Journal Name: Journal of Biomedical Research and Reviews
Article Type: Research
Received date: 22-February-2022
Accepted date: 31-March-2022
Published date: 07-April-2022
Citation: Wang H, Chen M, Sun H, Zhang L (2022) Diagnosis, Prognosis and Clinical Treatment of Refractory Atypical Chronic Lymphocytic Leukemia: A Case Report. J Biomed Res Rev Vol: 5, Issu: 1. (23-27).
Copyright: © 2022 Wang H et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most prevalent adult leukemia, and its incidence continues to rise year after year. Rapid and precise diagnosis is an essential element in effective case management, however, the clinical diagnosis, treatment, and prognosis of CLL/SLL are not fully elucidated. Case presentation: we report the case of a 66-year-old man with atypical CLL/SLL. The white blood cell (WBC) count (842.0 × 109/L), platelet count (30.6 × 109/L), and abnormal lymphocytes were increased in peripheral blood. Flow cytometry showed 98.34% of nucleated cells were malignant monoclonal mature B cell. Peripheral blood smear found the leukocytes and lymphocytes with abnormal morphology were increased. Fluorescence in situ hybridization showed CCND1 (11q23)/IGH (14q32) and abnormal chromosome 12 were negative, 91%~93% of interphase nuclei presented D13S319 and TP53, 17p13.1 loss. Histopathology analysis of bone marrow observed the proliferation centers with immunoblasts. Immunohistochemistry showed that bone marrow was positive for PAX-5, CD20, CD23, and CD5, negative for CD3, cyclinD1, and sox11, and partial positive for Ki67. The patient was diagnosed as CLL/SLL based on above clinical and laboratory findings. The patient was managed with oral 50 mg Vinetoc, fluid replacement, hydration and alkalization, and the symptoms were significantly relieved. Conclusions: This report further expands the knowledge of clinical diagnosis and treatment of atypical CLL/SLL.
Keywords
CLL/SLL, Peripheral blood cell, Histopathology, Cytogenetics, Immunophenotype.
Abstract
Background: Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most prevalent adult leukemia, and its incidence continues to rise year after year. Rapid and precise diagnosis is an essential element in effective case management, however, the clinical diagnosis, treatment, and prognosis of CLL/SLL are not fully elucidated. Case presentation: we report the case of a 66-year-old man with atypical CLL/SLL. The white blood cell (WBC) count (842.0 × 109/L), platelet count (30.6 × 109/L), and abnormal lymphocytes were increased in peripheral blood. Flow cytometry showed 98.34% of nucleated cells were malignant monoclonal mature B cell. Peripheral blood smear found the leukocytes and lymphocytes with abnormal morphology were increased. Fluorescence in situ hybridization showed CCND1 (11q23)/IGH (14q32) and abnormal chromosome 12 were negative, 91%~93% of interphase nuclei presented D13S319 and TP53, 17p13.1 loss. Histopathology analysis of bone marrow observed the proliferation centers with immunoblasts. Immunohistochemistry showed that bone marrow was positive for PAX-5, CD20, CD23, and CD5, negative for CD3, cyclinD1, and sox11, and partial positive for Ki67. The patient was diagnosed as CLL/SLL based on above clinical and laboratory findings. The patient was managed with oral 50 mg Vinetoc, fluid replacement, hydration and alkalization, and the symptoms were significantly relieved. Conclusions: This report further expands the knowledge of clinical diagnosis and treatment of atypical CLL/SLL.
Keywords
CLL/SLL, Peripheral blood cell, Histopathology, Cytogenetics, Immunophenotype.
Abbreviations
CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma; WBC: white blood cell; FISH: Fluorescence in situ hybridization; CBC: complete blood count; PLL: prolymphocytic leukemia; RMH: Royal Marsden Hospital.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most common malignancy in adults originating from blood or bone marrow, with the characteristic of a clonal expansion of mature B cells [1]. CLL/SLL is a highly heterogeneous clinical course, with an increasing incidence [2-4]. Therefore, rapid recognition and timely treatment are crucial in achieving optimal outcomes.
