Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 02 March, 2020
Accepted date: 20 December, 2021
Published date: 27 December, 2021
Citation: Adesina Felicia C, Monye Rita N, Ighodaro Osasenaga M, Adeosun Abiola M (2021) Thermo-Stable Alkali Pectinase from a Mesophilic Bacillus subtilis in Industrial Effluent J Appl Microb Res. Vol: 4 Issu: 2 (11-17).
Copyright: © 2021 Adesina Felicia C et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Highly thermostable and alkali pectinases produced by microorganisms are increasingly in demand for commercial biotechnological processes. In this study, enzyme extracts from Bacillus subtilis-BSP5 (accession number KP980585) had the highest pectinase activity at variable temperatures among those produced by other bacteria from effluents at Oluyole industrial estate Ibadan, Nigeria, latitude 7.3215°N and longitude 3.8708°E. The crude pectinase was purified to a final purification fold of 6.61 and recovery of 6.97%. The purified and characterized pectinase from B. subtilis-BSP5 showed a specific activity of 1380.28 U/mg proteins, an optimum pH and temperature of 8.1 and 60.0°C respectively. Its band weight was estimated as 37KDa. The enzyme was observed to be stable and active 2 hours after exposure to increased temperature up to 70°C for 60 minutes, retaining over 60% of its optimum activity. Similarly, over 70%, of its activity was retained post exposure to pH of 9.0 for 150 minutes. The Km and Vmax of the purified pectinase were 1.0mg/mL and 5.7μmol/min/mg protein respectively. Calcium, Sodium and Potassium ions were observed to be activators for the pectinase, they respectively increased its catalytic activity by 22, 9 and 16 %, whereas Mercury, Lead and Aluminium ions were noted to be powerful inhibitors to the enzyme, respectively lowering its catalytic activity by 86, 60 and 72 %. Overall, the present study suggests that the purified pectinase from B. subtilis-BSP5 strain possesses some physicochemical and kinetic characteristics that potentially describe it as a thermostable and alkali protease. In light of this, B. subtilis-BSP5 strain may be considered as one of the commercially viable organisms for biotechnological production of thermostable and alkali pectinases.
Keywords
Thermostable, B. subtilis, Enzyme, Pectinase, Alkali, Commercial production.
Abstract
Highly thermostable and alkali pectinases produced by microorganisms are increasingly in demand for commercial biotechnological processes. In this study, enzyme extracts from Bacillus subtilis-BSP5 (accession number KP980585) had the highest pectinase activity at variable temperatures among those produced by other bacteria from effluents at Oluyole industrial estate Ibadan, Nigeria, latitude 7.3215°N and longitude 3.8708°E. The crude pectinase was purified to a final purification fold of 6.61 and recovery of 6.97%. The purified and characterized pectinase from B. subtilis-BSP5 showed a specific activity of 1380.28 U/mg proteins, an optimum pH and temperature of 8.1 and 60.0°C respectively. Its band weight was estimated as 37KDa. The enzyme was observed to be stable and active 2 hours after exposure to increased temperature up to 70°C for 60 minutes, retaining over 60% of its optimum activity. Similarly, over 70%, of its activity was retained post exposure to pH of 9.0 for 150 minutes. The Km and Vmax of the purified pectinase were 1.0mg/mL and 5.7μmol/min/mg protein respectively. Calcium, Sodium and Potassium ions were observed to be activators for the pectinase, they respectively increased its catalytic activity by 22, 9 and 16 %, whereas Mercury, Lead and Aluminium ions were noted to be powerful inhibitors to the enzyme, respectively lowering its catalytic activity by 86, 60 and 72 %. Overall, the present study suggests that the purified pectinase from B. subtilis-BSP5 strain possesses some physicochemical and kinetic characteristics that potentially describe it as a thermostable and alkali protease. In light of this, B. subtilis-BSP5 strain may be considered as one of the commercially viable organisms for biotechnological production of thermostable and alkali pectinases.
Keywords
Thermostable, B. subtilis, Enzyme, Pectinase, Alkali, Commercial production.
