Epstein-Barr virus and cytomegalovirus infections and their clinical relevance in Egyptian leukemic pediatric patients. Virology Journal 14: 46. [ Ref ]
Karadağ Geçgel S, Ersoy A, Sevinir BB, Sınırtaş M, Göral G (2012) Evaluation of PCR results in the diagnosis of Epstein-Barr virus infections. Mikrobiyol Bul 46: 594-606. [ Ref ]
Babady E (2016) Laboratory Diagnosis of Infections in Cancer Patients: Challenges and Opportunities. J Clin Microbiol 54: 2635-2646. [ Ref ]
Lisboa LF, Asberg A, Kumar D, Pang X, Hartmann A, et al. (2011) The clinical utility of whole blood versus plasma cytomegalovirus viral load assays for monitoring therapeutic response. Transplantation 91: 231-236. [ Ref ]
Wada K, Kubota N, Ito Y, Yagasaki H, Kato K, et al. (2007) Simultaneous Quantification of Epstein - Barr virus, Cytomegalovirus, and Human Herpesvirus 6 DNA in Samples from Transplant Recipients by Multiplex Real-Time PCR Assay. J Clin Microbiol 45: 1426-1432. [ Ref ]
Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, et al. (2012) Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 22: 144-155. [ Ref ]
Nefzi F, Ben Salem NA, Khelif A, Feki S, Aouni M, et al. (2015) Quantitative analysis of human herpesvirus-6 and human cytomegalovirus in blood and saliva from patients with acute leukemia. J Med Virol 87: 451-460. [ Ref ]
Zheng Y, Zhao Y, Wang Y, Rao J (2021) A multiplex real-time PCR quantitation of human herpesvirus 6, 7, 8 viruses: application in blood transfusions. Virology Journal 18: 38. [ Ref ]
Wada K, Mizoguchi S, Yoshinori, Kawada J, Yamauchi Y, et al. (2009) Multiplex real-time PCR for the simultaneous detection of herpes simplex virus, human herpesvirus 6, and human herpesvirus 7. Microbiol Immunol 53: 22-29. [ Ref ]
Imene H, Bechir A, Manel M, Sana R, Hazgui O, et al. (2020) Co-infections of human herpesviruses (CMV, HHV-6, HHV-7 and EBV) in nontransplant acute leukemia patients undergoing chemotherapy. Virol J 17: 37. [ Ref ]