Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 27 December, 2022
Accepted date: 10 February, 2023
Published date: 17 February, 2023
Citation: Bekele D, Lata W (2023) Retrospective Study on Prevalence of Typhoid Fever Infection among Patients Visiting Ejere Health Center, West Shawa Zone, Ethiopia. J Appl Microb Res. Vol: 6 Issu: 1 (01-08).
Copyright: 2023 Bekele D et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Typhoid fever is a major public health problem in worldwide including Ethiopia. The study design used in this study was a retrospective study to assess typhoid fever disease among patients in age ranges 1–18 years, who visited Ejere health center during the past three years (2018–2020). This study would also seek to know the conditions that are favorable to high prevalence of typhoid in the district. The results showed that total of 1152 individuals suffered from typhoid fever during the period of the study. From all patient, 486 (42.18%) were males, and 1158 (57.98%) were females. The highest number of cases was accounted for 422(36.31) in 2020, while the lowest number of cases was 346 (30.03) in 2018. Among this, typhoid fever was higher in patients from rural than patients from urban. Among the age groups, the 14–18 age groups were the most affected. Rural communities living far from health centers were susceptible to typhoid fever. High prevalence was observed during the wet months, although cases occurred throughout the year. This indicated that the risk factors were still continued and that there were no effective control measures in place. Therefore, an understanding of factors that influence the occurrence of typhoid fever in Ejere District was important in the management of the typhoid fever and environmental sanitation by communities could minimizes the risk factors.
Keywords
Typhoid fever, Ethiopia, Patients.
Abstract
Typhoid fever is a major public health problem in worldwide including Ethiopia. The study design used in this study was a retrospective study to assess typhoid fever disease among patients in age ranges 1–18 years, who visited Ejere health center during the past three years (2018–2020). This study would also seek to know the conditions that are favorable to high prevalence of typhoid in the district. The results showed that total of 1152 individuals suffered from typhoid fever during the period of the study. From all patient, 486 (42.18%) were males, and 1158 (57.98%) were females. The highest number of cases was accounted for 422(36.31) in 2020, while the lowest number of cases was 346 (30.03) in 2018. Among this, typhoid fever was higher in patients from rural than patients from urban. Among the age groups, the 14–18 age groups were the most affected. Rural communities living far from health centers were susceptible to typhoid fever. High prevalence was observed during the wet months, although cases occurred throughout the year. This indicated that the risk factors were still continued and that there were no effective control measures in place. Therefore, an understanding of factors that influence the occurrence of typhoid fever in Ejere District was important in the management of the typhoid fever and environmental sanitation by communities could minimizes the risk factors.
Keywords
Typhoid fever, Ethiopia, Patients.
Background
Typhoid fever is common among crowded and impoverished populations with inadequate sanitation and is transmitted through ingestion of water or food that has been contaminated by faeces or less commonly, urine of infected humans. Typhoid fever is a systemic prolonged febrile illness caused by certain Salmonella serotypes including Salmonella typhi, S. paratyphi A,B,C [1]. Typhoid is an infection of the gut that affects the whole body. It is spread from feces-to-mouth in contaminated food and water and often comes in epidemics (many people sick at once). Typhoid fever is a systemic infectious disease characterized by high continuous fever, malaise, and involvement of lymphoid tissues and spleen. It is reported that humans are the only host for Salmonella typhi and there are no known environmental reservoirs [2]. Typhoid fever remains a major public health problem in many developing countries. It is a sporadic disease in developed countries, occurring mainly in travelers returning from overseas, it can also produce the occasional point-source epidemic [3]. The illness begins with mounting fever, headache, vague abdominal pain and constipation, which may be followed by appearance of rashes in some cases. During the third week, the patient reaches a state of prolonged apathy, toxemia, delirium, disorientation and coma followed by diarrhoea. Serious epidemic forms of diarrhoea, e.g. typhoid (enteric fever), need a co-ordinated community approach. If left untreated, it can lead to complications affecting various organ systems [4]. The disease is communicable for as long as the infected person excretes S. typhi or S. Paratyphi in the feces or urine. Theoretically, it is possible to eliminate the salmonellae that cause enteric fever since they survive only in human hosts and are spread by contaminated food and water. However, given the high prevalence of the disease in developing countries that lack adequate sewage disposal and water treatment, elimination is currently unrealistic [4].
