Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 13 May, 2019
Accepted date: 17 May, 2019
Published date: 24 May, 2019
Citation: Istifanus F.M, Umar AF, Ayika PD, Danladi MMA, Agbo EB (2019) Sensory Evaluation and Microorganisms Associated with Pap Produced from Acha Varieties. J Appl Microb Res. Vol: 2 Issu: 1 (41-46).
Copyright: © 2019 Francis IM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Fourteen different Acha varieties were processed to produce fermented starch and non- fermented flour which were further used to make non-fermented, wet and dry fermented pap. A total of nineteen (19) microorganisms were isolated from the products. They include: Bacillus species, Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella species, Streptococcus faecalis, Lactobacillus fermentum, Leuconostoc mesenteroides, Lactobacillus brevis, Mucor species, Rhodotorula species, Saccharomyces cerevisiae, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Trichophyton species, Trichophyton rubrum, Streptococcus species, Aeromonas species, and Lactobacillus species, the microbial load ranges between 1.1X101 to 4.6 X104cfu/g. The sensory evaluation test reveals that wet pap was more attractive and likened compared to the non-fermented dry grain flour pap. Considering its nutritional value and the acceptability by consumers the use of it as food supplement should be encouraged.
Keywords
Fermented Starch; Non-Fermented Flour; Consumer Preference; Pap Samples.
Abstract
Fourteen different Acha varieties were processed to produce fermented starch and non- fermented flour which were further used to make non-fermented, wet and dry fermented pap. A total of nineteen (19) microorganisms were isolated from the products. They include: Bacillus species, Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella species, Streptococcus faecalis, Lactobacillus fermentum, Leuconostoc mesenteroides, Lactobacillus brevis, Mucor species, Rhodotorula species, Saccharomyces cerevisiae, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Trichophyton species, Trichophyton rubrum, Streptococcus species, Aeromonas species, and Lactobacillus species, the microbial load ranges between 1.1X101 to 4.6 X104cfu/g. The sensory evaluation test reveals that wet pap was more attractive and likened compared to the non-fermented dry grain flour pap. Considering its nutritional value and the acceptability by consumers the use of it as food supplement should be encouraged.
Keywords
Fermented Starch; Non-Fermented Flour; Consumer Preference; Pap Samples.
Introduction
The increased concern on the rising cost of wheat and other cereals have led to the need to search for indigenous cheap and easily available low sugar foods, which became necessary to explore the full potential of Acha (Digitaria exilis) as an alternative to low sugar food to supplement the diet of the people especially the diabetic patients [1]. Jideani and Jideani [2], also reported that supplementing our diets with Acha products would lead to lowering of cholesterol levels, strengthening of the immune system as well as acting as food roughages which aid the digestive system.
Despite the nutritional health benefits of this grain, it is still being neglected by research and extension service which have led to a decline in its cultivation. As such it can no longer compete with other crops like rice, wheat, millet, sorghum and maize. This is because the production in Nigeria is exclusively in the hands of poor resource rural farmers [3].
However, the utilization of Acha grain products and especially the fermented product like pap would undoubtedly lead to the improvement in the economic status of the producers, as is being used by individuals of different societal status [4]. It is believed that the original constituents of food are enhanced during fermentation due to the biochemical activities of associated microorganisms which provide a wide diversity of flavor, aroma, tastes and textures that enriched the human diet as reported by Adeleke and Abiodun [5]. It is in view of this, that the present study has been designed to isolate and characterized the microorganisms associated with fermented and nonfermented products of different Acha varieties and to also evaluate the sensory attributes of the pap produced.
