Journal Name: Journal of Biomedical Research and Reviews
Article Type: Research
Received date: 25-February-2022
Accepted date: 23-June-2022
Published date: 30-June-2022
Citation: Zhou W, Zhang WB, Yu Y, Wang Y, Guo CB, et al. (2022) Outcome of Free-Flap Transfer for Head and Neck Reconstruction in Hypercoagulable Patients. J Biomed Res Rev Vol: 5, Issu: 1. (39-43).
Copyright: © 2022 Zhou W et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The purpose of this study was to examine the outcome and complications of hypercoagulable patients undergoing free-flap transfer in the head and neck region.
Methods: We retrospectively reviewed the data of 685 consecutive free-flap transfers in the head and neck region performed by a single surgical team at the Peking University School and Hospital of Stomatology between January 2013 and December 2018. Based on preoperative coagulation indices the patients were separated into two groups: those with hypercoagulablity (group A, n = 45) and those with normal coagulation indices (group B, n = 640). Demographic characteristics, thrombosis and flap failure were compared between the two groups. Chi-square test and repeated-measures ANOVA were used for data comparisons.
Results: Microvascular thrombosis rate (P = 0.42) and free-flap success rate (P = 0.38) were not significantly different between the groups. The platelet count and activated partial thromboplastin time changed significantly during the perioperative period in the hypercoagulable group (repeated-measures ANOVA, P < 0.001 for both).
Conclusion: Hypercoagulability does not seem to increase risk of free-flap failure in head and neck microsurgery provided standard anticoagulation protocols are followed.:
Keywords
Head and neck, Free flap, Hypercoagulability
Abstract
Background: The purpose of this study was to examine the outcome and complications of hypercoagulable patients undergoing free-flap transfer in the head and neck region.
Methods: We retrospectively reviewed the data of 685 consecutive free-flap transfers in the head and neck region performed by a single surgical team at the Peking University School and Hospital of Stomatology between January 2013 and December 2018. Based on preoperative coagulation indices the patients were separated into two groups: those with hypercoagulablity (group A, n = 45) and those with normal coagulation indices (group B, n = 640). Demographic characteristics, thrombosis and flap failure were compared between the two groups. Chi-square test and repeated-measures ANOVA were used for data comparisons.
Results: Microvascular thrombosis rate (P = 0.42) and free-flap success rate (P = 0.38) were not significantly different between the groups. The platelet count and activated partial thromboplastin time changed significantly during the perioperative period in the hypercoagulable group (repeated-measures ANOVA, P < 0.001 for both).
Conclusion: Hypercoagulability does not seem to increase risk of free-flap failure in head and neck microsurgery provided standard anticoagulation protocols are followed.:
Keywords
Head and neck, Free flap, Hypercoagulability
Background
With improvements in microsurgery techniques, free-flap transfer has become the standard treatment for head and neck defects. The success rates are high (ranging from 90% to 99%), but the occasional flap failure is devastating for both patient and surgeon [1-4].
Thrombosis is the leading cause of free-flap failure [5,6]. It is more likely to occur in hypercoagulable patients, especially those undergoing surgery for malignancy and therefore free-flap transfer in these patients is still considered a surgical challenge [7,8].
The literature on microsurgery in the hypercoagulable patient is scanty. There is still no international consensus on whether hypercoagulability influences free-flap outcomes. The purpose of this study was to determine the outcome and complications of hypercoagulable patients undergoing freeflap transfer in the head and neck region.
Methods
Patients
This retrospective study included a total of 685 consecutive patients treated with free-flap transfer in head and neck region by the same surgical team at Peking University School and Hospital of Stomatology between January 2013 and November 2018. Preoperatively, all patients underwent assessment for hypercoagulable state. Hypercoagulability was diagnosed in patients with one or more of the following: 1) platelet count >300 × 109/L, 2) prothrombin time (PT) <10 seconds, and 3) activated partial thromboplastin time (APTT) <23 seconds. Intraoperatively, all patients received topical irrigation of the donor and recipient vessels with heparinized saline solution; postoperatively, they received oral aspirin 40 mg and dextran-40 30 g intravenous injection once daily for 5 days.
Study Variables
Patient demographics (age, gender) and free-flap outcome (postoperative thrombosis, flap failure) were compared between patients with and without hypercoagulability. For hypercoagulable patients, the three coagulation indices (platelet count, PT, and APTT) before operation and after operation (at 24 hours, 48 hours, and 120 hours) were recorded and analyzed.