A study in Ethiopia indicated that, the prevalence of typhoid fever cases is high, so coordinated epidemiological surveillance is necessary to know the true burden of the disease [5,6]. Occurrence of typhoid can be reduced through improved sanitation and hygienic performance and access to clean water yet it is reported that typhoid prevalence is high. Increased health services, disease awareness and improved attitude of residents reduce the prevalence of typhoid fever. However, if water quality, sanitation facilities and hygienic practices are not constant, it would be difficult to control and prevent typhoid effectively. According to WHO, 2006, faecal pathogens are frequently transferred to the water borne sewage system, through flush toilets and pit latrines subsequently contaminating surface and ground water [7]. Typhoid outbreaks do occur if control and preventive measures are not taken in a timely manner. Poor waste disposal and hygiene of workers in food handling and preparation activities would provide an obvious infection route.
Typhoid fever is one of the leading causes of morbidity and mortality across the world [8]. Typhoid is caused by a bacterium of the genus Salmonella. Salmonella infection in humans can be categorized into two broad types, that caused by low virulence serotypes of Salmonella enteric which cause food poisoning, and that caused by the high virulence serotypes Salmonella enteric typhi (S. typhi), that causes typhoid, and a group of servers, known as S. paratyphi A, B and C, which cause Paratyphoid. Humans are the only host of this latter group of pathogens. Salmonella typhi is a highly adapted human-specific pathogen [9]. A recent estimate found that 22 million new typhoid cases occur each year in the world with some 200,000 of these resulting in death [1]. Indicating that the global burden of this disease has increased steadily from a previous estimate of 16 million [10]. However, case- fatality rates have decreased markedly [1]. Generally, typhoid is endemic in impoverished areas of the world where the provision of safe drinking water and sanitation is inadequate and the quality of life is poor. Although, contaminated food and water have been identified as the major risk factors for typhoid prevalence, a range of other factors have been reported in different endemic settings such as poor sanitation, close contact with typhoid cases or carriers, level of education, larger household size, closer location to water bodies, flooding, personal hygiene, poor life style, and travelling to endemic areas. In addition, climatic variables such as, rainfall, vapour pressure and temperature have an important effect on the transmission and distribution of typhoid infections in human population [11].
However, no previous study has been reported in the literature regarding to the prevalence of typhoid fever infection in the population of Ejere health center. Therefore, the aim of this study is to generatedata on the prevalence of Typhoid fever infection in Ejere health center. These data may help partly in the planning of Typhoid fever control and prevention for the districts’ health sector, both governmental and NGOs (Non-governmental organization), and also the studies was to encourage many researchers to investigate the prevalence of typhoid infection regarding to the study area.
However, despite all the efforts taken to control, the disease continues to occur in Ejere District leading to significant morbidity (District health workers, 2020 personal communication). The objective of this study was to assess the temporal occurrence of typhoid fever in Ejere District for the past three years.
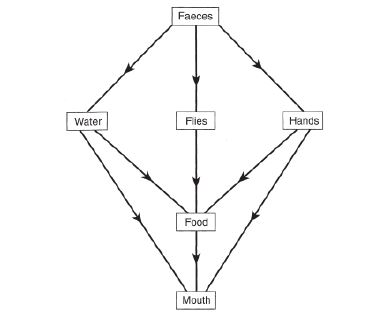
Many diseases are caused by food, water and hands that are contaminated by disease-causing organisms or “pathogens” that come from faeces. The diseases caused by these pathogens are called faecal–oral diseases because faecal material is ingested (Figure 1) [12]. These diseases, which include typhoid is responsible for much sickness and many deaths each year.
After ingestion in food or water, typhoid organisms pass through the pylorus and reach the small intestine. They rapidly penetrate the mucosal epithelium via either micro fold cells or enterocytes and arrive in the lamina propria, where they rapidly elicit an influx of macrophages that ingest the bacilli but do not generally kill them. Some bacilli remain within macrophage of the small intestinal [13].
Figure 1:Faecal-oral routes of typhoid transmission [12].
Water is a source of diseases of typhoid and paratyphoid which affect the alimentary canal. In home and school, it is essential that hands should be washed after defecating or urinating, for infection can be transferred in unclean hands used to prepare food or handle eating pots. Unwashed hands, exposed septic sores, contaminated water and flies can also spread infection to food during its preparation [14].
In areas where drainage and sanitation are poor, water runs over the ground during rainstorms, picks up faeces and contaminates water sources. This contributes significantly to the spread of diseases such as typhoid [15].