Materials and Method
Sample collection
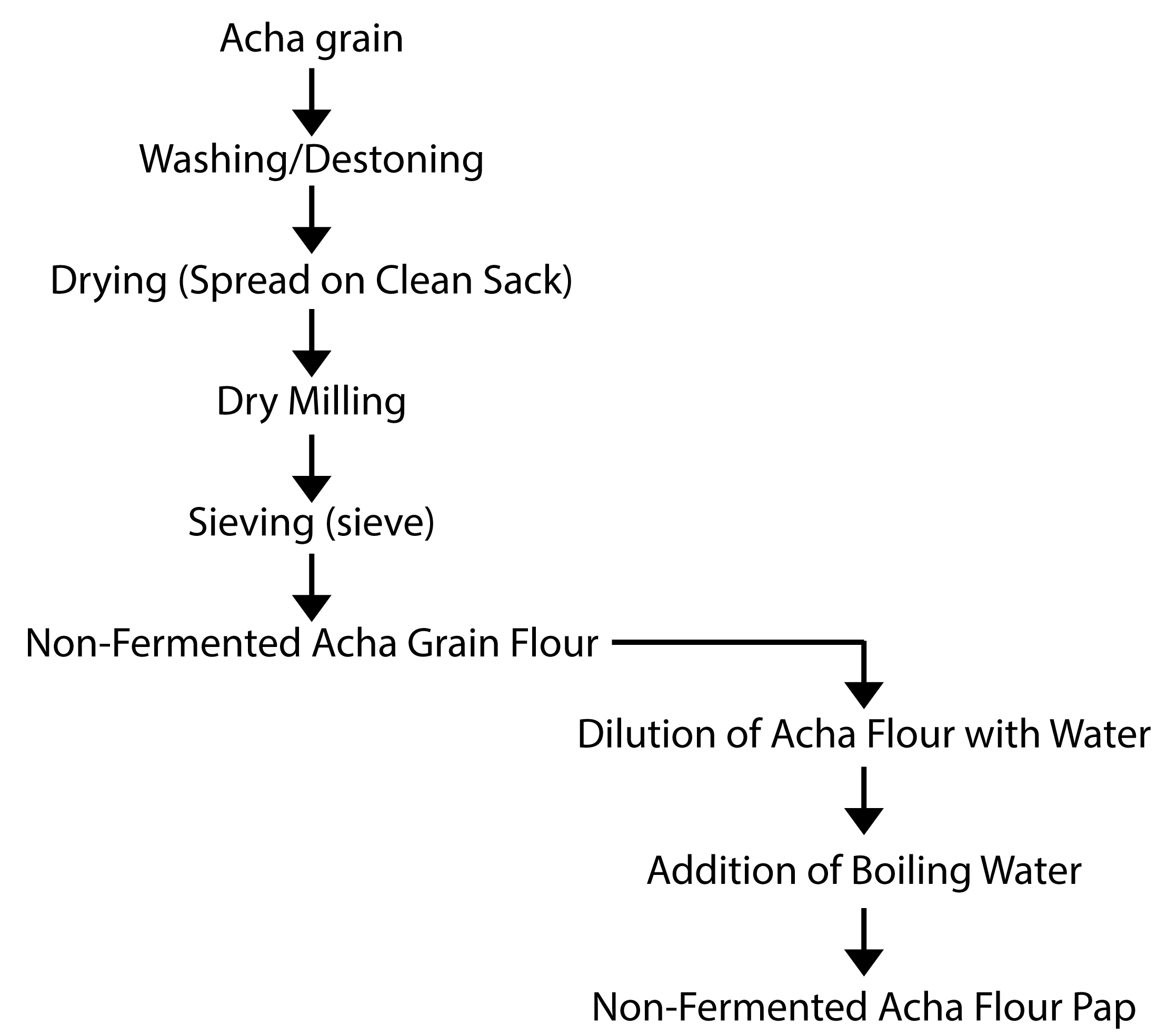
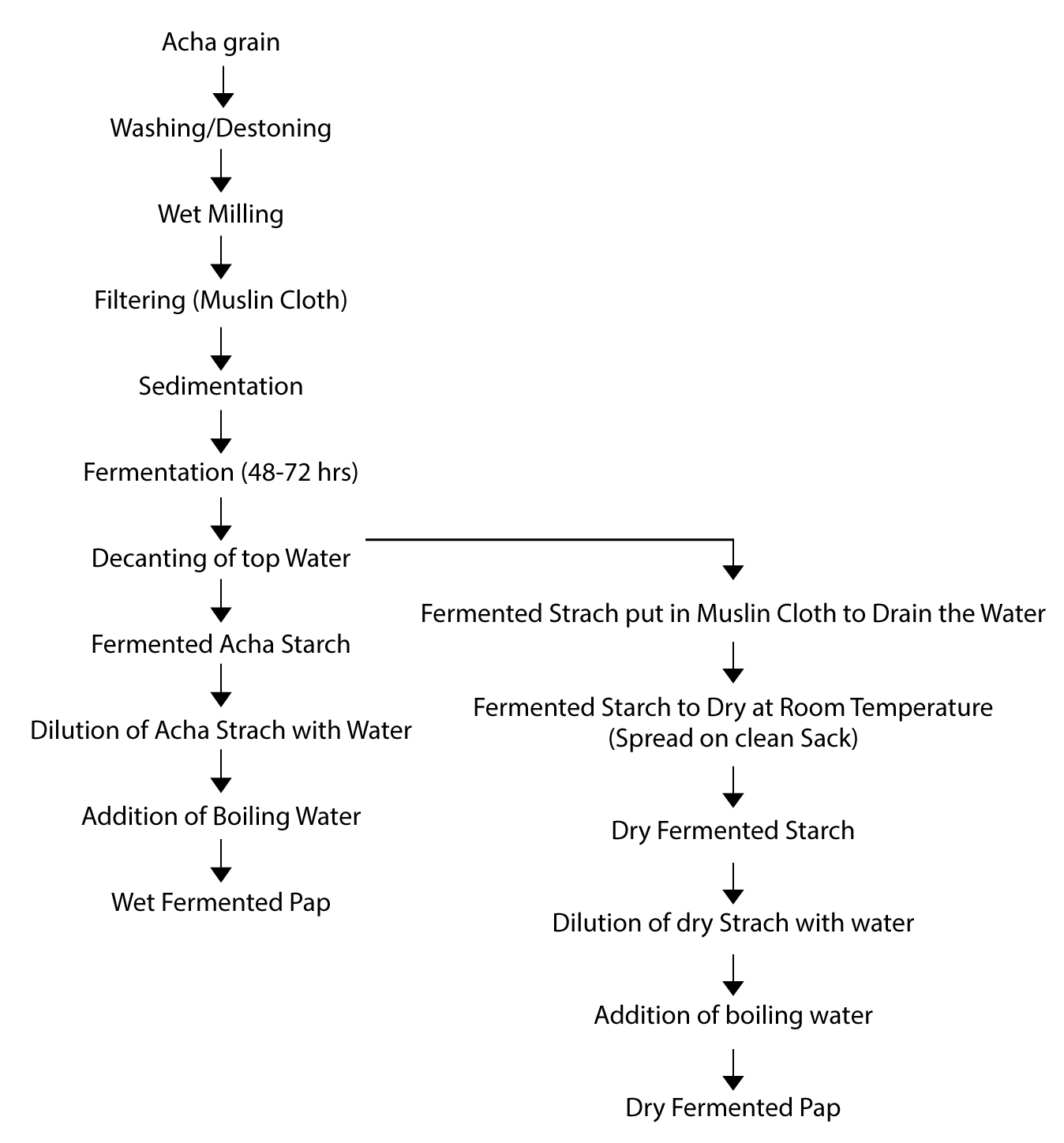
The study was carried out in four selected local governments, Bogoro in Bauchi state, Barkin Ladi, Bokkos and Mangu of Plateau state, Nigeria. A total of fourteen different varieties of Acha grains were obtained in their dehulled form from the producers, which were processed to produce fermented starch and non-fermented flour used to make pap. The preparation of the fermented starch, nonfermented flour and pap are described in figures 1 and 2 below.
Figure 1: Flow chart for the preparation of non – fermented Acha flour and pap.
Figure 2: Flow Chart for the preparation of wet and dry fermented Acha starch and pap.
Microbiological analysis of fermented starch and the non-fermented flour samples
Isolation and enumeration of microbial loads of samples: The starch and flour produced from the different Acha varieties were serially diluted. One ml of representative sample from the starch and flour was withdrawn aseptically into a 9ml of sterile distilled water to obtain a 1:10 dilution. The mixture was agitated gently to ensure uniform mixing. The dilution was repeated until 10-7 dilution was obtained. The diluents were plated out in triplicate using a sterile pipette; 1ml of 10-7 dilution was withdrawn aseptically into Petri dish. The media employed for the inoculation were the standard plate count agar and nutrient agar (oxiod) for isolation and enumeration of all bacteria, while for fungi and yeast isolation sabouraud and potato dextrose agar were used. The plates were swirled gently to ensure uniform mixing after which they were incubated at 37°C for 24-48hrs for bacteria and 28±2°C for 3-5 days for fungi. Preparation of all media types were according to the manufacturer’s instructions.
After 24 and 72 hours, the bacterial and fungal colonies that appeared on the plates were counted using a digital illuminated, colony counter. The average colony counts from the triplicate plated were obtained and were expressed as Colony Forming Unit (CFU) per millilitres of sample.
