Journal Name: Journal of Applied Microbiological Research
Article Type: Research
Received date: 27 February, 2023
Accepted date: 08 June, 2023
Published date: 15 June, 2023
Citation: Li J (2023) Two New Species of the Antipatharian-Inhabiting Barnacle, of the Genus Conopea (Arthropoda, Thecostraca, Balanomorpha), from the East China Sea and South China Sea, and a Way of Life for the Genus. J Appl Microb Res. Vol: 6 Issu: 1 (30-36).
Copyright: 2023 Li J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Two new species of Conopea (Say 1822) are described from the East China Sea and South China Sea: Conopea hongqi sp.nov. collected from the East China Sea, trawled in 350-450 m. It has a shape that is significantly different from that of other species of the genus Conopea. Conopea niuqv sp.nov collected from the South China Sea, local fishermen collect with crab nets in 20-25 m. Conopea hongqi have a lifestyle that has not been previously observed in the genus.
Keywords
Barnacle, Conopea hongqi, Conopea niuqv, East China Sea, morphology,
Abstract
Two new species of Conopea (Say 1822) are described from the East China Sea and South China Sea: Conopea hongqi sp.nov. collected from the East China Sea, trawled in 350-450 m. It has a shape that is significantly different from that of other species of the genus Conopea. Conopea niuqv sp.nov collected from the South China Sea, local fishermen collect with crab nets in 20-25 m. Conopea hongqi have a lifestyle that has not been previously observed in the genus.
Keywords
Barnacle, Conopea hongqi, Conopea niuqv, East China Sea, morphology,
Introduction
The genus Conopea Say, 1822 is a group of symbiotic barnacles associated with gorgonian octocorals (Cnidaria) such as sea fans and sea whips or antipatharians (black corals) [1]. There are currently 20 described species of Conopea. They are almost completely covered by the host coenenchyma tissue. The basis of the barnacle shell clasps the axis of the host, with only the opercular opening exposed. Conopea is a widespread genus that is found in temperate and tropical oceans around the world from shallow subtidal to deep waters.
Say (1822) erected a new genus Conopea to accommodate a new species, C. elongata. Say describes Conopea as ‘Shell sessile, fixed, composed of two cones joined by their bases, the lines of junction carinate each side: inferior cone entire, attached by its anterior side and tip to marine bodies; with an aperture at the summit, closed by a quadrivalved operculum [1].’ Darwin characterized these barnacles by “Parietes and basis sometimes permeated by pores, sometimes not; radii not permeated by pores, shell elongated in its rostrocarinal axis, basis boat-shaped, attached to Gorgoniae and Milleporae [2].” Carrison-Stone et al. noted that “Conopea is not a well-documented group [3].
There is very little data on host associations, species ranges are not well defined, and published descriptions are often incomplete and occasionally contain questionable information.” Van Syoc et al. indicated that
“The acastine species living in gorgonians differ from Conopea species in several ways, the most obvious is the form of their attachment to the host [4]. Conopea species are cemented directly, and firmly, to the surface of the gorgonian axis. The coenenchyme of the gorgonian overgrows the shell of Conopea species, but the proteinaceous axis generally does not. On the other hand, individuals of Acastinae become completely embedded in the proteinaceous axis rather than living attached to the surface of the gorgonian axis. The wall plates of most Conopea are heavily cemented to each other at the contact sutures, whereas those of acastines are loosely attached to each other and disarticulate very easily with handling or treatment in dilute sodium hypochlorite.”
Although the genus has a global distribution in tropical and warm temperate seas, only four species are known from the seas around China. Conopea are found from the Yellow Sea to the South China Sea, living in the intertidal to shallow depth range. The four known species of Conopea in China are: Conopea granulata, Conopea sinensis, Conopea cymbiformis, Conopea calceola [2,5-7]. Of these four species, C. granulata inhabits Antipathidae in the subtidal in the Taiwan Strait. Conopea sinensis also inhabits Antipathidae but lives in the intertidal to subtidal of the South China Sea. Conopea cymbiformis inhabits Gorgonacea living in the subtidal to shallow waters up to 450 meters deep in the South China Sea, while C. calceola also inhabit Gorgonacea but lives in the subtidal from the Yellow Sea to the South China Sea. This paper describes a new species of Conopea collected in the East China Sea and has a new way of life that has never been seen in Conopea.