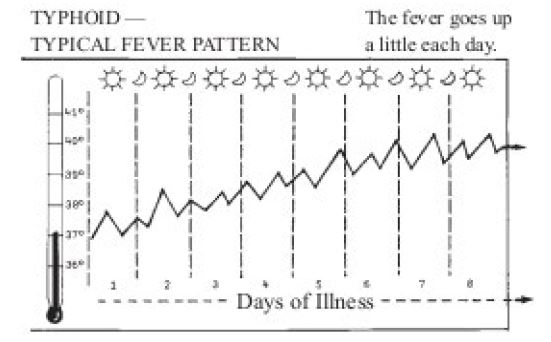
Typhoid begins like a cold. Temperature goes up a little more each day (Figure 2) [16]. Pulse rate relatively slow and sometimes diarrhea and dehydration. Trembling or delirium (mind wanders) and person very ill. Typhoid bacilli are drained into mesenteric lymph nodes where there is further multiplication and ingestion by macrophages. It is believed that typhoid bacilli reach the bloodstream principally by lymph drainage from mesenteric nodes, after which they enter the thoracic duct and then the general circulation. As a result of this silent primary bacteremia the pathogen reaches an intracellular haven within 24 hours after ingestion throughout the organs of the reticulo-endothelial system (spleen, liver and bone marrow), where it resides during the incubation period, usually of 8 to 14 days. The incubation period in a particular individual depends on the quantity of inoculums (the introduction of pathogenic organisms into body to produce immunity to the specific diseases), i.e. it decreases as the quantity of inoculum increases, and on host factors. The incubation periods ranging from 3 days to more than 60 days have been reported.
The clinical presentation of typhoid fever varies from a mild illness with low-grade fever, malaise, and slight dry cough to a severe clinical picture with abdominal discomfort and multiplecomplications. Many factors influence the severity and overall clinical outcome of the infection. They include the duration of illness before the initiation of appropriate therapy, the choice of antimicrobial treatment, age, the previous exposure or vaccination history, the virulence of the bacterial strain, the quantity of inoculums ingested, host factors (e.g. HLA type, AIDS or other immunosuppression) and whether the individual was taking other medications such as H2 blockers or antacids to diminish gastric acid. Patients who are infected with HIV are at significantly increased risk of clinical infection with S. typhi and S. paratyphi. Acute non-complicated disease: Acute typhoid fever is characterized by prolonged fever, disturbances of bowel function (constipation in adults, diarrhoea in children), headache, malaise and anorexia. Bronchitis cough is common in the early stage of the illness. During the period of fever up to 25% of patients are show exanthema (rose spots), on the chest, abdomen and back [17].
Figure 2:The fever pattern of typhoid [16].
Abdominal discomfort develops and increases. It is often restricted to the right lower quadrant but may be diffuse. The symptoms and signs of intestinal perforation and peritonitis sometimes follow, accompanied by a sudden rise in pulse rate, hypotension, marked abdominal tenderness, rebound tenderness and guarding, and subsequent abdominal rigidity. A rising white blood cell count with a left shift and free air on abdominal radiographs are usually seen. Altered mental status in typhoid patients has been associated with a high case-fatality rate. Such patients generally have delirium, rarely with coma [18].
The disease is communicable for as long as the infected person excretes S. typhi or S. paratyphi in the feces or urine. A study by Beyene et al. showed Salmonella typhus is widespread in the community [6]. Being an important communicable disease in the national list, typhoid fever has received considerable control efforts at national, regional and district levels. However, despite all the efforts taken to control, the disease continues to occur in Ejere district leading to significant morbidity.
The aim of this study was to assess the prevalence of typhoid fever infection in the Ejere health centers in the past three years (2018–2020). This study was given better information about the level of typhoid fever infections in the study area. Incorporating the result of this work with other similar findings to take appropriate health care measures will contribute for the social and economic wellbeing of the community in the region.
