Journal Name: Journal of Biomedical Research and Reviews
Article Type: Research
Received date: 10-August-2020
Accepted date: 14-October-2020
Published date: 21-October-2020
Citation: Ujvary I, Lopata A (2020) Phenytoinlike Antiepileptic Effect of Cannabidiol and Related Phytocannabinoid Metabolites: Structural Insights from Molecular Modeling. J Biomed Res Rev Vol: 3, Issu: 2 (32- 40).
Copyright: © 2020 Ujvary I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cannabidiol (CBD), a non-psychotropic phenolic terpene constituent of Cannabis sativa, was approved in 2018 by FDA in the USA (Epidiolex®) and, in 2019, by EMA in the European Union (Epidyolex®) as an adjunct for the treatment of certain forms of pediatric epilepsy. The mechanism(s) by which CBD exerts its antiepileptic effects is not known. There is also a paucity of data on the fate and biological activity of human metabolites of CBD. However, similarities have been noted between the anticonvulsant properties of the antiepileptic drug phenytoin (PHT) and CBD. Herein we describe the results of molecular modeling studies comparing the stereoelectronic properties of PHT with those of CBD and its carboxylic acid metabolite (7-COOH-CBD), and of 7-hydroxycannabidivarin. The similar electrostatic potential maps of PHT and the Phase I cannabinoid metabolites suggest analogous hydrogen bonding interactions at a potential common target site which involve the hydantoin moiety of PHT on the one hand and the polar groups of the Phase I metabolites on the other. Superposition of PHT and 7-COOH-CBD reveals similarities in the spatial arrangement of their respective polar and hydrophobic moieties. Furthermore, as shown by their perfect overlay, the 1-cyclohexenecarboxylic acid core of 7-COOHCBD mimics Δ2(E)-valproic acid, a non-teratogenic bioactive metabolite of the commonly used antiepileptic valproic acid. It is proposed that C–7 oxidized phytocannabinoid metabolites are involved in the observed phenytoin-like anticonvulsant effects of the parent phytocannabinoid drugs.
Graphic abstract
Keywords
Antiepileptic, Cannabidiol, Cannabidivarin, Hydantoin, Molecular modeling, Oxidative, metabolites, Phenytoin.
Abstract
Cannabidiol (CBD), a non-psychotropic phenolic terpene constituent of Cannabis sativa, was approved in 2018 by FDA in the USA (Epidiolex®) and, in 2019, by EMA in the European Union (Epidyolex®) as an adjunct for the treatment of certain forms of pediatric epilepsy. The mechanism(s) by which CBD exerts its antiepileptic effects is not known. There is also a paucity of data on the fate and biological activity of human metabolites of CBD. However, similarities have been noted between the anticonvulsant properties of the antiepileptic drug phenytoin (PHT) and CBD. Herein we describe the results of molecular modeling studies comparing the stereoelectronic properties of PHT with those of CBD and its carboxylic acid metabolite (7-COOH-CBD), and of 7-hydroxycannabidivarin. The similar electrostatic potential maps of PHT and the Phase I cannabinoid metabolites suggest analogous hydrogen bonding interactions at a potential common target site which involve the hydantoin moiety of PHT on the one hand and the polar groups of the Phase I metabolites on the other. Superposition of PHT and 7-COOH-CBD reveals similarities in the spatial arrangement of their respective polar and hydrophobic moieties. Furthermore, as shown by their perfect overlay, the 1-cyclohexenecarboxylic acid core of 7-COOHCBD mimics Δ2(E)-valproic acid, a non-teratogenic bioactive metabolite of the commonly used antiepileptic valproic acid. It is proposed that C–7 oxidized phytocannabinoid metabolites are involved in the observed phenytoin-like anticonvulsant effects of the parent phytocannabinoid drugs.
Graphic abstract
Keywords
Antiepileptic, Cannabidiol, Cannabidivarin, Hydantoin, Molecular modeling, Oxidative, metabolites, Phenytoin.
Introduction
Epilepsy is one of the world’s oldest recognized diseases. It is a life-shortening, chronic neurological disorder globally affecting around 50 million people, of which over 8 million are below the age of 19 years [1,2]. Epilepsy is characterized by partial or generalized spontaneous seizures which sometimes are accompanied by loss of consciousness, and control of bowel and bladder functions. Seizure episodes are repetitive and are a result of abnormal neuronal discharges in a group of brain cells.