Systematics
Class Thecostraca Gruvel, 1905
Subclass Cirripedia Burmeister, 1834 [8]
Infraclass Thoracica Darwin, 1854 [2]
Superorder Thoracicalcarea Gale, 2015
Order Balanomorpha Pilsbry, 1916 [9]
Superfamily Balanoidea Leach, 1817
Family Balanidae Leach, 1817
Subfamily Archaeobalaninae Newman & Ross, 1976
Genus Conopea Say, 1822 [1]
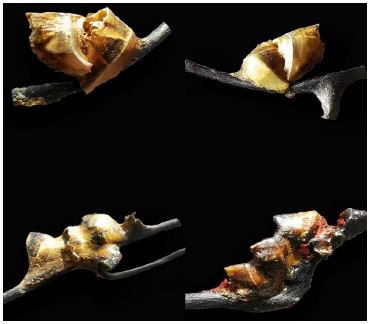
Conopea hongqi sp. nov.
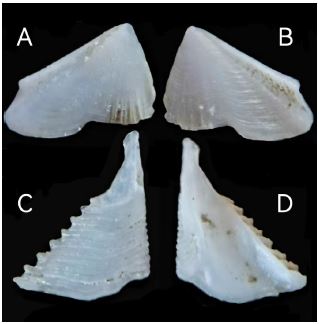
Figure 1
Figure 1:Conopea hongqi, holotype.
Type locality: China. the East China Sea, fisheries zone 1956, region near 30ºN, 128ºE, trawled in 350-450 m, May 2012, collected by local fishermen, on antipatharians.
Holotype National Animal Collection Resource Center, Institute of Zoology, Chinese Academy of Sciences (NACRCCAS), CIR1.
Paratypes: 12 specimens, same data as holotype.
Diagnosis.— Shell elongated in basis, rostrum and carina. Basis cruciform, basis center pale purplish red with pale radial radiation pattern, margin purplish red with light spots. Carina gently sloping from summit to basal margin where it contacts host axis, gradually thinning until sharp edge formed, purplish red with light spots near lower edge. Rostrum pale purplish red to white, angle near apex decreasing sharply, gradually diminishing until just a sharp edge; rostrum wider than carina. Triangular area from center to basal margin of two lateral plates protruding outwards, forming long, triangular, pyramidal thorn, thorn sweeping back in direction of carina, end sharp; thorn in corresponding position of basis inverted with thorn of lateral plate forming long, bird-like beak, thorns hollow. Thickness of ‘beak’ greater than width, width of end in some individuals reduced to nothing, but thickness remains, making end of ‘beak’ a sharp ridge; length less than length of carina. Basis not wrapping or embedded host protein axis, but protein axis of host extended into yellow membrane surrounding basis of barnacle.
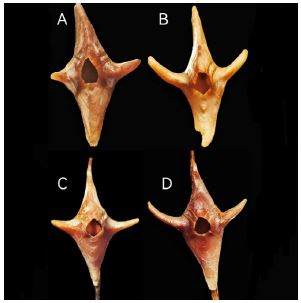
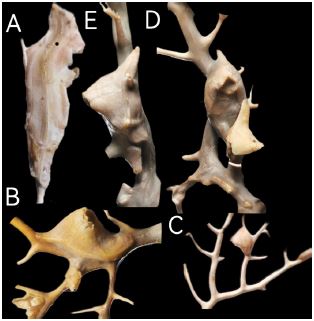
Figure 2
Figure 2:Conopea hongqi, paratypes 1 to 4, top view B rostrum broken, C and D on the axis of the host.
Description.—Shell elongated along basal, rostral and carinal axis; basis cruciform, center pale purplish red with pale radial radiation pattern and concentric circular pattern, edge folded up to connect with wall plates, latter coloured purplish red with pale spots center of basis with greatest depth, the upper edge falls to tips along axis of rostrum-carina, raised ridge present beneath carina; carina gently sloping from summit to basal margin where it contacts host axis, gradually thinning out until sharp edge formed, purplish red with pale spots near the lower edge; rostrum pale purplish red to white, height near apex decreasing sharply, gradually sloping until sharp end formed, rostrum wider than carina. Carino-latus broader at basal margin, narrowing to summit, purplish red with pale spots arranged longitudinally; latus rostral margin and carina lateral margin long, sloped, radii with oblique downward pale stripes, closed at basal margin, broadening toward summit; triangular area from center to basal margin of two lateral plates protruding outward, forming long, triangular, pyramidal thorn sweeping back in direction of carina, end is sharp; thorn in corresponding position of basis inverted with thorn of lateral plate, forming long thorn, thickness of thorn greater than width, with width of end of thorn reduced to nothing in some individuals, but thickness making sharp ridge at end of thorn; length of thorn less than carina; a unique structure not seen previously in Conopea species. Surfaces of wall plates smooth. Upper margin of carinal latus same width as basal margin; white dura mater, sometimes covering all wall plates. Wall plates heavily cemented to each other at contact sutures, orifice smooth or even, not dentate radii with summits parallel to basal margin of pariete and denticulated sutural margins. Basis of Conopea hongqi not wrapping or embedded host protein axis. Instead, protein axis of host extended into yellow membrane surrounding basis of Conopea hongqi.