Methods
Description of the study area
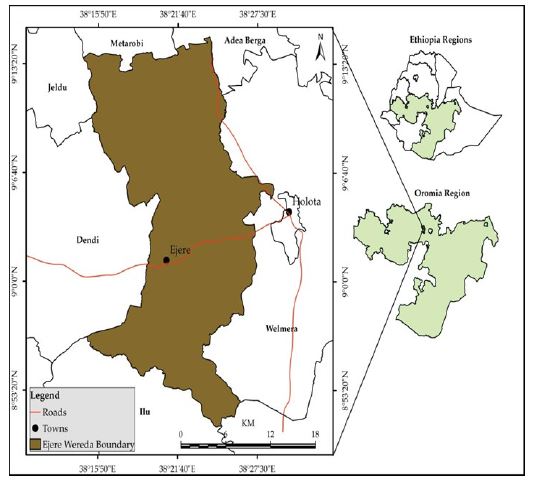
This study was conducted from September 2018-august 2020 in Ejere health center, Ejere District (Figure 3) which is located in west Shawa zone, Oromia Regional state, Ethiopia. The area is bordered on the west by Ejersa Lefo, on the south by Sebata Awas, on south west by Ilu, on the north by Adda Berga, on the North West by Meta Robi and on the east by Walmara districts. With regards to health service status of the District, there are 3 health centers such as Gorba health center, Amaro health center and Ejere health center with total coverage of 97%. It gives services for cases like; emergency, pre-natal, deliver service and for different chronic diseases like intestinal parasitic infections. The major water sources for human consumption are deep wells (from pulling system). Rivers and spring water are also available in seasonal pools and minor rivers; the total water coverage of the District is 63% among which almost 50% is river and ground water. The district is located at about 42 km to the west of capital city of the country Addis Ababa. The area has an elevation of about 2495m above sea level with longitude of 90 2’N and 32024 ‘E. It has an estimate total population of 115029 according to central statistical agency 2007 with growth rate of 2.9% increase per year consisting of 58504 males and 56525 females (Ejere district administration office, 2020).
Figure 3:Map of the study area.
All patients of age1-18 years were suspected of typhoid fever who visited the clinic during the years between 2018– 2020. The sampling frame for the study was, therefore, the number of cases of typhoid that had been recorded during the study period at Ejere health center. Inclusion criteria: Patients who are suspected to have typhoid infection in age1-18 were assessed. Exclusion criteria: Patients whose bloods are not tested for typhoid infection were excluded.
A secondary data was collected using the prepared check list like individuals with age groups of 1– 18 years and sex from log books of clients of patients treated in Ejere Health center between 2018 and 2020.
Laboratory diagnosis
The Widal slide agglutination test was done using S. typhi O and H antigens according to the instructions of the manufacturer. The antigen suspension commercially available in 5 ml volume from spin react Reagent Ltd. (Spain) was used. A direct slide agglutination technique was used in this study for qualitative determination of the agglutination ability of sera. In brief, the test was done by mixing one drop of serum with one drop each of O and H antigens separately on slide. After shaking the slide back and forth for 1 min, the mixture was observed for macroscopic agglutination. If there was agglutination within 1 min it was reported as reactive, otherwise, non- reactive.
Results
The present study attempted to assess the prevalence of typhoid fever among children of 1–18 years and associated risk factors in Ejere Health center. The results of the data extracted from patient records in Ejere health center presented as follows. Of the total population (1152) diagnosed during past three years for typhoid fever 487 (42.27%) were males and 668 (57.98%) were females. Out of the total population 431(37.41) patients were from Ejere Urban area of which 168(14.58) were males while 263(22.82) were females (Table 1). Regarding their age distribution ranged from 1–18 years; 210 (18.22%) students were in the age group 1–5 years, 279 (24.21%) were 6–9 years, 317 (27.51%) were 10–13 years and 346 (30.04%) were 14–18 years old. The highest number of cases was found as 422(36.63%) seen in 2020, whereas the lower number of cases 346(30.04%) was seen in 2018 year and followed by 384 (33.33%) in 2019 year.
Prevalence of typhoid infections among patients visited Ejere health center
Of the total 1152 patients examined for typhoid fever 487 (42.27%) and 668 (57.98%) males and females, respectively. Although gender was not a significant risk factor for the prevalence of typhoid infections, in the present study, females showed high prevalence of infections than males. The number of typhoid cases in the year 2018 relatively low, followed by 2019 high and highest in 2020 (Table 2).