Introduction
Pectinases are a group of enzymes that degrade complex polysaccharide, precisely pectins that are present in plants. Microorganisms such as yeast, fungi, and actinomycetes are capable of inducibly producing these enzymes [1,2]. Pectinases are becoming one of the leading commercialized enzymes with annual production growth rate of 2.86% between 2013-2016, and the market size is projected to reach 3.67% before 2021 [3]. These classes of enzymes are widely used in textile production, food and feedstock processing, biofuel, textile and paper production and waste management [2-4]. Several reports have documented the production of pectinases [5- 8]. Microorganisms are preferred source for the production of these enzymes because they are easily cultured in
large quantities within a short period, and they can easily be manipulated to derive enzyme of interest in greater [9].
In bacteria, pectinases are majorly produced from Bacillus and Erwinia spp. Bacillus are about the most abundant soil bacteria, they produce several bioactive substances with high pharmaceutical and biotechnological use [10]. Enzymes produced by Bacillus spp. constitute about 50% of the total enzyme market [11]. They are superior strain in industrial biotechnology because they their simple nutritional needs (require cheap substrate) for growth [12].
Bacillus spp. produces all classes of pectinases including hydrolases, lyases, esterases, and protopectinases. However, thermostable and alkali pectinases are not readily available in enzyme market. In view of this, pectinase produced by Bacillus subtilis obtained from waste was purified and characterized to determine its thermostability and stability at alkali pH range.
Materials and Methods
Production and purification of crude pectinase by Bacillus subtilis-BSP5
Vincent’s mineral salt medium was prepared and 1mL culture broth of Bacillus subtilis-BSP5 was aseptically introduced into it, tightly covered while incubation was done at 50°C for 48 hours under aerated and agitated condition in a rotary shaker set at 150 rpm [13]. After the incubation period, the crude culture was centrifuged at 10,000 x g for 15 minutes at 4°C and the cell-free supernatant obtained was used as extracellular crude pectinase solution.
The crude pectinase without B. subtilis cells obtained by centrifugation was purified using a 4-step purification process comprising of saturated ammonium sulfate precipitation, 65% dialysis ion-exchange chromatography on SP Sephadex C-50 column (bead 40-125uM, pore size 200,000, and capacity 2.0-2.6 meq/g) and gel filtration on Sephadex G-50 column [14-16]. Pectinase activity and protein concentration of crude and purified solutions were determined at each step and used to measure specific pectinase activity.
Pectinase activity measurement
Activity of crude and purified pectinase by Bacillus subtilis-BSP5 (accession number KP980585) was measured by adding 1 mL of the enzyme solution with an equal volume of a pH 5 aqueous solution of citrus pectin (10 g/L) as substrate. The mixture was incubated at 50°C for 15 minutes. Dinitrosalicylic acid (DNSA) reagent (2 mL) was then added and the reaction mixture was boiled for 5 minutes. The absorbance of the cooled reaction mixture was read at 540 nm against a blank. A solution containing 2ml DNSA reagent diluted with 2 mL distilled water represented the blank solution [17]. One unit of pectinase activity was defined as the amount of enzyme required for liberating 1 μg of galacturonic acid per minute under the assay conditions. Protein concentration was also determined [18].
Characterisation of pectinase from Bacillus subtilis- BSP5 (accession number KP980585)
The molecular or band weight of the Bacillus subtilis-BSP5 pectinase was determined using Sodium Dodecyl Sulphate Polyacrilamide Gel Electrophoresis (SDS-PAGE) technique. The process was carried out with a 10% polyacrylamide gel using Tris-Glycine-SDS buffer system according to the method described by Laemmli [19].
Determination of optimum temperature and thermal stability of pectinase
Aliquots of purified pectinase from Bacillus subtilis-BSP5 (accession number KP980585) was used for this assay. The effects of temperature on pectinase were investigated at temperature values of 30, 40, 50, 60, 70, 80 and 90 °C.
Diluted purified enzyme (200 μL) was pipette into a test tube containing 1800 μL of standard citrus pectin solution (10 g/L W/V) and the mixture was incubated at each of the above temperatures for 15 minutes. The thermal stability of the purified pectinase was determined by incubating the dilute enzyme solution at varying temperatures (30–90°C) for 120 minutes. Aliquot of 200 μL was withdrawn at 30 minutes’ interval to determine the residual activity of the purified pectinase [9].