Approximately 25% -50% present of patients with CLL/ SLL are asymptomatic at diagnosis, and was incidentally found by blood routine examination [5]. CLL/SLL patients typically present with fatigue, decreased physical activity and weakness, which generally occur before anemia or lymphadenopathy and hepatosplenomegaly. Clinically, 80% of patients with CLL/SLL possess painless lymphadenopathy upon diagnosis, and about half of CLL/SLL patients have mild or moderate hepatosplenomegaly at diagnosis. Lymphadenopathy is a relatively advanced clinical presentation, which has become an important limiting factor for disease treatment. The diagnosis of CLL/SLL relies on flow cytometric immunophenotyping [6]. Combined flow cytometry, cytomorphological analysis, and molecular genetic have been the powerful methods for high sensitivity and precise diagnosis of CLL/SLL.
Case Report
A 66-year-old man was admitted to our hospital because of two-month history of asthenia, night sweats, vomiting, and hepatosplenomegaly. Presenting complete blood count (CBC) showed 842.0×109/L, white blood cell (WBC), 91 g/L hemoglobin, and 88×109/L platelet count. Microscopic examination of peripheral blood smears observed abnormal lymphocytes was present in 95%. The serum biochemical indices of the patient (Supplementary Table S1) were as follows: Total protein 57.2 g/L, cholinesterase 4883 U/L, calcium ion 2.08 mmol/L, urea 8.89 mmol/L, sodium ion 148.3 mmol/L, bicarbonate 32.4 mmol/L, uric acid, 459.2 Umol/L, lactate dehydrogenase 544 U/L, potassium ion 330 mmol/L, alkaline phosphatase 192 U/L, β2-microglobulin 5127.59 Ug/L.
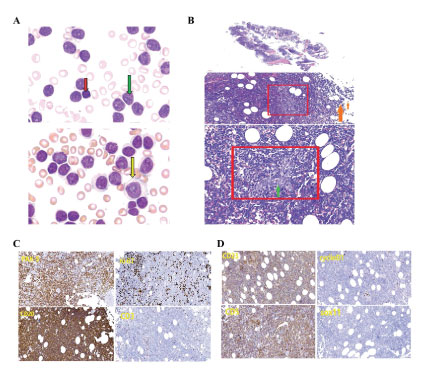
Peripheral blood smear (Figure 1A) showed that the abnormal leukocytes and lymphocytes were markedly increased. Approximately 93% of the cells were prolymphocytes (green arrow) with large cell bodies, round or irregular nuclei, accumulated chromatin, obvious nucleoli. We also found 7% of immunoblast (yellow arrow) and small lymphocytes (red arrow, <1%). In the French American British classification, prolymphocytes ≥55% were considered as prolymphocytic leukemia (PLL) [7]. Here, although prolymphocytes accounted for 93% of nuclear cells, morphological analysis observed the cell body of low proportion cells was smaller than that of CLL cells with normal lymphocytes.
Table 1:The results of biochemical test.V
| Name | Content |
|---|---|
| Total protein | 57.2g/L |
| cholinesterase | 4883U/L |
| calcium ion | 2.08mmol/L |
| urea | 8.89mmol/L |
| sodium ion | 148.3mmol/L |
| bicarbonate | 32.4mmol/L |
| uric acid | 459.2umol/L |
| lactate dehydrogenase | 544U/L |
| potassium ion | 330mmol/L |
| alkaline phosphatase | 192U/L |
| β2-microglobulin | 5127.59ug/L |
For precise diagnosis, we performed MICM diagnosis. Histopathology analysis (Figure 1B) of bone marrow observed large cells with median nucleoli (i.e., paraimmunoblast) in proliferation centers, small chronic lymphocytes with deeply stained in the periphery. Immunohistochemistry (Figure 1C) found that bone marrow was positive for PAX-5, CD20, CD23, and CD5, negative for CD3, cyclinD1, and sox11, and the expression of Ki67 is partial positive with the positive rate of 30%.