This retrospective study was approved by the Ethics Committee for Human Experiments at the Peking University School and Hospital of Stomatology (PKUSSIRB-201631114). The need for informed consent was waived in view of the retrospective nature of the study.
Data analysis
Statistical analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The chi-square test and Fisher’s exact test were used to compare the rates of microvascular thrombosis and flap failure between the two groups. The repeated-measures analysis of variance (ANOVA) was used to analyze the changes in coagulation indices (platelet count, PT, and APTT). P ≤ 0.05 was considered statistically significant.
Results
A total of 685 patients (401 males and 284 females) with a mean age of 49.8 ± 15.5 years (range, 16–83 years) were included in this study. The free flaps used for reconstruction included 394 (57.5%) fibula flaps, 142 (20.7%) anterolateral thigh flaps, 117 (17.1%) radial forearm free flaps, 18 (2.6%) iliac crest free flaps, 11 (1.6%) submental free flaps, and three (0.4%) rectus abdominis flaps (Table 1).
Among the 685 patients, 45 patients (group A) met the criteria for hypercoagulability: 33 patients had preoperative platelet count >300 × 109/L, seven patients had preoperative APTT <23 seconds, two patients had preoperative platelet count >300 × 109/L and PT <10 seconds, two patients had preoperative platelet count >300 × 109/L and APPT <23 seconds, and one patient had preoperative PT <10 seconds and APTT <23 seconds. Preoperative blood coagulation indices were normal in 640 patients (group B).
Postoperative microvascular thrombosis occurred in 3/45 (6.7%) patients in group A vs. 25/640 (3.9%) patients in group B (P = 0.42). Free-flap success rate was 95.6% (43/45 patients) in group A vs. 97.2% (622/640 patients) in group B (P = 0.38; Table 2). These differences between the groups were not statistically significant. In all, 20 flaps were lost; the total free flap success rate was 97.1%. Venous thrombosis was the main cause of free flap failure in 2/2 (100%) patients in group A vs. 15/18 (83.3%) patients in group B. The other three flap loss in group B was result from arterial thrombosis.
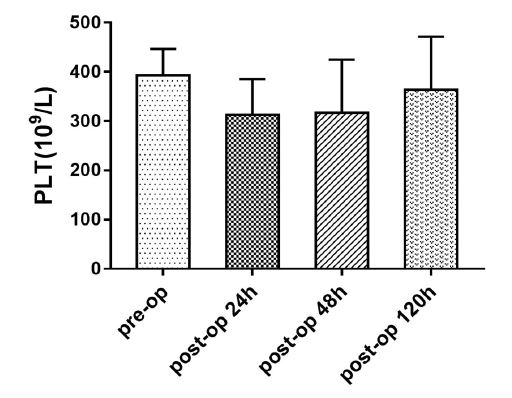
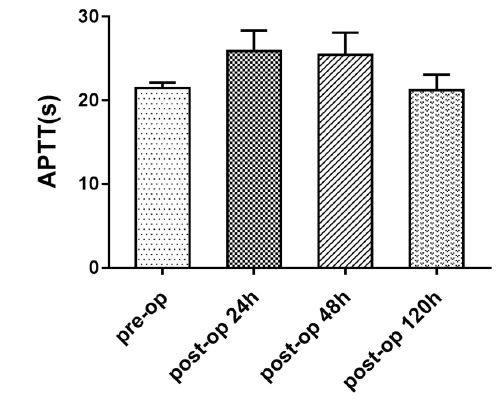
The changes in coagulation indices (platelet count, PT, and APTT) in the perioperative period were examined in hypercoagulable patients. Platelet count was low during postoperative days 1–2, but began rising on postoperative day 5. The APTT was high during postoperative days 1–2, but began decreasing on postoperative day 5. The changes in platelet count and APTT were statistically significant (repeated-measures ANOVA, P < 0.001; Table 3; Figures 1, 2).
Discussion
The hypercoagulable state, also called prethrombotic state, is a pathological state induced by a combination of vascular endothelial cell injury and anticoagulant and fibrinolytic system dysfunction. It may be caused by a variety of pathological conditions but, since it is not a disease per se, there is no consensus on the criteria for its diagnosis. Many of the literature reports of hypercoagulability in surgical patients refer to microvascular thrombotic events. However, intraoperative or postoperative thrombotic events do not necessarily indicate the presence of hypercoagulability; radiation, hypertension, diabetes, and old age may also be responsible for thrombosis.