Identification of bacterial isolates: Cultural characteristics of discrete colonies such as colour, shape, elevation, pigmentation, opacity and nature of the edge of the colonies were observed and noted. This was followed by characterization of the isolates using microscope for morphology. Microscopic examination for detailed cell morphology and Gram reaction were carried out on a 24hrs old culture [6]. Biochemical test carried out on all the bacterial isolates were based on utilization of glucose, lactose, sucrose, maltose, and fructose. Other tests carried out were the indole, methyl-red, voges-proskaver, catalase, urease activity, hydrogen sulphide- production and coagulates test. (The official methods for micro biological analyses of food, 2013) was adopted for the entire test [7]. Final identification of isolates was by comparison of results obtained with literature standards using Bergey’s Manual of Determinative Bacteriology [8].
Identification of fungal isolates: The colonial morphology of each fungal isolate was examined at both vegetative and sporulating stages, by their gross structured stained with lactophenol under the microscope. For the microscopic examination of moulds, a portion of mycelium was picked from the colony and placed on a slide, to which a drop of alcohol was applied and teased out carefully. When the alcohol was almost evaporated a drop of lactophenol was then covered with a cover-slip and allowed the stained sample to stay for 30 minutes before observing under microscope (X40 objective) as described by [9]. While for the isolation of yeast, 1g of the sample was poured into Triton soye broth (TSB) and incubated for 24hrs for the yeast to multiply before it was inoculated into sabouraud dextrose agar. The isolated yeast was then stained and subjected to fermentative tests using glucose, fructose, sucrose and maltose as substrates. The identification was done by comparing the observed morphological characteristics and the biochemical reaction with those described by [10]. References were made to fungal stock cultures.
Sensory evaluation of non-fermented, wet and dry fermented pap
The acceptability and preference of the pap types (Nonfermented, wet and dry fermented pap) were assessed using 50 judges comprising male and females. The sensory evaluation was carried out for colour, taste, aroma (flavour) and overall acceptability. A 5 point hedonic scale 1 and 5 representing “extremely like and extremely dislike” as described by Potter and Hotchkiss was used to rate the samples [11]. The mean scores were subjected to analysis of variance at P-value confidence level to determine the degree of preference between samples. The Likert scale for ordinal value was used where increasing numbers represented decrease in preference.
Results and Discussion
Microbial load and isolates from the samples
Ten (10) microorganisms were isolated from the fourteen (14) varieties of non-fermented grain flour, whereas nineteen (19) were isolated from the fermented starch; the details are presented in tables 1 and 2. The increase number of microorganisms observed in the fermented starch was due to the free access of organisms to the nutrient in fermenting medium [12]. It can also be attributed to the increase in the microbial biomass as a result of growth and the lactic acid bacteria as well as the enzymes secreted by microorganisms during the fermentation process [13].
Table 1: Microbial load and microorganisms isolated from the fourteen non-fermented grain flour varieties.
| S/NO | Sample | Microbial load | Microbial Isolates | |
|---|---|---|---|---|
| Bacteria | Fungi | |||
| 1. | BD | 3.0X102 | Bacillus sp, Staphylococcus aureus | Aspergillus flavus, Trichophyton rubrum |
| 2. | WS | 4.0X102 | Bacillus sp, Klebsiella aerogenes | Aspergillusflavus,Tric hophyton rubrum |
| 3. | WR | 1.0X102 | Bacillus sp, Staphylococcus aureus | Aspergillus niger |
| 4. | CR | 3.2X103 | Bacillus sp, Staphylococcus epidermidis | Aspergillus niger, Trichophyton rubrum |
| 5. | CK | 2.4X103 | Staphylococcus epidermis | Aspergillus niger |
| 6. | WD | 3.3X103 | Staphylococcus aureus, Bacillus sp | Mucor sp, Trichophytton sp |
| 7. | CF | 2.2X102 | Bacillus sp, Staphylococcus epidermidis | Trichophyton sp, Aspergillus flavus |
| 8. | AR | 3.2X102 | Bacillus sp, Staphylococcus aureus, klebsiella aerogenes | Aspergillus flavus |
| 9. | JM | 3.6X101 | Bacillus sp, Staphylococcus aureus | Aspergillus flavus |
| 10. | ZR | 4.0X102 | Aeromonas sp, Bacillus sp, Staphylococcus aureus | Trichophyton rubrum, mucor sp |
| 11. | NB | 1.6X103 | Bacillus sp, Aeromonas sp | Trichophyton sp |
| 12. | NH | 1.2X101 | Bacillus sp | Trichophyton sp |
| 13. | KR | 4.0X103 | Aeromonas sp, Bacillus sp | Aspergillus niger, Aspergillus flavus |
| 14. | SN | 1.1X101 | Bacillus sp, Staphylococcus aureus | Aspergillus niger |
BD: Badama; CK:Chidt Kusun/Kukwom; JM: Jakalak/Mara; WS: Whey Swello; WD: Wandat; ZR: Zor; WR: Whey Rwey; CF: Chidt Fyali/Kall;
NB: Nhibang; CR: Chin Ryey; AR: Chikaraya; NH: Nhin; KR: Kurep; SN: Sun
Table 2: Microbial load and microorganism isolated from the fourteen fermented acha starch varieties.