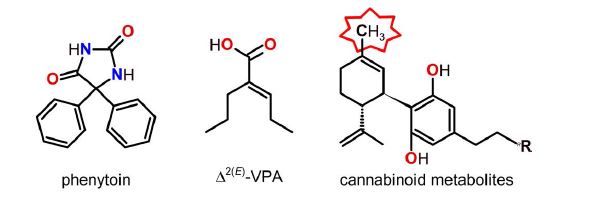
Since the landmark discovery by Merritt and Putnam of the anticonvulsant effect of phenytoin (PHT; Figure 1) over 80 years ago [3], more than two dozen of clinically approved antiepileptic drugs (AEDs) have become available and several others are under development yet definitive treatment for epilepsy is still lacking. The currently used AEDs are known to act at one or more targets, which include voltage- and ligand-gated ion channels, GABA or glutamate transporters or receptors, and certain enzymes such as carbonic anhydrase [4–8]. While epileptic seizures can often be treated or prevented satisfactorily – and often affordably – by an appropriately selected AED alone or in combination, a significant number of patients are resistant to current pharmacotherapies; side effects from AEDs are also common. This is the case for valproic acid (VPA; Figure 1) which is a frequently prescribed, broad-spectrum AED but may cause serious side effects including hepatotoxicity and teratogenicity; one of its bioactive metabolites, Δ2(E)-valproic acid (Δ2(E)-VPA; Figure 1), however, appears to be safer [9– 11]. Therefore, there is an urgent need for the development of new AEDs which are not only effective but also devoid of side effects especially upon chronic treatment.
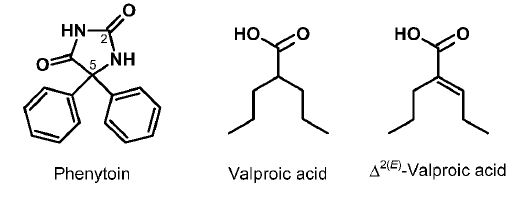
One of the promising new antiepileptic drugs is cannabidiol (CBD, Figure 2A), a non-intoxicating constituent of Cannabis sativa L. CBD was first isolated in 1940 [12,13], and its structure determined in 1963 [14]. Being relatively safe CBD offers a wide range of therapeutic applications [15– 19]. CBD (Epidiolex®) was approved by the US FDA in June 2018 and, as Epidyolex®, by the European Medicine Agency in September 2019 to treat seizures associated with Lennox- Gastaut syndrome and Dravet syndrome, in conjunction with the antiepileptic clobazam [20,21]. In August 2020, Epidiolex received authorization also for the treatment of seizures associated with tuberous sclerosis complex in children [20]. Recently, another phytocannabinoid, cannabidivarin (CBDV; Figure 2A), the n-propyl homologue of CBD, has also been investigated in clinical trials [6,22].
Figure 1: Structures of antiepileptic drugs discussed. Δ2(E)-Valproic acid is a bioactive metabolite of valproic acid.
Both Epidiolex and Epidyolex are prescription medicines used as oral solutions in daily doses up to 20 mg/kg. However, due to regulatory ambiguities in many countries various artisanal cannabis preparations, such as ‘CBD oils’, of mostly unknown quality and with unproven medical claims are being openly marketed [23,24].
The exact mechanism of anticonvulsant action of CBD is not known [25,26]. Prompted by the pharmacological and certain structural similarities between PHT, Δ2(E)-VPA, and primary oxidative metabolites of CBD and CBDV, we have compared by molecular modeling key stereoelectronic features of these substances. In this communication we survey relevant literature and report the results of our studies.
Phytocannabinoids as anticonvulsant agents
Preparations of various parts of hemp, C. sativa, have been used for religious, medicinal and recreational purposes for centuries. The first methodical studies on the therapeutic properties, including anti-seizure effects, of hemp preparations, in the form of tinctures, were carried out by O’Shaughnessy in the late 1830s [27]. By now, over 140 cannabinoids have been isolated from C. sativa [28]. Until recently, research has mainly focused on Δ9-tetrahydrocannabinol (THC; Figure 2A), the main psychoactive principle of cannabis [29,30]. However, THC appears to have paradoxical pro- and anticonvulsant effects which, along with its psychotropic properties, render this phytocannabinoid unsuitable as an AED [31–34]. Interestingly, 11-hydroxy-Δ9-THC, which is a short-lived psychotropic Phase I metabolite of THC, was more active as an anticonvulsant in mice with an earlier peak-effect than the parent drug suggesting its involvement in the anticonvulsant action of THC [35].