Several authors have examined blood coagulation and fibrinolysis factors in patients undergoing microsurgery [8-13]. Wang et al. retrospectively reviewed 2032 free-flap surgeries performed at the University of Pennsylvania, between January 1, 2005, and October 1, 2010. They identified 41 hypercoagulable patients, the diagnosis being based on presence of factor V Leiden mutation, protein C deficiency, hyperhomocysteinemia, antiphospholipid antibody syndrome, prothrombin gene mutation, factor VIII elevation, anticardiolipin antibody syndrome, or essential thrombocytosis [14]. In a prospective study, Kloeters et al. collected blood samples of patients preoperatively, intraoperatively, and postoperatively (at 3, 6, 12, 24, 36, 48, 72, 96, and 120 hours after the operation). They assessed the presence of a hypercoagulable state by analyzing markers of coagulation such as prothrombin fragment 1+2, thrombin–antithrombin III-complex, and antithrombin, as well as markers of fibrinolysis such as plasminogenactivator inhibitor-I, tissue-plasminogenactivator, and plasminogen [15].
Table 1: Demographic characteristics and donor sites of patients in the two groups.
| Group A (Hypercoagulable patients) | Group B (Non-hypercoagulable patients) | Total | |
|---|---|---|---|
| Sample size (n) | 45 | 640 | 685 |
| Age, years, mean ± SD | 49.0 ± 13.7 | 49.8 ± 15.6 | 49.8 ± 15.5 |
| Gender, n (%) | |||
| Male | 26 (57.8%) | 375 (58.6%) | 401 (58.5%) |
| Female | 19 (42.2%) | 265 (41.4%) | 284 (41.5%) |
| Donor site | |||
| Fibula flap | 23 (51.1%) | 371 (58.0%) | 394 (57.5%) |
| ALTF | 13 (28.9%) | 129 (20.2%) | 142 (20.7%) |
| RFFF | 5 (11.1%) | 112 (17.5%) | 117 (17.1%) |
| Iliac crest flap | 1 (2.2%) | 17 (2.7%) | 18 (2.6%) |
| Submental free flap | 2 (4.4%) | 9 (1.4%) | 11 (1.6%) |
| RAF | 1 (2.2%) | 2 (0.3%) | 3 (0.4%) |
| ALTF, anterolateral thigh flap; RFFF, radial forearm free flap; RAF, rectus abdominis flap; SD, standard deviation. | |||
Table 2: Outcomes and complications in the two groups.
| Group A (Hypercoagulable group) | Group B (Non-hypercoagulable patients) | P | |
|---|---|---|---|
| Sample size | 45 | 640 | |
| Thrombosis | 3 | 25 | 0.42 |
| Flap failure Flap success rate | 2 95.6% | 18 97.2% | 0.38 |
Table 3: Changes in platelet count and activated partial thromboplastin time of hypercoagulable patients during the perioperative period.
| Platelet count (×109/L) | APTT (seconds) | |
|---|---|---|
| Preoperative | 392.91 ± 53.33 | 21.48 ± 0.63 |
| 24 h postoperatively | 313.32 ± 71.79 | 25.87 ± 2.46 |
| 48 h postoperatively | 317.13 ± 107.60 | 25.44 ± 2.64 |
| 120 h postoperatively | 363.94 ± 107.68 | 21.21 ± 1.85 |
| P | P < .001 | P < .001 |
| All values are means ± standard deviations APTT, activated partial thromboplastin time | ||
Figure 1: Platelet count in the hypercoagulable group during the perioperative period.
Figure 2: Activated partial thromboplastin time in the hypercoagulable group during perioperative period.
In our institution preoperative laboratory investigations usually include complete blood counts, coagulation function tests, and biochemical indicators. Platelet count, PT, and APTT are used to identify patients with hypercoagulable state. We have found that these examinations are generally sufficient to assess the patient’s preoperative condition and to ensure that the operation can be performed safely. Moreover, these tests do not unduly prolong hospital stay or increase treatment costs.
Thus far, there is no consensus regarding routine use of antithrombotic agents for preventing thrombosis after head and neck reconstructive surgery [16-20]. Most microsurgeons agree that for hypercoagulable patients’ antithrombotic agents should be used intraoperatively or postoperatively to prevent thrombosis, but there is no standard regimen. The antithrombotic regimen varies between institutions and surgeons, usually involving different combinations of oral aspirin, low molecular weight dextran, and low molecular weight heparin.