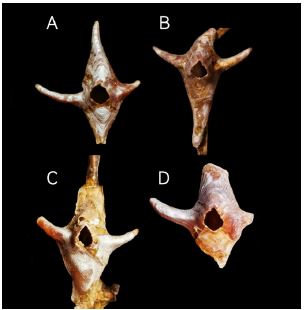
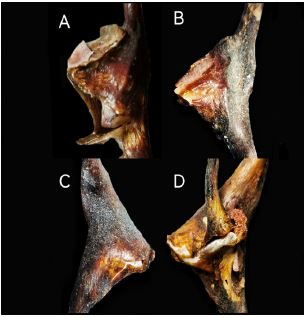
Figure 3
Figure 3:Conopea hongqi, paratypes 5 to 8, B and C, on the axis of the host.
Scutum height greater than width; lateral depressor muscle pits and adductor muscle pits shallow, lateral depressor muscle pits open to basal margin; height of articular ridge about half that of tergal margin, external parallel horizontal growth ridges parallel to basal margin, radial ridges absent; apex acute, light purplish red; interior surface lightly textured, growth ridges visible internally; basal margin curved, tergal and occludent margins relatively straight, tergal margin shorter than occludent margin, occludent margin with dentate structure. Tergum width similar to height, width slightly longer; external horizontal growth ridges parallel to basal margin; apex acute, white, some areas translucent, spur wider than long, slightly less than half width of basal margin, very short, end flat, lateral depressor ridge shallow; lateral depressor ridge shallow, generally of six articles, basiscutal angle obtuse, articular furrow wide; articular ridge minimal, resembling small arc, scutal margin straight, carinal margin slightly convex.
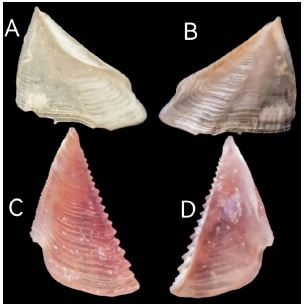
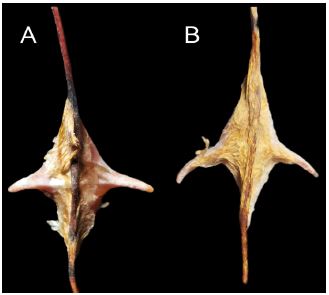
Figure 4
Figure 4:Conopea hongqi, opercular plates of paratypes. A tergum interior; B tergum exterior; C scutum exterior; D scutum interior.
Etymology. — This species is named in honor of Hongqi Chen (陈鸿琪), my grandfather.
Remarks. — All specimens were from the same antipatharians. Fishermen do not freeze antipatharians after catching it. It will take a long time for fishing boatsdepending on whether they go fishing in other areas-to return to the harbor from area 1956.This leads to the decay of all the software parts of barnacles, and the loss of tergum and scutum in most barnacles (Figures 5-7).
Conopea niuqv sp.nov
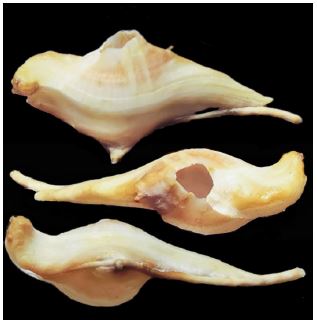
Figure 5
Figure 5:Conopea niuqv, holotype.
Type locality: China. the South China Sea, near Danzhou in the Beibu Gulf, 20-25 m, May 2022, local fishermen use crab nets collected, on gorgonian.