| S/NO | Sample | Microbial load | Microbial Isolates | |
|---|---|---|---|---|
| Bacteria | Fungi | |||
| 1. | FBD | 1.8X104 | Bacillus sp, Lactobacillus fermentum | Aspergillus niger, Saccharomyces cerevisiae, Trichophyton rubrum |
| 2. | FWS | 4.6X104 | Bacillus sp, Klebsiella aer ogenes, Lactobacillus sp | Saccharomyces cerevisiae, Aspergillus niger, Trichophyton sp |
| 3. | FWR | 3.8X104 | Bacillus sp, Streptococcus Sp, Lactobacillus sp, Staphylococcus aureus | Rhodotorula graminis, mucor sp, Trichophyton sp |
| 4. | FCR | 3.5X104 | Bacillus sp, Lactobacillus Sp, Staphylococcus epidermidis | Saccharomyces cerevisiae, Aspergillus niger, Trichophyton rubrum |
| 5. | FCK | 2.8X104 | Bacillus sp, Klebsiella aer ogenes, Leuconostoc mes enteroides, Staphlococcus epidermidis | Rhodotorula graminis, Mucor sp, Aspergillus niger |
| 6. | FWD | 2.5X103 | Bacillus sp, Lactobacillus Fementus, Staphylococcus aureus | Rhodotorula graminis, Aspergillus niger |
| 7. | FCF | 4.2X103 | Bacillus sp, Streptococcus facealis, Leuconostoc mesenteroide | Saccharomyces cerevisiae, Aspergillusflavus |
| 8. | FAR | 4.5X103 | Bacillus sp, streptococcus Sp, Lactobacillus brevis |
Saccharomyces cerevisiae, Trichophyton sp, Aspergillus fumigatus |
| 9. | FJM | 3.7X102 | Lactobacillus sp, Bacillus sp, Staphylococcus aureus | Rhodotorula graminis, Trichophyton rubrum |
| 10. | FZR | 2.8X104 | Bacillus sp, Streptococcus Faecalis, lactobacillus fermentum | Rhodotorula graminis, Trichophyton rubrum |
| 11. | FNB | 3.0X102 | Aeromonas sp, Bacillus sp, Lactobacillus brevis | Aspergillus fumigatus, Saccharomyces cerevisiae |
| 12. | FNH | 1.9X102 | Bacillus sp, Lactobacillussp, Staphylococcus epidermidis | Aspergillus flavus, mucor sp Saccharomyces cerevisiae |
| 13. | FKR | 1.8X104 | Bacillus sp, Lactobacillus fermentum | Trichophyton sp, Rhodotorula graminis |
| 14. | FSN | 2.0X102 | Bacillus sp, Leuconostoc mensenteroides | Saccharomyces cerevisiae, Aspergillus niger |
FBD: Fermented Badama; FCR: Fermented Chin ryey; FCF: Fermented Chidt fyali/kall ;AR: Fermented Chikaraya; FSN: Fermented Sun;
FWS: Fermented Whey swello; FNB: Fermented Nhibang; FNH: Fermented Nhin; FJM: Fermented Jakalak/mara; FWD: Fermented Wandat;
FKR: Fermented kurep; FWR: Fermented Whey rwey; FZR: Fermented zor; FCK: Fermented Chidt kusun/Kukwom
The observed increase also in the microbial load during fermentation was mainly due to the proliferation of microorganisms and their metabolic activity that produces carbon dioxide and water as reported by Chutmanop et al [14]. However, the microbial loads of both the fermented and non-fermented products were relatively low for the fourteen Acha varieties, because all the total plate count were not above 105cfu/ml. Egbere; Microbiological quality guide for ready to eat foods; 2009 and Microbiological guidelines for food, 2014) reported that the total plate count accepted in most foods especially ready to eat food with minimum handling prior to consumption should not be more than 105cfu/ml [15-17]. The low count could be as a result of the high level of hygiene observed during the production process, the materials and equipment used.