Pannucci et al. have reported good results with the use of antithrombotic therapy for high-risk patients [21]. Their practice is to use intravenous unfractionated heparin, run at 800 U/h during the operation and for 24 hours after surgery. Subsequently, the patient is started on prophylactic-dose enoxaparin, which is continued at least until discharge. In a retrospective study of hypercoagulable patients who underwent free-flap transfer Nelson et al. reported the use of an anticoagulation protocol that included an intravenous bolus of 2000 U of unfractionated heparin prior to microvascular pedicle anastomosis, followed by heparin infusion at 500 U/hour, which was postoperatively increased to therapeutic levels [22]. The authors reported that while the regimen was effective for preventing thrombosis, the hematoma rates were unacceptably high. In the present study all hypercoagulable patients received intraoperative topical irrigation of the donor and recipient vessels with heparinized saline solution, followed by postoperative oral aspirin 40 mg and intravenous dextran-40 30 g once daily for 5 days. No patient had side effects such as pulmonary edema, gastritis, or allergic reactions.
A hypercoagulable state is estimated to exist in 5%-10% of the general population, but it may be unrecognized by common preoperative laboratory tests. In Wang et al.’s review of 2032 free-flap surgeries performed at the University of Pennsylvania, 58 were identified as hypercoagulable. Thrombosis occurred in 12 of these cases—a perioperative thrombosis rate of 20.7%. They concluded that free-flap transfer in the hypercoagulable patient was feasible but was associated with a much higher risk of thrombosis.
In our institution, all patients were monitored immediately after the reconstruction surgery. Trained nurses examined the patients for flap color and temperature every hour for the first 72h and every 2h for the next 48h. Once flap crisis was confirmed, the exploratory surgery was performed immediately. Patients who underwent exploratory surgery were administered postoperative anticoagulation with subcutaneous injection of 4100 U LMWH once daily for 5 days. In our study, three patients with hypercoagulability had venous anastomotic thromboses postoperatively; however, the free-flap success rate was 95.6% (43/45 patients). There were no significant differences in thrombosis and flap success rates between hypercoagulable and normal patients. Thus, a hypercoagulable state does not appear to increase the risk of thrombosis or flap failure in head and neck reconstruction.
An interesting finding in this study was the change in platelet count and APTT of hypercoagulable patients during the perioperative period. The mechanism of this phenomenon is not entirely clear, but it may be associated with the activation of some self-regulatory mechanism or it may be the effect of intravenous fluid administration. In a previous study we have found a similar pattern in patients with normal coagulation status [23]. We believe that the significance of this change needs further study. We did not analyze the changes in PT because abnormal PT was seen in only three patients in this study.
Conclusion
In the present study the thrombosis rate and free-flap rate were comparable in hypercoagulable patients and normal patients. Thus, we conclude that hypercoagulability does not affect outcomes of free-flap transfer in the head and neck region if standard intraoperative and postoperative anticoagulation protocols are followed.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee for Human Experiments at the Peking University School and Hospital of Stomatology (PKUSSIRB-201631114).
Consent for publication
The need for informed consent was waived in view of the retrospective nature of the study.
Availability of data and material
According to the regulations of our institution for protecting patient privacy, we have present part of our datasets in additional supporting files. We will provide rest of datasets if necessary.
Competing interests
All the authors declare that they have no competing interests.
Funding
Not applicable.
Authors’ contributions
Wei Zhou is the first author of the manuscript and in charge of study conception and design, acquisition of data and drafting the manuscript. Wen-Bo Zhang, Yao Yu, Yang Wang, Chi-Mao, Chuan-Bin Guo and Guang-Yan Yu performed the operation. Xin Peng is the corresponding author and substantially contributed to the revision of the manuscript.
Acknowledgements
Not applicable.