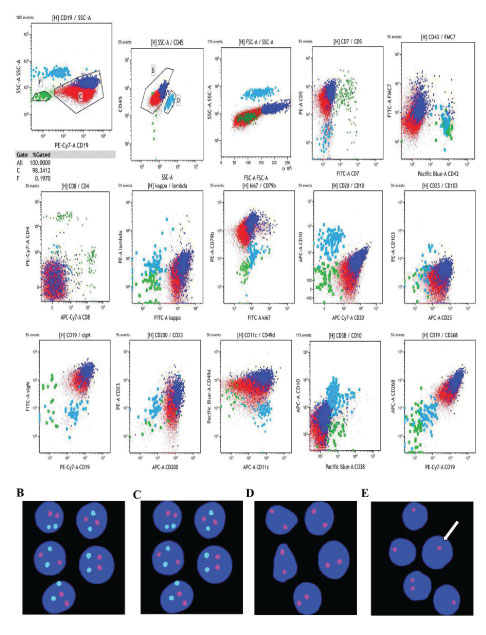
Flow cytometry (Figure 2A) of peripheral blood showed that 98.34% of nucleated cells was CD19+ cells with heterogeneous cell size. They coexpressed CD19, CD5, kappa bri, CD20, CD79b bri, CD25, sIgM bri, CD200, CD23, CD11c, CD49d, and CD268, not expressed CD7, CD43, CD4, CD8, lambda, Ki67, CD10, CD103, and CD38, and partially expressed FMC7, indicating these cells were malignant monoclonal mature B cell. PLL cells usually has CD5- CD23- FMC7+ immunophenotype, which is different from CLL cells. CLL can’t transform into PLL, and the increase of precursor cell only indicates the increased progression of CLL, which often accompanied by NOTCH1 or TP53 aberration [8]. Therefore, although prolymphocytes accounted for 93% in this patient, the possibility of PLL was still excluded. The Royal Marsden Hospital (RMH) score is used for the diagnosis of CLL/SLL by flow cytometry [9]: CD5 positive, CD23 positive, FMC7 negative, CD79b or CD22 weakly positive, kappa/lambda monoclonal and weakly positive or membrane immunoglobulin weakly positive. For this patient, the RMH score was 3-4, the atypical chronic lymphocytic, atypical mantle cell lymphoma, and other CD5 + small B-cell lymphomas cannot be excluded by 3-4 score and strong positive expression of CD200 [10,11].
Fluorescence in situ hybridization (FISH) (Figure 2B- 2E) revealed that the fusion genes of CCND1 (11q23)/ IGH (14q32) were not found, chromosome 12 showed no abnormality, 91% of interphase nuclei detected loss of D13S319, and 93% of interphase nuclei detected loss of TP53, 17p13.1. Combination of negative expressing cyclinD1and sox11 (Figure 2D) indicated the atypical CLL.
It has been reported that the gene expression (un-mutated immunoglobulin heavy-chain variable gene, CD38, CD49d, ZAP-70), trisomy 12 abnormalities, 11q-, 13q-, 6q-, 17p- or TP53 mutations, and serum β2-microglobulin deposition are associated with CLL progression and poor prognosis [12]. When CLL patients suffer from high proportion of atypical prolymphocytes in the circulation, clinicians should be alert to the possibility of disease progression [8]. The amount of mitotic cells in proliferation center more than 2.4, or Ki-67 > 40% per proliferation center is considered a propensity for accelerated disease [13]. The lymph node and tissue biopsy are used for histopathological analysis of large cell lymphoma transformation. In this case, this patient expressed CD49d, which is a poor predictor of CLL and involved in tumor invasion [14]. Although the number of chromosome 12 were not observed in this patient, other poor prognostic factors, including D13S319 loss, TP53 gene loss, higher Ki67 positive rate, significant myeloproliferative centers, and cell transformation, were observed as factors indicating poor prognosis. The patient developed B symptoms, such as progressive enlargement of the liver, spleen, and lymph nodes, fever, abdominal pain, weight loss, progressive anemia and thrombocytopenia, rapid increase in peripheral blood lymphocytes and newly diagnosed β2-microglobulin up to 5127.59 Ug/L, was suspicious for refractory disease progression.
Figure 1: Analysis of peripheral blood and histopathology. (A) Peripheral blood was stained by Wright. (B) H&E staining in bone marrow. Red box, proliferation center; green arrow, immunoblast; orange arrow, chronic lymphocyte. (C) Immunohistochemistry analysis for PAX-5, CD20, CD23, CD5, CD3, cyclinD1, sox11, and ki67 expression in bone marrow.
The patient was diagnosed with CLL/SLL (BinetC, RaiIV; IPI7 polarization). He was treated with oral 50 mg Vinetoc, fluid replacement, hydration, and alkalinization. After 15 days, the WBC decreased to 25.0 × 109/L, the symptoms were significantly relieved. The patient was discharged after the first course of chemotherapy.
Conclusion
In conclusion, when a high proportion of circulating precursor cells exist in rare cases, a comprehensive analysis of peripheral blood cell, immunophenotype, tissue biopsy, and cytogenetics is critical for disease diagnosis. This study elaborated the diagnosis, prognosis, and clinical standardized treatment of CLL/SLL, and illustrated the significance and value of complete MICM diagnosis.