Determination of optimum pH and stability of pectinase
Pectinase activity was measured under standard assay conditions at different pH values using the following buffers; 50mM glycine-HCl buffer (pH 3.0), 50mM sodium acetate buffer (pH 4.0–5.0), 50mM phosphate buffer (pH 6.0–7.0), 50mM Tris-HCl buffer (pH 8.0–9.0) and 50mM glycine- NaOH (pH 10.0–12.0). The effect of pH on the stability of the purified pectinase was determined by incubating the purified enzyme with the substrate in solution with relevant buffers (pH 3.0–12.0) for 120 minutes, with the periodic withdrawal of aliquot enzyme every 30 minutes to measure the residual activity of the purified pectinase [9].
Determination of Km and Vmax of pectinase
Citrus pectin was used as substrate to determine Km and Vmax of the pectinase. The kinetic data obtained from the reaction between the enzyme and its substrate at different concentrations was compared to the Michaelis-Menten model, with the aid of Graphpad prism software (version 6.05) San Diego, U.S.A, www.graphpad.com.
Effect of metallic ions on the activity of pectinase from Bacillus subtilis-BSP5 (accession number KP980585)
The effect of different metal ions (MnCl2, CuCl2, PbCl2, NaCl, MgCl2, HgCl2, CoCl2, KCl, AlCl3, and CaCl2) on the activity of the purified pectinase was determined by pre-incubating the enzyme with the metal ions at a final concentration of 10mM using their chloride salt prepared in 50mM Tris- HCl buffer, pH 8.0. After 30 minutes of incubation at 60°C, pectinase activity relative to the control without metal ions was determined [20].
Data analyses
Analyses of data were done on Microsoft Excel 2013 (Microsoft INC., USA) and (version 6.05) San Diego; U.S.A. Results were expressed as mean ± standard deviation of replicates.
Results
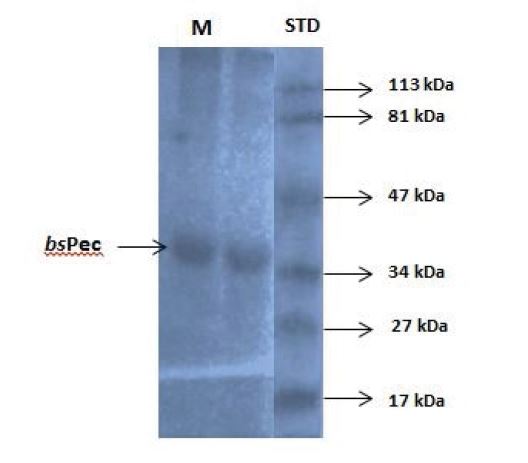
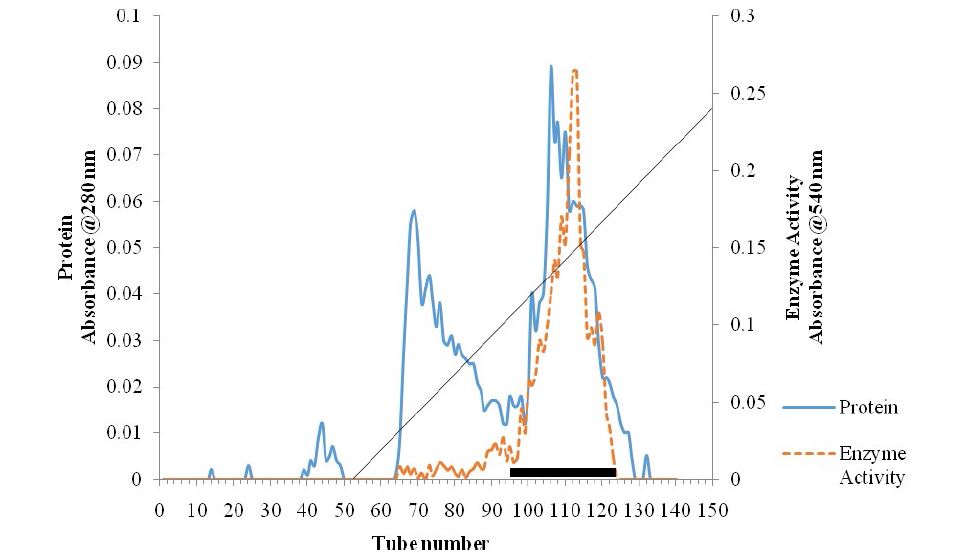
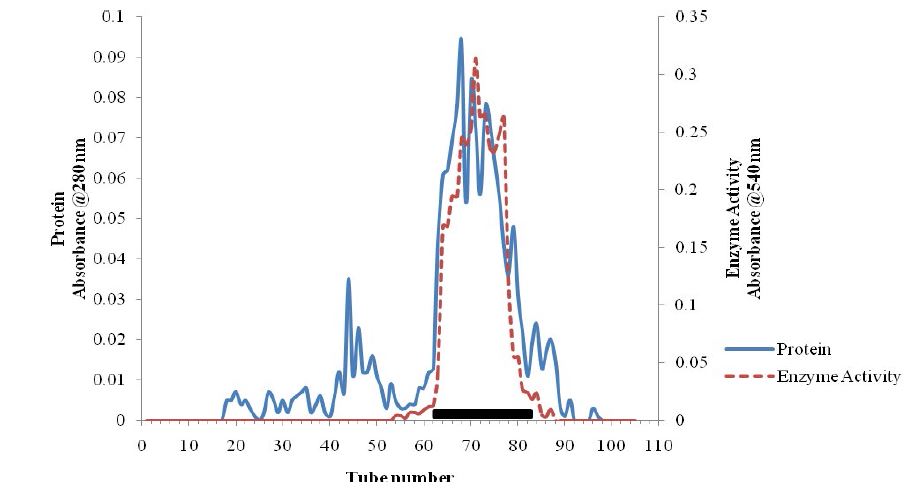
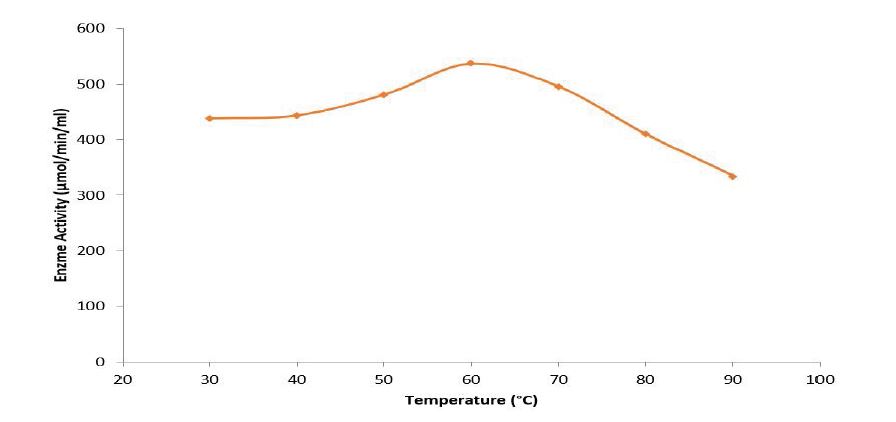
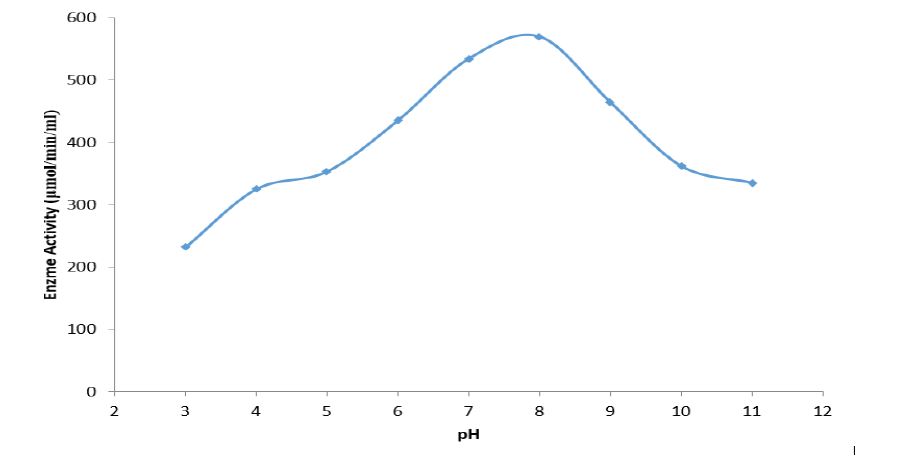
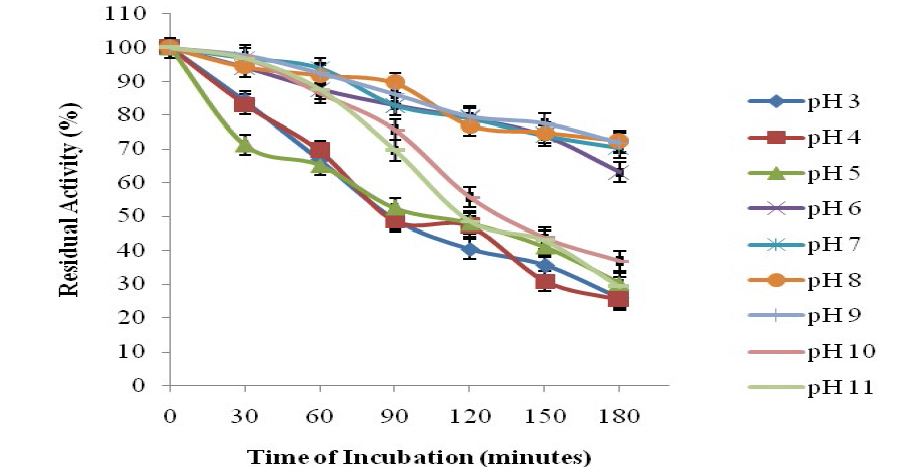
Total activity of the crude pectinase obtained from B. subtilis -BSP5 (accession number KP980585) was 442781U as shown in table 1. Sequel to ion exchange chromatography (IEC) purification step, pooled fraction from tube numbers 100 to 120 had peak enzyme activity and highest protein concentration (Figure 1). Similarly, pooled fractions of tube and numbers 62 to 82 from Gel filtration chromatography (GFC) purification step demonstrated highest pectinase activity protein concentration (Figure 2). The pectinase produced from Bacillus subtilis-BSP5 was purified to a final purification fold of 6.61 and recovery of 6.97% as shown in Table 1. The total activity of the enzyme decreased from 442781 to 30863 U (30.3 %) while its specific activity increased from 208.8 to 1380.3 U/mg proteins (561.1 %) at final purification step (Table 1). The Bacillus subtilis-BSP5 pectinase was purified to almost homogeneity and its band or molecular weight was estimated as of 37kDa (Plate 1). The activity of the purified pectinase was optimum at 60°C (Figure 3) and over 60% of this activity was retained on exposure to a temperature of 70°C for 60 minutes (Figure 4). The enzyme had an optimum pH of 8.0 (Figure 5), with over 70% of the activity retained after exposure to pH 9 for 150 minutes (Figure 6). The Km and Vmax of the enzyme were 1.0mg/mL and 5.7μmol/min/mg protein respectively. Among the metal ions evaluated for their effects on the purified pectinase, calcium, sodium and potassium ions were observed to be activators or positive effectors for the pectinase, as they respectively increased its catalytic activity by 22, 9 and 6 %. Conversely, mercury, lead and aluminum ions had significant inhibitory effects on the enzyme, respectively lowering its catalytic activity by 86, 60 and 72 % (Table 2).