Chien W, Varvares MA, Hadlock T, Cheney M, Deschler DG (2005) Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope 115: 973-976. [ Ref ]
Eckardt A, Fokas K (2003) Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg 31: 197-201. [ Ref ]
Kruse AL, Luebbers HT, Grätz KW, Obwegeser JA (2010) Factors influencing survival of free-flap in reconstruction for cancer of the head and neck: a literature review. Microsurgery 30: 242-248. [ Ref ]
Lueg EA (2004) Comparing microvascular outcomes at a large integrated health maintenance organization with flagship centers in the United State. Arch Otolaryngol Head Neck Surg 130: 779-785. [ Ref ]
Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, et al. (2007) Free flap reexploration: Indications, treatment and outcomes in 1193 free flaps. Plast Reconstr Surg 119: 2092-2100. [ Ref ]
Yii NW, Evans GR, Miller MJ, Reece GP, Langstein H, et al. (2001) Thrombolytic therapy: what is its role in free flap salvage? Ann Plast Surg 46: 601-604. [ Ref ]
Mingozzi F, Legnani C, Lunghi B, Scanavini D, Castoldi E, et al. (2003) A FV multiallelic marker detects genetic components of APC resistance contributing to venous thromboembolism in FV Leiden carriers. Thromb Haemost 89: 983-989. [ Ref ]
Olsson E, Svartling N, Asko-Seljavaara S, Lassila R (2001) Activation of coagulation and fibrinolysis during reconstructive surgery in patients with cancer. Microsurgery 21: 208-213. [ Ref ]
Davison SP, Kessler CM, Al-Attar A (2009) Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg 124: 490-495. [ Ref ]
Höijer P, Olsson E (2006) Elevated coagulation factor VIII, postoperative thrombosis and flap failure in late breast reconstruction with a free TRAM flap: a case report. J Plast Reconstr Aesthet Surg 59: 102-104. [ Ref ]
Komuro Y, Sekiguchi J, Nomura S, Ohmori K, Takasugi Y, et al. (1998) Blood coagulation activity during microsurgery. Ann Plast Surg 40: 53-58. [ Ref ]
Olsson E, Svartling N, Asko-Seljavaara S, Lassila R (2001) Activation of coagulation and fibrinolysis in microsurgical reconstructions in the lower extremities. Br J Plast Surg 54: 597-603. [ Ref ]
Olsson E, Svartling N, Asko-Seljavaara S, Lassila R (2001) Activation of coagulation and fibrinolysis during reconstructive microsurgery in patients with cancer. Microsurgery 21: 208-213. [ Ref ]
Wang TY, Serletti JM, Cuker A, McGrath J, Low DW, et al. (2012) Free Tissue Transfer in the Hypercoagulable Patient: A Review of 58 Flaps Plast. Reconstr. Surg 129: 443-453. [ Ref ]
Kloeters O, Vasilic D, Hupkens P, Ulrich D (2017) Markers of blood coagulation and fibrinolysis in patients with early and delayed microsurgical reconstructions in the lower extremities. J Plast Surg Hand Surg 51: 420-426. [ Ref ]
Conrad MH, Adams WP Jr. (2001) Pharmacologic optimization of microsurgery in the new millennium. Plast Reconstr Surg 108: 2088- 2096. [ Ref ]
Dassonville O, Poissonnet G, Chamorey E, Vallicioni J, Demard F, et al. (2008) Head and neck reconstruction with free flaps: a report on 213 cases. Eur Arch Otorhinolaryngol 265: 85-95. [ Ref ]
Pohlenz P, Blessmann M, Heiland M, Blake F, Schmelzle R, et al. (2007) Postoperative complications in 202 cases of microvascular head and neck reconstruction. J Craniomaxillofac Surg 35: 311-315. [ Ref ]
Chernichenko N, Ross DA, Shin J, Chow JY, Sasaki CT, et al. (2008) Arterial coupling for microvascular free tissue transfer. Otolaryngol Head Neck Surg 138: 614-618. [ Ref ]
Fukuiwa T, Nishimoto K, Hayashi T, Kurono Y (2008) Venous thrombosis after microvascular free-tissue transfer in head and neck cancer reconstruction. AurisNasus Larynx 35: 390-396. [ Ref ]
Pannucci CJ, Kovach SJ, Cuker A (2015) Microsurgery and the Hypercoagulable State: A Hematologist’s Perspective. Plast Reconstr Surg 136: 545e-552e. [ Ref ]
Nelson JA, Chung CU, Bauder AR, Wu LC (2017) Prevention of thrombosis in hypercoagulable patients undergoing microsurgery: A novel anticoagulation protocol. J Plast Reconstr Aesthet Surg 70: 307- 312. [ Ref ]
Zhou W, Zhang WB, Yu Y, Wang Y, Mao C, et al. (2019) Are antithrombotic agents necessary for head and neck microvascular surgery? Int J Oral Maxilloffac Surg 48: 869-874. [ Ref ]