The commonest organism identified in both the fermented and non-fermented products are: Bacillus sp, Staphylococcus aureus, Klebsiella aerogenes, Staphylococcus epidermidis, Aeromonas sp, Aspergillus niger, Trichophyton rubrum, Aspergillus flavus, Mucor sp, and Trichophyton sp. While the few not common to the two types of samples include: - Lactobacillus fermentum, Lactobacillus sp, Streptococcus sp, Lactobacillus brevis, Streptococcus faecalis, Leuconostoc mesenteroides, Saccharomyces cerevisiae, Rhodotorula graminis and Aspergillus fumigatus.
The isolation of lactic acid bacteria and yeast are due to the fact that they are responsible for the fermentation of most legumes and cereals. Their presence also confirms that they grow in close association with the food substrate [18,19]. These organisms are obligate fermenters, flavourful organisms not harmful to the consumers but produce enzymes that hydrolyze food complexes into simple non-toxic products with desirable textures and aroma that makes them palatable [5,20,21]. While the presence of other organisms could be through various sources especially when strict hygienic practices are not adhered to, which could be attributed to the raw materials used, processing environment, human involvement, milling machine employed, muslin clothes used in filtering, source of water and utensils used [22].
Bacillus sp, being the most predominant bacterial flora isolated in both products of the samples, may be due to their ability to survive both slightly acidic and alkaline environment. They are saprophytic and prevalent in water, soil, air and plant compost, they have also been reported to survive many foods processing like, pasteurization, cooking and drying [23,24].
Sensory evaluation of the non-fermented wet and dry fermented pap
The summary of the result in Table 3 reveals that wet fermented pap was most preferred for colour and taste, followed by dry fermented. The overall acceptability was found to be the wet fermented pap. In comparing the three types of pap between and within groups, colour for wet and dry fermented are not significantly different and so also aroma for all the three types of pap, while taste and overall acceptability for the three types of pap were significantly different from each other at P-value confidence level.
Table 3: Sensory parameters of non: fermented, wet and dry fermented pap.
| Parameters | Non: fermented | Wet fermented | Dry fermented |
|---|---|---|---|
| Colour | 2.64+1.208a | 1.48+0.789b | 1.84+0.710b |
| Aroma | 2.02+1.270a | 1.80+0.639a | 1.80+1.245a |
| Taste | 2.88+1.282a | 1.32+0.741b | 1.92+1.027c |
| Overall Acceptability | 2.58+0.936a | 1.32+0.626b | 1.813+0.127c |
Values are means of triplicate determination + Standard Deviation. Means with same superscript in each row are not significantly different from one another (LSD, p<0.05).
The preference of the fermented product was simply due to the fact that fermentation makes food palatable by improving its nutritional content, enhancing its taste, texture, and aroma [25,26]. These organoleptic properties make fermented food more popular than the non-fermented one in terms of consumer acceptance [27]. However, the panel preference of the wet over the dry fermented pap was because moisture content of food material affects the suitability of the food for consumption as well as the physical (texture) and chemical (composition) properties of food which relates to the freshness and stability of the food, [25].
Conclusion
The species of microorganisms involved in the fermented starch are lactic acid bacteria and yeast, while the presence of other organisms in the product could stem from contamination of raw materials used or from processing equipment. It could also be from the environment and flora of the grain. However, production of the pap under hygienic condition will greatly reduce or prevent microbial contamination of the product. All the grains gave good pap product which was accepted by the consumers during the acceptability test.
Significance of the Study
The study revealed the suitability of Acha in nonfermented, wet and dry fermented pap production and the acceptability by consumers. This will help to promote the use of the grain and reduce the over dependence of the people on other cereals like maize, millet and sorghum for pap production, which will also improve the economic status of the producers and eventually stimulate action to increase the commercial production.
Recommendation
Awareness should be created by the food industry on the use of Acha to produce non- fermented, wet and dry fermented pap like any other cereals and the strict adherence to the hygienic techniques of the production be followed. There is therefore need for researchers to provide information and technologies that will enhance Acha production to meet the demands of the people in Africa and even for exportation, considering its nutritional and health benefits.
There are no references