Table 1: Purification Table for Pectinase from Bacillus subtilis.
| PurificationSteps | Volume (mL) | Total activity (U) | Protein conc. (mg) | Specific activity (U/mg) | % yield | Purification fold |
|---|---|---|---|---|---|---|
| Crude | 900.0 | 442781.0 ± 2.5 | 2121.6 ± 0.5 | 208.8 ± 3.0 | 100.0 ± 0.0 | 1.0 ± 0.0 |
| NH4 Sulphate Precipitation (60%) | 86.0 | 59260 ± 4.2 | 109.3 ± 0.3 | 542.0 ± 7.2 | 13.4 ± 1.3 | 2.6 ± 0.0 |
| Dialysis against buffer | 62.0 | 46861.0 ± 6.7 | 61.0 ± 0.5 | 767.7 ± 8.1 | 10.6 ± 2.5 | 3.7 ± 0.5 |
| Ion-Exchange on SP-Sephadex C-50 | 48.0 | 38009.0 ± 3.4 | 29.9 ± 0.4 | 1272.9 ± 7.4 | 8.6 ± 1.2 | 6.1 ± 0.5 |
| Gel Filtration on Sephadex G-50 | 44.0 | 30863.0 ± 5.5 | 22.4 ± 0.3 | 1380.3 ± 10.5 | 7.0 ± 0.6 | 6.6 ± 0.4 |
| Replicate results were presented as mean± standard deviation, conc. = concentration | ||||||
Table 2: Effects of metallic ions on the activity of purified Pectinase from Bacillus subtilis-BSP5 (accession number P980585).
| Metal ion | Relative Activity (%) |
|---|---|
| Control | 100 ± 0.01 |
| MnCl2 | 85 ± 0.69 |
| CuCl2 | 71 ± 0.67 |
| PbCl2 | 40 ± 0.55 |
| NaCl | 109 ± 0.82 |
| MgCl2 | 98 ± 0.13 |
| HgCl2 | 24 ± 0.07 |
| CoCl2 | 66 ± 0.15 |
| KCl | 106 ± 0.82 |
| AlCl3 | 28 ± 0.12 |
| CaCl2 | 122 ± 1.15 |
Plate 1: Polyacrylamide gel electrophorectogram of purified pectinase from Bacillus subtilis-BSP5 under denaturing conditions (SDS-PAGE). STD: standard molecular weight marker lane, M: purified pectinase lane, bsPec: Band of pectinase by Bacillus subtilis-BSP5
Figure 1: Ion-exchange Chromatogram of the Pectinase Purification.
Figure 2: Gel Fitration Chromatogram of the Pectinase Purification.
Discussion
Based on the increasing demand for alkali and thermostable pectinases by textile, biopulping and animal feed industries, identifying microbial enzymes with such potentials for commercial processes becomes imminent in daily research.
Ion exchange and gel filtration purification techniques were of choice in this study, because they are commonly used techniques for purification of pectinases. This is basically due to their ability to effectively remove both unbound and bound molecules, including related proteins from the enzyme of interest. Besides, the techniques facilitate recovery of the target pectinase in its absolute form [21]. The latter, which is necessary to ensure correct data on the native characteristics of the enzyme, is interestingly an integral part of our study objectives.
Generally, pectinases have been observed over time to have band weights ranging from 30 to 80 kDa but the band weight of exopolygaraturonases have been particularly reported to be between 30-50 kDa [8]. Interestingly the purified pectinase from Bacillus subtilis-BSP5 in this study had a band weight of 37KDa, a value which apparently portends it as an exo-pectinase. The band weight obtained in this work is similar to that reported by Mukesh et al. for a B.subtilis but in disparity with the 60kDa observed by Mercimek and Filiz for another strain of B. subtilis. It appears proteases from microbes of the same species do not necessarily share similarity in molecular weights. This is probably due to environmental differences and variation in mechanisms of action of alkali pectinases [14,22,23].
Figure 3: Effect of temperature on pectinase activity of pectinase from Bacillus subtilis-BSP5 (accession number KP980585).
Figure 4:Effect of Temperature on the Stability of purified Pectinase from Bacillus subtilis-BSP5 (accession number KP980585).
Figure 5:Effect of pH on the pectinase activity.
Figure 6:Effect of pH on Stability on the Pectinase activity.
The desirable characteristics, including thermo-stability, alkali nature, relatively high specific activity exhibited by the Bacillus subtilis-BSP5 pectinase in this study, makes the enzyme a potential candidate for flax retting in textile industries, in degumming rannie in the pulp industry, production of liquedised vegetable and cotton scouring [6,24, 25]. The stability of the enzyme at relatively high temperature and alkali pH is of immense relevance in industrial applications of enzymes. Thermo and pH stability of pectinases from other strains of Bacillus subtilis have been reported in previous studies [26]. Particularly, the optimum pH and temperature obtained in this study are consistent with those earlier reported by Akinyemi et al. for B. megaterium pectinase [27].