Period of prevalence of typhoid fever in Ejere health center from 2018-2020
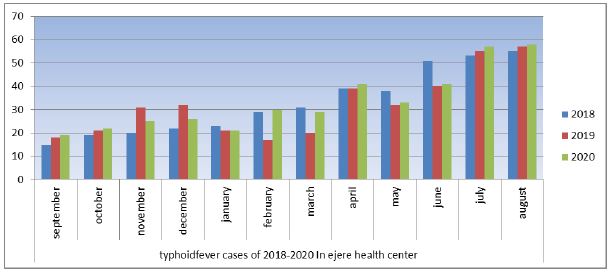
In 2018 cases gradually increased after September in that year recorded were 21cases. Generally, cases recorded during this month were low. October recorded 29 cases, and then reduced in November by 3. The same number was recorded in December. From there, cases gradually increased month by month, until it reached a peak in august. The figure 1 represents the number of typhoid cases in the year 2018–2020 with August recording the highest, followed by July, June, October and September producing the lowest number of cases. The year 2019 was in sharp contrast to the previous year since the disease peaked in august lowest in the following month. Subsequent months recorded fluctuations in the number of typhoid fever cases until the turn of the year. August produced the first-highest number of typhoid fever cases (Figure 1). In 2020, cases gradually increased before peaking in August, reducing slightly in September and recording the highest in august (Figure 1). In all years After March, numbers gradually increased. Of all year 2020 rerecord the highest cases. Typhoid fever began to increase from 2018 through 2019 and 2020. Across all the years, the period from September- August produced the highest number of cases. In all years the incidence of typhoid fevers increased gradually over the twelvemonth period was seen. The highest incident of typhoid fever was recorded at end year in August and the lowest recorded in middle of the year in September (Figure 4).
Table 1:Age category among male and female in rural and urban population areas in the year between 2018 and 2020.
| Year | Age | Male | Female | Total No. | ||
|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | |||
| 2018(n=346) | 1-5 | 13 | 15 | 12 | 28 | 68 |
| 6-9 | 9 | 21 | 16 | 32 | 78 | |
| 10-13 | 10 | 30 | 23 | 30 | 93 | |
| 14-18 | 13 | 32 | 25 | 40 | 110 | |
| 2019(n=384) | 1-5 | 11 | 16 | 20 | 27 | 74 |
| 6-9 | 19 | 22 | 21 | 37 | 99 | |
| 10-13 | 13 | 30 | 29 | 30 | 102 | |
| 14-18 | 14 | 35 | 27 | 33 | 109 | |
| 2020(n=422) | 1-5 | 10 | 12 | 20 | 26 | 68 |
| 6-9 | 18 | 32 | 19 | 33 | 102 | |
| 10-13 | 17 | 37 | 20 | 48 | 122 | |
| 14-18 | 21 | 37 | 31 | 41 | 130 | |
| Total | 155 | 329 | 263 | 405 | 1152 | |
Discussion
The present study finding showed that the majority of patients were within age groups of 10–13. The reason for which may be attributed to causative factors such as increased consumption of unhygienic food and water, bathing/swimming in ponds, with a higher possibility of exposure to S. typhi infection and consequently higher incidence of typhoid fever in this particular age group. The study agrees with the study done by Rahman et al. in Bangladesh where the prevalence of typhoid fever was highest (56.25%) among the school age patients compared to adolescents (16.67%) and preschool age children (14.58%) [19]. And study done in Kolkata, India by Ghosh et al., revealed that the most vulnerable age group was found between 10–14 years [20]. The variations might be due to different demographic characteristics, study setting, their perceptions and economic status of the country.
A total of 1152 people were found to have suffered from typhoid fever during the study period. Of these numbers, 487 (42.27%) were males and 668 (57.98%) females. Out of the total population 418 patients were from Ejere Urban area where as 734 patients were come from rural area of Ejere town. In this study, the prevalence of typhoid fever was higher among rural as compared to the urban. This is due to the lack of sufficient safe drinking water, toilet and water for hand washing after the toilet. This probably creates a greater opportunity for person-to-person transmission in these congregated sites. In each year, females were more affected than males. Regarding to sex group females were at high risk of being infected by the disease, this might be due to they eat uncooked fruits and raw meat when they cook food. This study is in contrast to the study finding by showed that males and females were at risk with relatively equal chances of being infected by the disease and therefore equally susceptible. Other study reported that high prevalence among female than male [5,21,22].