Holotype National Animal Collection Resource Center, Institute of Zoology, Chinese Academy of Sciences (NACRCCAS), CIR2.
Paratypes: 4 specimens, same data as holotype.
Diagnosis.— Shell with elongated basis and rostrum. Center of barnacle always at bifurcation of twigs of gorgonian, causing basis to bend upward with host branch as it extends toward rostrum and carina, with V-shaped bottom edge rather than straight line as in Conopea hongqi. Basis very high, accounting for half height of barnacle, including basis below t rostrum (in C. hongqi, basis below rostrum with almost no height.) basis below carina not closely connected to host axis, basis under rostrum wrapping all or almost all of host axis, causing basis below rostrum to distort with distortion of host’s axis, also distorting rostrum to some extent. Broad purplish red band parallel lower edge of wall plates in middle of carina, rostral latus, lateral and carinal latus. And the rest of each plate, radius and whole rostrum white.
Figure 6
Figure 6:Conopea niuqv, opercular plates of paratypes. A tergum interior; B tergum exterior; C scutum exterior; D scutum interior.
Description. —Shell elongated in basis, carina and rostrum. The center of the barnacle is always at the bifurcation of the twigs of the antipatharians. This causes basis to bend upward with the host branch as it extends toward rostrum and carina, with a V-shaped bottom edge rather than a straight line like Conopea hongqi. The basis is very high, accounting for half the height of the barnacle, including the basis below the rostrum. (in Conopea hongqi, the basis below the rostrum has almost no height.) the basis below the carina is not closely connected to the host axis, and the basis under the rostrum wraps all or almost all of the host axis. This causes the basis below the rostrum to distort with the distortion of the host’s axis, and distorts the rostrum to some extent. There is a broad purplish red band parallel to the lower edge of wall plates in the middle of Carina, rostral latus, lateral plate and carinal latus, and the rest, radius and rostrum white. The upper edge of rostrum is almost horizontal, and the upper edge of carina drops gently. Wall plates surface is smooth heavily cemented to each other at the at the contact sutures, orifice that is not dentate but smooth or even.
Scutum width greater than height; lateral depressor muscle pits deep, adductor muscle pits shallow, lateral depressor muscle pits open to basal margin; articular ridge is slightly longer than half the length of tergal margin, external parallel horizontal growth ridges parallel to basal margin, radial ridges absent; apex acute, white; basal margin curved, tergal and occludent margins relatively straight, tergal margin shorter than occludent margin, occludent margin with dentate structure. Tergum width greater than height; external horizontal growth ridges parallel to basal margin; apex blunt, white, spur wider than long, slightly longer than half width of basal margin, lateral depressor ridge deep, can be seen from the other side; lateral depressor ridge shallow, generally of seven articles, basiscutal angle obtuse, articular furrow wide; articular ridge shallow and straight, scutal margin straight, carinal margin slightly convex.
Soft parts of specimen damaged and cannot be described.
Remarks. — For the same reason as Conopea hongqi, its software and tergum and scutum are completely missing.
Etymology. — niuqv(扭曲)means twist in Chinese. Because the basis and rostrum of this barnacle bend as the host axis bends.
Figure 7-10
Figure 7:Conopea niuqv, paratypes 1 to 4, A Breakage C smaller adult, B, D and E in the axis of the host, D and E is the same specimen, D can see that the opening for cirrus stretching is sealed.
Figure 8:Conopea hongqi, paratypes 9 to 12, in the axis of the host.
Figure 9:Conopea hongqi, paratypes 3-4 In the back view, the basis that extends from the host axis and wraps the barnacle.
Figure 10:Conopea calceola, attach to other Conopea calceola, and in the axis of the host.
Key to Conopea Species found in China
The following key includes only Conopea species found in China. The distribution range is after species level taxa.
1. Radius narrow and completely covered by adjacent shell plates or extensions. . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C. sinensis [7]
(South China Sea, intertidal zone or subtidal zone) Radius wide, exposed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
2. Small thorns or particles on the outer surface of the shell plate. . . . . . . . . . . . . C. granulata [5]
(Taiwan Strait, southern Japan, subtidal zone)
No small thorns or particles on the outer surface of the shell plate. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
3. Carina-rosttum axis lengthened without angular protuberance, shell not fusiform.. . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C. calceola [5]
(Yellow Sea to South China Sea, Japan, Malaysia, Australia, Indian Ocean, Mediterranean, West Africa, Subtidal Zone)
Carina-rosttum axis extends into an angular process, shell is fusiform. . . . . . . . . . . . . . . . . . . . . . . . . . .4
4. Two lateral plates and basal stick out a long thorn to both sides. . . . . . . . . . . . . . C. hongqi Jie Li sp.nov.
Basal not extend beyond the scope of carina, not extend upward to replace part of carina. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. niuqv Jie Li sp.nov.