While the endocannabinoid system is known to be involved in the regulation of neuronal network excitability [36], the multimodal anticonvulsant effects of CBD are not directly mediated by cannabinoid receptors. In preclinical studies CBD has been shown to reduce neuronal hyperexcitability through modulation of voltage- or ligandgated ion channels, by blocking orphan GPR55 receptor, the transient receptor potential vanilloid 1 channel, by inhibiting certain enzymes, as well as through modulation of adenosine mediated signalling, cytokine expression and TNFα release, and phosphatidylinositol 3-kinase signaling pathways [15,25,26,37,38]. Nevertheless, the precise mechanisms of action(s) of CBD are yet to be elucidated. In several animal models of epilepsy, the anticonvulsant properties of PHT and CBD have shown similarities in many, though not all, respects [15]. However, while both PHT and VPA affect voltage-gated sodium ion channels, the action of CBD at this latter target is equivocal [39–43].
Many drugs used in therapy are metabolically converted into active metabolites and interindividual variations in the formation and fate of such active metabolites may cause variability in the response to treatment of different individuals [44,45]. Information on the human metabolism of CBD is limited [46–48] and data on its pharmacokinetics have only been recently emerging [49–58]. CBD appears to have poor bioavailability: in general, therapeutic efficacy could only be reached with relatively high daily doses. A large portion of administered CBD is excreted intact or as its glucuronide. Phase I metabolism of CBD involves CYP450 isoenzymes: sequential oxidation of CBD at the 7-C atom affords 7-hydroxy-CBD (7-OH-CBD) and 7-nor-1- carboxy-CBD (7-COOH-CBD) (Figure 2B) as primary human metabolites. A recent study with adults given a single oral dose of 1500 mg CBD reported mean total (free + protein-bound) peak blood plasma concentrations of 292, 239 and 3060 ng/mL for CBD, 7-OH-CBD and 7-COOH-CBD, respectively [49]. Additional, though less explored, biotransformations include oxidations at other single and/or multiple sites and truncation of the n-pentyl side chain and afford >30 oxidative metabolites [46–48]. Little attention has been paid to the biological activity of CBD metabolites [48]. Anticonvulsant effects of 7-OH-CBD and 7-hydroxycannabidivarin (7-OHCBDV; Figure 2B) against pentylenetetrazole-induced acute seizures in mice were claimed in a recent patent [59], while 7-OH-CBD was reported to be more effective than CBD or 7-COOH-CBD in preventing MES-induced generalized seizures in the mouse [60].
The paucity of data on the anticonvulsant activity of CBD metabolites prompted us to examine in silico the structural similarities of some currently used AEDs and the two primary metabolites of CBD as well as of the related Phytocannabinoid 7-OH-CBDV.
Methods
Molecular modeling calculations were performed on laptop computers. Initial structural conformations of PHT, Δ2(E)-VPA, and (–)-(R,R)-CBD (the natural product), were manually built relying on data from earlier crystallographic and NMR experiments and/or molecular modeling studies.
Figure 2: Structures of phytocannabinoids (A) and some of their main metabolites (B).
Electrostatic potential maps (EPMs) projected onto the electron density surface of the energy minimized molecules were calculated by ωB97X-D/6-31G* density functional method using the C-PCM continuum solvation model (water) by Spartan’16 software (Version 2.0.7, Wavefunction, Inc., Irvine, CA, USA). The surfaces were color-coded according to the potential with electron rich regions coloured red and electron poor regions coloured blue.
Superpositions were done using Discovery Studio Visualizer 4.1 (Accelrys/BIOVIA, San Diego, CA, USA) software. Once again, the molecules were manually built, their geometry optimized (CHARMm); alignments were done either by balanced (50:50) steric + electrostatic fields overlay or by three to five selected atom pair tethers according to command options in the Structure/Superimpose/Molecular Overlay menu of the software.
For further details of methodology and additional references on previous structural and/or molecular modeling studies with PHT and VPA and their analogues as well as with CBD, see Supplementary material.
Results and Discussion
Despite their apparent structural differences, certain similarity between the stereoelectronic properties of CBD and PHT has been noted [61]: each drug contains two similarly oriented hydrophobic rings and, according to this proposal, two similarly positioned electron-donating functionalities, that is two phenolic hydroxyls in CBD and two carbonyl groups of the hydantoin ring in PHT. Phenytoin contains two geminal phenyl rings attached to a hydantoin core, which is capable of both accepting (C=O) and donating (NH) hydrogen bonds at the target site. PHT is semi-rigid and, in fact, is perfectly superimposable with the three-ring system-containing carbamazepine, an AED also targeting the sodium ion channel [62–64].