Acknowledgement
Not applicable.
Authors’ Contribution
Hui Wang, corresponding author, designed the research and revise paper. Xiaoge Zhou, Ping Wu,Tong Wang, Hongxing Liu, revise paper. Man Chen, First Author, Statistical Analysis, analyzed data and wrote the paper. Huipeng Sun, Lina Zhang, Minjing Fu and Haiyan Gao gather clinical data. Aixian Wang, Xueying Wu, Junyi Zhen, Meiwei Gong, Qing Du, test samples and report results.
Funding
Not applicable.
Figure 2: Analysis of immunophenotype and cytogenetics. (A) The immunophenotype of cells in the peripheral blood was analyzed by flow cytometry. (B) FISH analysis showing the t(11; 14) (q13; q32) translocation. Green signal is CCND1, red signal is IGH. (C) The red probe detects the centromere of chromosome 12. (D) FISH detected loss of D13S319. Red signal and white arrow indicates del(13q14). (E) Detection of TP53/CEP17 site. Red signal is TP53, green signal is CEP17, white arrow indicates the loss of 17p13.1 and TP53.
Availability of Data and Materials
The data and materials used and analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participates
The procedures reported in this manuscript were approved by the Ethics Committee of the General Hospital of Chinese People’s Liberation Army and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant prior to specimen collection.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Trimech M, Letourneau A, Missiaglia E, De Prijck B, Nagy-Hulliger M, et al. (2021) Angioimmunoblastic T-Cell Lymphoma and Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Novel Form of Composite Lymphoma Potentially Mimicking Richter Syndrome. Am J Surg Pathol 45: 773-786. [ Ref ]
Nadeu F, Delgado J, Royo C, Baumann T, Stankovic T, et al. (2016) Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood 127: 2122-2130. [ Ref ]
Szoltysek K, Ciardullo C, Zhou P, Walaszczyk A, Willmore E, et al. (2020) DAP Kinase-Related Apoptosis-Inducing Protein Kinase 2 (DRAK2) Is a Key Regulator and Molecular Marker in Chronic Lymphocytic Leukemia. Int J Mol Sci 21: 7663. [ Ref ]
Kapoor I, Bodo J, Hill BT, Hsi ED, Almasan A (2020) Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis 11: 941-941. [ Ref ]
Atwal D, Raval M, Firwana B, Ramos J, Sasapu A (2017) An unusual presentation of chronic lymphocytic leukemia. Avicenna J Med 7: 133- 136. [ Ref ]
Goshaw JM, Gao Q, Wardrope J, Dogan A, Roshal M (2020) 14-Color single tube for flow cytometric characterization of CD5+ B-LPDs and high sensitivity automated minimal residual disease quantitation of CLL/SLL. Cytometry B Clin Cytom 100: 509-518. [ Ref ]
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, et al. (2018) iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 131: 2745-2760. [ Ref ]
Oscier D, Else M, Matutes E, Morilla R, Strefford JC, et al. (2016) The morphology of CLL revisited: the clinical significance of prolymphocytes and correlations with prognostic/molecular markers in the LRF CLL4 trial. Br J Haematol 174: 767-775. [ Ref ]
Quinquenel A, Aurran-Schleinitz T, Clavert A, Cymbalista F, Dartigeas C, et al. (2020) Diagnosis and Treatment of Chronic Lymphocytic Leukemia: Recommendations of the French CLL Study Group (FILO). Hemasphere 4: e473. [ Ref ]
Matutes E, Owusu-Ankomah K, Morilla R, Garcia Marco J, Houlihan A, et al. (1994) The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia 8: 1640-1645. [ Ref ]
Ting YS, Smith SABC, Brown DA, Dodds AJ, Fay KC, et al. (2018) CD200 is a useful diagnostic marker for identifying atypical chronic lymphocytic leukemia by flow cytometry. Int J Lab Hematol 40: 533-539. [ Ref ]
Oscier DG, Gardiner AC, Mould SJ, Glide S, Davis ZA, et al. (2002) Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood 100: 1177-1184. [ Ref ]
Giné E, Martinez A, Villamor N, López-Guillermo A, Camos M, et al. (2010) Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica 95: 1526-1533. [ Ref ]
Jain N (2015) New developments in Richter syndrome. Clin Adv Hematol Oncol 13: 220-222. [ Ref ]