Information on the kinetic parameters of the studied pectinase is crucial in rating its catalytic efficiency and projecting its industrial utilization or exploitation. The Km and Vmax values obtained indicate that the pectinase has high affinity for its substrate and thus can attain substrate saturation at relatively good rate. This implies that the enzyme can rapidly attain maximal velocity even at low substrate concentration. Moreover, the Km value (1mg/ mL) of the pectinase is similar to that associated with good microbial pectinases such as the one produced from P. chrysogenum, and interestingly lower than that of a Bacillus subtilis Btk27 which was reported to be 1.891mg/mL [28,29].
Metal ions influence enzyme activities in different ways. In this study sodium potassium and calcium chloride were noted to increase pectinase activity. Our finding on the effect of potassium ion correlates with studies on other Bacillus strains like B. megaterium and Bacillus cereus [27,30]. Previous experiments have observed monovalent ions to have positive influence on activity of pectinases, also calcium ions have been noticed to increase the activity of alkali pectinases, in vitro [31,32]. It is interesting that Manganese noticed to inhibit the activity of this pectinase was previously reported to activate pectinase from Bacillus species isolated from a variety agrowaste in a study by Torimiro and Okonji [32]. Thus, the features associated with B. subtilis-BSP5 pectinase in this study are promising, and thus suggest that more attention should be directed at the enzyme as a probable candidate for industrial and biotechnological applications.
Conclusion
B. subtilis-BSP5 produced pectinase which is highly thermostable and alkali in nature. The enzyme possesses potential for use in textile, pulping and other related industries. Further characterization and optimization studies on the enzyme are being considered in our laboratory and we therefore recommend same to other laboratories.
Adesina FC, Olutola AA, Adeyefa OA, Ummi H, Shadrach OA (2013) Production of Pectinase by Fungi isolated from Degrading Fruits and Vegetable. Nature and Science 11: 102-108. [ Ref ]
Bijesh K, Denoj S (2018) Review on bacterial production of alkaline pectinase with special emphasis on Bacillus species. Bioscience Biotechnology Research Communications 11: 18-30. [ Ref ]
Yvonne L, Marc H, Karl S, Philippe S, Marc Van M, et al. (2018) Biotechnology and the bioeconomy—Towards inclusive and sustainable industrial development. New biotechnology 40: 5-10. [ Ref ]
Simran Jot K, Vijay Kumar G (2017) Production of pectinolytic enzymes pectinase and pectin lyase by Bacillus subtilis SAV-21 in solid state fermentation. Annals of microbiology 67: 333-342. [ Ref ]
Blanco P, Carmen S, Tomás GV (1999) Production of pectic enzymes in yeasts. FEMS Microbiology Letters 175: 1-9. [ Ref ]
Ranveer Singh J, Shivalika S, Reena G (2005) Microbial pectinolytic enzymes: a review. Process Biochemistry 40: 2931-2944. [ Ref ]
Biscaro PD, Costa MA, Eleni G, Eleonora Cano C (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnology Journal: 3: 9-18. [ Ref ]
Nevadita S, Madhu R, Mukesh S (2013) Microbial pectinase: sources, characterization and applications. Reviews in Environmental Science and Bio/Technology 12: 45-60. [ Ref ]
Martin FC, Christopher B (1990) Recent Advances in Enzyme Technology. Enzyme Technology. Cambridge University Press Archive. [ Ref ]
Torsten S (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular microbiology 56: 845-857. [ Ref ]
Marcus S, Ajay S, Owen PW (2004) Developments in the use of Bacillus species for industrial production. Canadian journal of microbiology 50: 1-17. [ Ref ]
Muhammad S, Zakia L (2016) Phylogenetic analysis of polygalacturonaseproducing Bacillus and Pseudomonas isolated from plant waste material. Jundishapur journal of microbiology 9: 16-21. [ Ref ]