We have recently suggested that metabolites of CBD may contribute to or even responsible for some of the pharmacological properties of the parent phytocannabinoid in vivo [48]. We now have tested by molecular modeling the hypothesis that C–7-oxidized metabolites of CBD are involved in its observed phenytoin-like effects. This hypothesis has been inspired by the reported bioisosterism [65–67] of the hydantoin ring, present in PHT, and the carboxylic acid functionality, present in dominant human metabolites of CBD. The early observation in cats that diphenylacetic acid possessed anticonvulsant activity similar to PHT [68] also indicates that carboxyl groups could function as hydantoin equivalents. Herein we report the results of molecular modeling studies comparing the electrostatic and conformational features of CBD and main phytocannabinoid metabolites with those of PHT. We also demonstrate the recently noted [48] structural similarity of the branched 2-alkenecarboxylic acid Δ2(E)-VPA (Figure 1) and the 1-cyclohexenecarboxylic acid moiety of 7-COOHCBD.
There have been several computational approaches to understand the mechanism of antiepileptic action of PHT and VPA and their analogues but none for CBD (see, however, [43]). For PHT, CoMFA studies have revealed that one of the hydantoin-attached phenyl rings can be replaced by alkyl groups with a 6–7 carbon chain length being optimal for binding to voltage-gated sodium ion channels in vitro and for anticonvulsant activity in a mouse model of epilepsy [69,70]. For example, replacement of one of the phenyl groups by a cyclohexyl ring provided a sodium ion channel blocker with respectable activity: IC50 = 58 ΔM for the racemic cyclohexyl analogue versus IC50 = 40 μM for phenytoin [69]. Relevant bioactivity data for the known [71] 5-cyclohexen-1-yl PHT analogue, which may be considered a simplified CBD-type compound, are lacking. Interestingly, 5-(o-hydroxyphenyl) hydantoin PHT analogues were reported to have anticonvulsant activity in the mouse [72]. Pharmacophore studies on the flexible VPA and its amides acting as PHT-like anticonvulsants though scarce established the importance of electrostatic interactions involving their polar headgroup and the requirement of at least one lipophilic moiety attached to it [62,63,73].
We have hypothesized that the 7-OH- or 7-COOH moieties of primary CBD metabolites mimic the hydantoin ring of PHT. Though the electrostatic properties of these functionalities differ in terms of acidity, there is similarity in terms of hydrogen bonding ability: both the PHT-hydantoin moiety (pKa = 8.31 [74,75]), either as a lactam or as its lactim tautomer [76], and the carboxylic acid of the CBD metabolite are capable of forming multiple hydrogen bonds. The electrostatic similarity of Δ2(E)-VPA and of the polar head group of 7-COOH-CBD is obvious. The pKa values for VPA and its α,β-unsaturated metabolite are 4.95 and 4.36, respectively [77]; relevant data are not available for the phytocannabinoid metabolites, however, the unsubstituted α,β-unsaturated cyclohexenecarboxylic acid, present as a core fragment in 7-COOH-CBD, has a pKa of 3.88 [78]. The phenolic groups of the resorcinol moiety as well as the nonacidic 7-hydroxylated side chain of the metabolites may also participate in hydrogen bonding interactions.
To shed light on the structural relatedness of PHT and the cannabinoids, various alignments have been explored in silico. (A review on unrelated molecular modeling studies on the interaction of CBD and various other pharmacological protein targets has recently been published [79]; see also [43].) Based on the known pharmacological and the observed spatial similarities between PHT and CBD on one hand, and on the reported hydantoin–carboxylic acid bioisosterism on the other, we compared by molecular modeling the stereoelectronic properties of PHT, CBD and related cannabinoid metabolites. We note that the energyminimized structures of the molecules could not be perfectly overlaid but the relatively low rotational barrier about the respective C(sp2)–C(sp3) pivot bonds, which connect the two phenyl groups and the hydantoin ring of PHT [64,80,81], and the resorcinol and cyclohexenyl moieties of the cannabinoids [61,82,83], render the conformation of each drug relatively flexible.