Discussion
In addition to the genus Conopea, a few species of the subfamily Acastinae are symbionts of antipatharians or gorgonians. There are several obvious differences between Acastinae species and Conopea species: the wall plates of most Conopea are heavily cemented to each other at the contact sutures, whereas those of Acastinae are loosely attached to each other. The shape of Conopea is slender, the axis of rostrum-carina is obviously elongated, while in species of the Acastinae it is more rounded, the axis of rostrum-carina is not particularly elongated. Conopea has an orifice that is not dentate but smooth or even, radii with summits parallel to the basal margin of the pariete, and denticulated sutural margins, Acastinae has a dentate orifice, and radii with oblique summits. Conopea hongqi has all the features Conopea and is attached to antipatharians. Of the 20 species of Conopea, only C. cornuta from the Gulf of Guinea in Africa has a special structure similar to C. hongqi, with two sharp corners extending from the side to the side. But C. hongqi is thinner and longer than C. cornuta, and the thorns protruding to the left and right sides of lateral plate and basis are much longer than those of C. cornuta. The axis of the basis of C. cornuta is an obvious arc, nearly semicircular, while the axis of C. hongqi is a straight line. Conopea cornuta’s wall plates are red, yellow, and white, and C. hongqi is fuchsia. The scutum of C. cornuta is distinctly convex, with rather prominent growth ridges. The basal margin is sinuous. The articular ridge occupies two thirds of the length of the tergal margin, and is reflexed, but narrow. The adductor ridge is confluent with the articular ridge in the upper part and quite indistinct in the lower part. The tergum has its apex pointed but not extended. There is no longitudinal furrow, and the spur, the width of which is approximately half that of the whole valve, is low and obliquely rounded. The scutal margin is nearly straight; there is hardly any distance between the basi-scutal angle and the spur. The basal margin is slightly hollowed [10]. The scutum of C. hongqi is relatively flat, the height of the articular ridge is approximately half that of tergal margin, spur wider than long, slightly less than half the width of the basal margin; it has a flat end and is very short, approximately one-quarter of the height of tergum, the scutal margin straight, and the carinal margin is slightly convex.
The habitats of C. hongqi and C. cornuta are also different: C. cornuta was collected from the Gulf of Guinea, Africa, 32 meters deep, attached to gorgonians, while C. hongqi comes from the 1956 area of the East China Sea, 350–450 m deep, attached to antipatharians.
Van Syoc et al. indicated that “The acastine species living in gorgonians differ from Conopea species in several ways, the most obvious is the form of their attachment to the host. Conopea species are cemented directly, and firmly, to the surface of the gorgonian axis [4]. The coenenchyme of the gorgonian overgrows the shell of Conopea species, but the proteinaceous axis generally does not. On the other hand, individuals of Acastinae become completely embedded in the proteinaceous axis rather than living attached to the surface of the gorgonian axis. The wall plates of most Conopea are heavily cemented to each other at the contact sutures, whereas those of acastines are loosely attached to each other and disarticulate very easily with handling or treatment in dilute sodium hypochlorite.”
But C. hongqi and C. niuqv appeared the above two life styles at the same time-C. hongqi, which lives on the finer antipatharians branches, only attached to the surface of the branches, was covered by coenenchyme, and was not embedded in the protein axis of antipatharians. On the other hand, C. hongqi, which lives on the thicker antipatharians branch, is embedded in the protein axis of antipatharians, showing only the opening.
It should be noted that although C. hongqi and C. niuqv wrapped by the protein axis of the host, but the causes of the two are probably not the same. The basis of C. hongqi does not wrap the protein axis of the host. On the contrary, the protein axis of the host forms a yellow membrane that wraps the basis of C. hongqi. This means that completely wrapped C. hongqi may be just an extreme example of the first way of life-the host’s membrane is so long and thick that it wraps not only the basis of C. hongqi, but also the rest of C. hongqi, leaving only an opening for cirrus to stretch. For C. hongqi, this means that being wrapped in the axis of the host is a normal state of life, and the wrapped C. hongqi can still live well. There are several pieces of evidence to prove this-all fully wrapped C. hongqi have openings for cirrus to stretch. The lateral spines of two fully wrapped C. hongqi punctured theprotein axis, and the other part wrapped it with a crack. This means that the three barnacles are still alive and well after being wrapped, and even grow up to destroy the axis that wraps them. Traces of physical decay were found in more than one completely wrapped C. hongqi [11-15].