Table 2:Prevalence of typhoid infections among the study population in Ejere health center.
| Year | Age | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Urban | Percent (%) | Rural | Percent (%) | Urban | Percent (%) | Rural | Percent (%) | ||
| 2018(n=346) | 1-5 | 13 | 19.11 | 15 | 22.05 | 12 | 17.64 | 28 | 41.47 |
| 6-9 | 9 | 11.5 | 21 | 26.93 | 16 | 20.5 | 32 | 41.02 | |
| 10-13 | 9 | 11.53 | 30 | 32.22 | 23 | 24.73 | 30 | 32.60 | |
| 14-18 | 10 | 12.82 | 32 | 29.09 | 25 | 22.72 | 40 | 37.38 | |
| 2019(n=384) | 1-5 | 13 | 11.81 | 16 | 21.62 | 20 | 27.02 | 27 | 35.52 |
| 6-9 | 11 | 14.86 | 22 | 22.22 | 21 | 21.21 | 37 | 40.65 | |
| 10-13 | 19 | 19.19 | 30 | 29.41 | 29 | 28.43 | 30 | 27.77 | |
| 14-18 | 13 | 12.74 | 35 | 32.11 | 27 | 24.77 | 33 | 30.55 | |
| 2020(n=422) | 1-5 | 14 | 12.84 | 12 | 17.64 | 20 | 29.41 | 26 | 36.11 |
| 6-9 | 10 | 14.70 | 32 | 26.22 | 19 | 15.57 | 33 | 35.10 | |
| 10-13 | 18 | 14.75 | 37 | 30.32 | 20 | 16.39 | 48 | 39.02 | |
| 14-18 | 16 | 12.8 | 37 | 28.46 | 31 | 23.8 | 41 | 32.53 | |
| Total | 155 | 13.45 | 329 | 27.69 | 263 | 22.83 | 405 | 35.16 | |
Figure 4:Typhoid fever cases in Ejere health center from 2018-2020.
Regarding to the distribution of typhoid fever in this study it varied from year to year. The number of typhoid cases in the year 2018 relatively low, followed by 2019 high and highest in 2020. This shows an increasing rate of typhoid fever in study area as the years go by, and from this, it could be predicted that of 2021 and subsequent years could be higher if measures were not put in place to deal with it. A similar increase in Salmonella cases year by year was observed by another study [23]. The prevalence of typhoid fever in the age group of the age distribution ranged from 1-18 years; 210 (18.22%) students were in the age group 1-5 years, 279 (24.21%) were 6-9 years, 317 (27.51%) were 10-13 and 346 (30.04%) were 14-18 years old. In the present finding, the majority aged between 14- 18 years suffered from typhoid than others in other age groups in their life time. This is due to children under these ages may play on faecally contaminated area. As reported in other study children usually do not take care of their personal hygiene. For instance, they play in contaminated outdoor environments, in and around disposal sites (which can certainly cause serious health problems), face problem because of absence of latrine and lack of life skills such as washing hands before and after meals [24]. Furthermore, according to Gebre conditions of cooking utensils, food storage systems (time and temperature) as well as food handlers’ knowledge and practices affect food safety directly or indirectly [25]. More over a study conducted in India identified that, improved personal hygiene and intensive community health education are the public health measures that could help to prevent and control Typhoid Fever [26].
The present study finding revealed that, most patients (63.9%) were from rural residents and (36.1%) were from urban areas. This study finding was in line with the report from Birhanie et al. in which most of the patients were farmers (71.5%), rural residents (89%) [27]. Also, the finding supports the study done by Sharma of Lakhimpur District of Assam where people residing in rural areas were highly afflicted with typhoid as compared to urban people [28]. This is due to use of unsafe drinking water, improper sanitation and inadequate medical care, using raw fruits and vegetables by those people, unhygienic conditions in their surrounding environment.
Although studies reported that there is no significant difference in the occurrence of typhoid between urban and rural environments, in this study, since most of the patients are from rural residents’ higher risk of typhoid fever as compared to urban residents [29]. Although studies reported that there is no significant difference in the occurrence of typhoid between urban and rural environments. In this study, since most of the patients are from rural resident’s higher risk of typhoid fever was occurring as compared to urban residents. This could be explained by suboptimal access to safe water and lack of hygienic education which was supported by the high prevalence of typhoid among farmers with no formal education [17]. Moreover, the reason why the prevalence of typhoid lower in urban area is since there is a greater centralization of services and many clinics are associated with large hospitals. The results of this study concur with the study carried out, where a high prevalence of typhoid fever occurred due to overcrowding with poor access to clean water and sanitation [22,30].