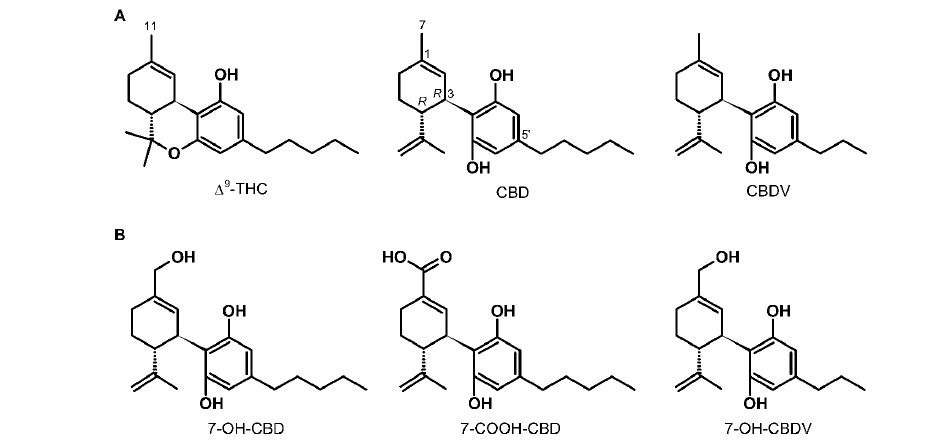
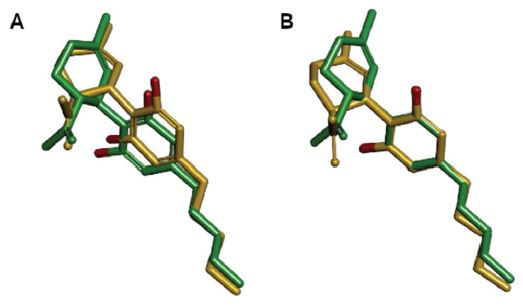
First, we examined the spatial relation of PHT and CBD as proposed earlier [61]. The original study pointed out the presence of two freely rotatable hydrophobic rings in both PHT and CBD as well as the “similar orientations and positions of the[ir] two electron-rich groups”; no superposition was, however, attempted. In our hand, examining various overlays of the two drugs according to this early proposal satisfactory alignment was obtained only by tethering: (1) the two carbonyl oxygens of PHT to the two resorcinol oxygens of CBD, and (2) the hydantoin C-5 atom of PHT to the cyclohexenyl C-3 atom of CBD; this alignment positioned both phenyl rings of PHT over the limonenyl moiety of CBD (Figure 3A). However, as seen in figure 3B, much better overall alignment was obtained by tethering the respective carbonyl groups of the isosteric hydantoin and carboxylic moieties of PHT and 7-COOH-CBD instead of the above hydantoin–resorcinol tethering. For comparisons of the electrostatic potential surfaces of PHT and selected cannabinoid metabolites, this latter alignment was used.
Figure 3: Alternative superpositions of PHT (gold carbon atoms) and CBD (green carbon atoms) by tethering the hydantoin C-5 atom and the cyclohexenyl C-3 atom, and (A) by tethering the two carbonyl and resorcinol oxygens of PHT and CBD, respectively, according to Ref. 61; or (B) by tethering the C-2 carbonyl group of PHT and the carbonyl group of 7-COOHCBD. Hydrogens are shown on heteroatoms only. Two views (left and right) are rotated by 90° about the vertical axis.