Safia, K Zahid A, Zafar I, Awais A, Tahir A, et al. (2014) Utilization of Aspergillus oryzae to produce pectin lyase from various agro-industrial residues. Journal of Radiation Research and Applied Sciences 7: 327-332. [ Ref ]
Aysun MTH, Turkmen FU (2016) Extracellular pectinase production and purification from a newly isolated Bacillus subtilis strain. International Journal of Food Properties 19: 2443-2450. [ Ref ]
Virgen-Ortíz JJ, Ibarra-Junquera V, Osuna-Castro JA, Escalante- Minakata P, Mancilla-Margalli NA, et al. (2012) Method to concentrate protein solutions based on dialysis–freezing–centrifugation: Enzyme applications. Analytical biochemistry 426: 4-12. [ Ref ]
Anjana DN, Appu Rao AG (1996) Fractionation, purification, and preliminary characterization of polygalacturonases produced by Aspergillus carbonarius. Enzyme and Microbial Technology 18: 59-65. [ Ref ]
Lorenz MG (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry 31: 426-428. [ Ref ]
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265‐275. [ Ref ]
Laemmli Ulrich K (1970) Cleavage of the Structural Proteins During Assembly of the Head of Bacteriophage T4. Nature 227: 680-685. [ Ref ]
Francesco R, Paolo V, Eugenio G (1999) What students must know about the determination of enzyme kinetic parameters. Biochemical Education 27: 87-91. [ Ref ]
Punekar NS (2018) Quantification of Catalysis and Measures of Enzyme Purityin ‘Enzymes: Catalysis, Kinetics and Mechanisms. Springer Nature, Singapore. [ Ref ]
Mukesh Kumar DJ, Saranya GM, Suresh K, Andal Priyadharshini D, Rajakumar R, et al. (2012) Production and optimization of pectinase from Bacillus sp. MFW7 using Cassava waste. Asian Journal of Plant Science Resources 2: 369-375. [ Ref ]
Jacob N, Poorna CA, Prema P (2008) Purification and partial characterisation of polygalacturonase by Stretomyces lydicus. Bioresource Technology 99: 6697-6701. [ Ref ]
Kalantzi S, Mamma D, Kalogeris E, Kekos D (2010) Improved properties of cotton fabrics treated with lipase and its combination with pectinase. Fibres Text East European 18: 86-92. [ Ref ]
Guo F, Zou M, Li X, Zhao J, Qu Y (2013) An effective degumming enzyme from Bacillus sp. y1 and synergistic action of hydrogen peroxide and protease on enzymatic degumming of ramie fibers. BioMed Resources International. [ Ref ]
Margarita S, Pilar D, Javier PFI (2005) Pectinolytic systems of two aerobic sporogenous bacterial strains with high activity on pectin. Current Microbiology 50: 114-118. [ Ref ]
Akinyemi BT, Buraimoh Olanike M, Ogunrinde Olayemi O, Olukayode OA (2017) Pectinase production by Bacillus megaterium, Bacillus bataviensis, and Paenibacillus sp. isolated from decomposing wood residues in the Lagos lagoon. Journal of Tropical Life Science 7. [ Ref ]
Laha S, Sarkar D, Chaki S (2014) Optimization of production and molecular characterization of pectinase enzyme produced from penicillium chrysogenum. Scholars Academic Journal of Biosciences 2: 326-335. [ Ref ]
Oliyad Jeilu O, Dawit A (2017) Characterization of Pectinase from Bacillus subtilis Strain Btk 27 and Its Potential Application in Removal of Mucilage from Coffee Beans. Enzyme Research. [ Ref ]
Sangeeta Y, Pramod Kumar Y, Dinesh Y, Singh YKD (2008) Purification and characterization of an alkaline pectin lyase from Aspergillus flavus. Process Biochemistry 43: 547-552. [ Ref ]
Herron SR, Scovatta RD, Garrett M, Legner M, Jurnak F (2003) Characterisation and implication of calcium ion binding to pectate lyase. Canadian Journal of Biological Chemistry 278: 12271-12277. [ Ref ]
Torimiro N, Okonji RE (2013) A comparative study of pectinolytic enzyme production by Bacillus species. African Journal of Biotechnology 12: 6498-6503. [ Ref ]