In this study the assessment of typhoid data over age above 16 ages were mostly affected than the rest of age group. However, in 2018, 2019 and 2020 relatively below 14 ages were decreased, whereas between 14 and 18 increased in 2020. Typhoid cases are observed throughout the year, the peak incidence of typhoid fever is reported during the summer in endemic areas as the secondary data indicates, during the rainy season from year to year was high due to high transmission time of typhoid fever from (2018–2020). In this study there was a high occurrence of typhoid fever in July and August. This supports the study done by Sharma in Lakhimpur District of Assam, where there was an increase in the incidence of typhoid during the month of July-September [28]. But it was against the finding by Badaru et al. in Ejule (Nigeria) indicates that the month of January and February during the 2011 periods, January and December during the 2012 periods and January during 2013 periods having the highest occurrences [31]. Moreover, the highest numbers of cases of typhoid fever are recorded during the rainy season in tropical and developing countries [1].
These results concur with the study carried out in Kibera, where a high prevalence of typhoid fever (24.7%) occurred due to overcrowding with poor access to clean water and sanitation [30]. According to Ashebir et al., the health extension workers promote the awareness among the rural communities to construct latrines but have been less active in teaching proper use and maintenance [32]. Inorder to combat diseases caused by inadequate sanitation more efficiently, the installation of sanitary excreta facilities should be encouraged with measures taken to dispose of wastes and improve personal and food hygiene [33].
Conclusion
The findings of the present study showed that typhoid fever infections were prevalent and are found to be one of the health problems in the study area. The 14-18 age group and females were highly associated with infections and this problem can create condition on people to be exposed to typhoid fever infections. Providing well treated water, hygienic practice, encouraging healthy behaviors and environmental sanitation in the study area could help in reducing the prevalence of typhoid fever,
Recommendations
Health education should be offered to the society to raise their attitude, knowledge, and practice on the prevention of the typhoid fever. To prevent the prevalence of typhoid fever, personal hygiene and environmental sanitation should be practiced. The change in habit of defecation, using latrines instead of open fields, will create a less contaminated environment. Protection of water sources from contamination is a proven way of reducing infections of all sorts. The health organization has responsibility for all the officially controlled medical care within the rural area, including school medical services, clinics and health centers.
Declarations
Ethical approval
The study protocol was approved by a college of Natural and Computational Science, Biology Department using an agreement letter prepared from Ambo University. All the information that was obtained about the subjects was kept confidential.
Consent for publication
Not applicable
Availability of data and material
Data generated or analyzed during this study are included in this article.
Competing interests
Authors have declared that no competing interests exist.
Funding
The study was granted from Research and Community Services Office, Ambo University, Ethiopia.
Authors’ contributions
DB: Conceived designed, data management, analysis, and interpretation of the findings and drafting the manuscript. DB and WL: analysis, interpretation, and reviewing the manuscript. The two authors read and approved the final manuscript.
Acknowledgement
Authors thank to the Ejere Woreda Health Office for their enthusiastic cooperation on typhoid fever data collection and we also thank to Biology Department of Ambo University to finalize this research.
Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82: 346-353. [ Ref ]
World Health Organization (2003) Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. Geneva. [ Ref ]
Ackers ML, Puhr ND, Tauxe RV, Mintz ED (2000) Laboratory-based surveillance of Salmonella serotype typhi infections in the United States: antimicrobial resistance on the rise. JAMA 283: 2668-2673. [ Ref ]
Fauci AS, Kasper DL, Longo DL, Hauser B, Loscalzo J (2008) Harrison’s Principles of Internal Medicine. McGraw-Hill Professional, New York, USA. [ Ref ]
Crump JA, Mintz ED (2010) Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50: 241-246. [ Ref ]
Beyene G, Asrat D, Mengistu Y, Aseffa A, Wain J (2008) Typhoid fever in Ethiopia. J Infect Dev Ctries 2: 448-453. [ Ref ]