For C. niuqv, its basis will be embedded or even completely wrapped around the host axis. This means that being wrapped in the host’s axis is unlikely to be a continuation of its first way of life. At the same time, all the wrapped C. niuqv were completely wrapped, not even leaving an opening protruding from the total cirrus. They are fully encased in coral transition growth after they die. This situation is not only on C. niuqv and C. calceola, but also on Conopea other than C. hongqi.
C.calceola is not only attached to black coral. C. calceola may also be attached to other C. calceola. In this case, both C. calceola can live well. But this situation is very rare. Of the 121 C. calceola specimens I have, only 8 live in this way.
WoRMS (2022) Conopea Say, 1822. [ Ref ]
Darwin CR (1854) A Monograph on the Sub-Class Cirripedia, with Figures of All the Species. The Balanidae (or Sessile Cirripedes); the Verrucidae, etc. The Ray Society, London. [ Ref ]
Carrison-Stone D, Van Syoc R, Williams G, Simison B (2013) Two new species of the gorgonian inhabiting barnacle, Conopea (Crustacea, Cirripedia, Thoracica), from the Gulf of Guinea. ZooKeys 270: 1-20. [ Ref ]
Van Syoc RJ, Carrison-Stone D, Madrona L, Williams GC (2014) Barnacle symbionts of gorgonian sea fans: description of seven new species (Archaeobalanidae: Cirripedia) from the Philippines, including a key to western Pacific species of Conopea. The Coral Triangle: The 2011 Hearst Philippine Biodiversity Expedition. California Academy of Sciences, San Francisco. [ Ref ]
Hiro F (1937) Studies on Cirripedian fauna of Japan. II. Cirripeds found in the vicinity of the Seto Marine Biological Laboratory. Mem Coll Sci, Kyoto Imp Univ, Ser. B 12: 385-478. [ Ref ]
Ren X, Liu R (1978) Studies on Chinese Cirripedia (Crustacea). I. Genus Balanus. Studia Marina Sinica 13: 119-196. [ Ref ]
Ellis J (1758) CXIII. An account of several rare species of barnacles. In a letter to Mr. Isaac Romilly, F. R. S. from John Ellis, Esq; F. R. S. Philosophical Transactions 50: 845-855. [ Ref ]
Burmeister H (1834) Beitrage zur Naturgeschichte der Rankenfusser (Cirripedia). Reimer, Berlin, Germany. [ Ref ]
Pilsbry HA (1916) The sessile barnacles (Cirripedia) contained in the collections of the U.S. National Museum: including a monograph of the American species. Bulletin of the United States National Museum 93: 1-366. [ Ref ]
Hoek PPC (1913) The Cirripedia of the Siboga Expedition. B. Cirripedia Sessilia. Siboga Expeditie Monograph 31b: 129-275. [ Ref ]
Broch H (1922) Studies on Pacific cirripeds. Papers from Dr. Th. Mortensen’s Pacific Expedition 1914–1916, No. X. Videnskabelige Meddelelser Dansk Naturhistorisk Forening 73: 215-358. [ Ref ]
Kolbasov GA, Chan BKK, Molodtsova T N, Achituv Y (2016) Revision of the coral-inhabiting genus Conopea (Cirripedia: Archaeobalanidae) with description of two new species of the genera Conopea and Acasta. Zootaxa 4178: 182. [ Ref ]
Nilsson-Cantell CA (1921) Cirripeden Studien. Zur Kenntnis der Biologie, Anatomie und Systematik dieser Gruppe. Zool Bidrag Uppsala 7: 75-390. [ Ref ]
Ren X, Liu R (2016) Fauna sinica invertebrate vol. 42 Crustacea Cirripedia Thoracica Chinese Academy of Science, Science Press, Beijing. [ Ref ]
Say T (1822) An account of some of the marine shells of the United States. Journal of the Academy of Natural Sciences of Philadelphia. 2: 221-248, 257-276, 302-325. [ Ref ]