WHO (2006) Preventing diseases through healthy environment towards an estimate of the environmental burden of diseases. World Health Organization, Geneva, Switzerland. [ Ref ]
Nagashetty K, Channappa ST, Gaddad SM (2010) Antimicrobial susceptibility of Salmonella typhi in India. J Infect Dev Ctries 4: 070–073. [ Ref ]
Bhan M, Bahl R, Bhatnagar S (2005) Typhoid and paratyphoid fever. Lancet 366: 749-762. [ Ref ]
World Health organization (WHO) (1997) Typhoid fever. WHO, Geneva. [ Ref ]
Wang LX, Li XJ, Fang LQ, Wang DC, Cao WC (2012) Association between the incidence of typhoid and paratyphoid fever and meteorological variables in Guizhou, China. Chinese Med J 125: 455-460. [ Ref ]
Howard G (2002) Healthy villages: a guide for communities and community health workers. World Health Organization, Geneva. [ Ref ]
Black RE, Cisneros L, Levine MM, Banfi A, Lobos H (1985) Case-control study to identify risk factors for pediatric endemic typhoid fever in Santiago, Chile. Bull World Health Organ 63: 899-904. [ Ref ]
Soper R, Smith ST (1986) Biology: An integrated approach for East African Schools. Macmillan publishers, London and Basingstoke. [ Ref ]
Kolsky P (1998) Storm drainage: an intermediate guide to the lowcost evaluation of system performance. Intermediate Technology Publications, London. [ Ref ]
Werner D, Thuman C, Maxwell J (2015) Where there is no doctor: a village health care handbook. Hesperian Health Guides 1919 Addison, Berkeley, California, USA. [ Ref ]
Andualem G, Abebe T, Kebede N, Gebre-Selassie S, Mihret A, et al. (2014) Prevalence and antimicrobial susceptibility patter of Salmonella typhi and Salmonella paratyphi isolates from patients with clinical symptom compatible with typhoid fever. 2nd International Congress on Bacteriology and Infectious Diseases. J Bacteriol Parasitol 5: 183. [ Ref ]
Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, et al. (1999) Typhoid fever in children aged less than 5 years. Lancet 354: 734-737. [ Ref ]
Rahman A, Ahmad M, Begum RS, Hossain MZ, Hoque SA, et al. (2011) Prevalence of Typhoid fever among the children in a semi urban area of Bangladesh. J Dhaka Med Coll 20: 37-43. [ Ref ]
Ghosh S, Batabyal P, Rajendran K, Palit A (2010) Typhoid fever in rural communities, division of epidemiology and bacteriology National Institute of cholera and enteric diseases. Jpn J Infect Dis 63: 220. [ Ref ]
Youssef FG, Daba AS, Kabeil SS, Parker TM (2010) A Comparative study of blood culture and antibody response with the duration of illness in diagnosis of typhoid fever. Australian Journal of Basic and Applied Sciences 4: 609-614. [ Ref ]
Eba K, Bekele D (2019) Prevalence of Typhoid Fever and its Risk Factors in Lalo Assabi District, West Wollega, Oromiya, Ethiopia. Journal of Bacteriology & Parasitology 10: 365. [ Ref ]
Tadesse G (2014) Prevalence of human Salmonellosis in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis 14: 88. [ Ref ]
Abu Mourad T (2004) Palestinian refugee conditions associated with intestinal parasites and diarrhoea: Nuseirat refugee camp as a case study. J Public Hlth 118: 131-142. [ Ref ]
Gebre ET (1997) Public Food Service Establishment Hygiene. Food Hygiene: Principles and Methods of Food-borne Diseases Control with Special Reference to Ethiopia. Addis Ababa University, Ethiopia. [ Ref ]
Sur D, von Seidlein L, Manna B, Dutta S, Deb AK, et al. (2006) The malaria and typhoid fever burden in the slums of Kolkata, India: data from a prospective community-based study. Trans R Soc Trop Med Hyg 100: 725-733. [ Ref ]
Birhanie M, Tessema B, Ferede G, Endris M, Enawgaw B (2014) Malaria, Typhoid Fever, and their coinfection among febrile patients at a rural Health Center in Northwest Ethiopia: A Cross-Sectional Study. Adv Med 2014: 531074. [ Ref ]
Sharma J (2013) Distribution of Typhoid fever in different rural and urban areas of Lakhimpur District of Assam. Int J Res Dev Health 1: 109-114. [ Ref ]
Whitaker JA, Franco-Pardes C, del Rio C, Edupuganti C (2009) Rethinking typhoid fever vaccines: implications for travelers and people living in highly endemic areas. J Travel Med 16: 46-52. [ Ref ]
Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, et al. (2012) Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya:Implications for typhoid vaccine use in Africa. PloS One 7: e29119. [ Ref ]
Badaru YU, Olayemi IK, Spencer O, Yakubu M (2014) Assessment of a vulnerable rural community to typhoid fever using geospatialtemporal analysis: Case Study of Ejule, Kogi State of Nigeria. Journal of Environment and Earth Science 4: 22. [ Ref ]
Ashebir Y, Sharma HR, Alemu K, Kebede G (2013) Latrine use among rural households in northern Ethiopia: a case study in Hawzien District, Tigray. International Journal of Environmental Studies 70: 629-636. [ Ref ]
Charles K (1995) Community Health and Sanitation Intermediate technology Publications, London, United Kingdom. [ Ref ]