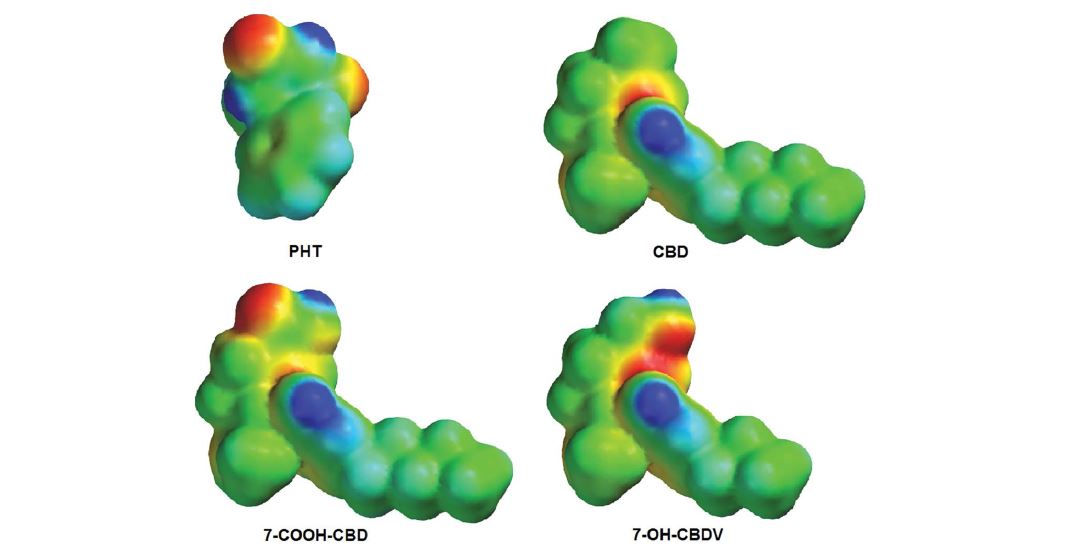
Figure 4 shows the EPMs projected onto the electron density surface of PHT, CBD, 7-COOH-CBD and 7-OHCBDV. As seen, the EPMs of PHT and CBD show significant differences. For the weak acid PHT, the hydantoin ring, which would correspond to the branched allylic system bearing the 7-CH3 of CBD, is able to participate in multiple hydrogen bond interactions: the exposed C=O groups, as the most electron-rich regions (red), may act as hydrogen bond acceptors while the amide/imide hydrogen atoms (blue) are available as hydrogen bond donors. In CBD, the area around the 7-CH3 group bears essentially ‘zero potential’ (green); the lone pairs of electrons (electron rich regions; red) of the resorcinol oxygens point toward limonenyl moiety of CBD and appear to be buried between the two rings thus likely inaccessible for hydrogen bonding as acceptors; consequently, in the minimum-energy conformation the two phenolic hydrogens (blue) point away from the limonenyl moiety and may act as hydrogen bond donors only. Compared to the sterically more compact PHT, the hydrophobic pentyl side chain of CBD could occupy an additional hydrophobic cavity of the target site and may facilitate membrane penetration also. However, the remarkable similarities in the EPMs of the polar functionalities of PHT and 7-COOHCBD are readily recognizable: the hydantoin system and the carboxylic acid moiety show similar electron distribution (compare the respective red and blue regions in figure 4) which supports our hypothesis on the hydantoin–carboxylic acid bioisosterism for this pair of compounds. Likewise, the EPMs of PHT and 7-OH-CBDV also show a certain degree of similarity around the polar functionalities (Figure 4); the 7-OH group of the cannabinoid metabolite can be involved in hydrogen bonding interactions though not as extensively as a carboxylic acid species.
Insufficient structure-activity relationship (SAR) data preclude the formulation of a rigorous pharmacophore model for the anticonvulsant cannabinoids. Notwithstanding, the present study have revealed steric and electrostatic similarities between the cannabinoid metabolites and PHT which suggest a common pharmacophore orientation shown in figure 3B. Accordingly: (1) similar to the hydantoin ring in PHT, the exposed 7-OH or 7-COOH polar headgroups of the cannabinoid metabolites function as hydrogen bond donors and/or acceptors; and (2) similar to the spatial arrangement of the two phenyl rings in PHT, the positioning of the limonenyl and the substituted-aryl groups of the cannabinoids facilitate hydrophobic interactions, which are likely strengthened by hydrogen bonds involving the phenolic hydroxyl(s) oriented to the target site. The importance of at least one free phenolic group in the antiseizure effect of CBD or metabolites cannot be neglected: upon sequential O-methylation of the resorcinol moiety the activity gradually diminishes [84].
Although not related to anticonvulsant effects, it is interesting to note that 5,5-diphenyl( thio)hydantoins [85,86] alkylated at the imidic nitrogen atom have been found to be CB1 receptor ligands, typically antagonists; no data for N-unsubstituted hydantoins, including PHT, have been reported.
We have also examined the similarity of 7-COOHCBD and the anticonvulsant drug metabolite Δ2(E)-VPA by superimposing their minimum-energy structures (Figure 5). Not surprisingly, there is an excellent overlap between the respective α,β-unsaturated alkenoic and cycloalkenoic acid fragments of Δ2(E)-VPA and 7-COOH-CBD. As noted earlier, diphenylacetic acid, which is an aromatic analogue of VPA and Δ2(E)-VPA as well as a carboxylic acid bioisostere of PHT, was shown to be anticonvulsant in cats decades ago [68]. It is of note, that a molecular model-based pharmacophore for the anticonvulsant potency and sodium ion channel binding efficiency has been proposed for PHT, carbamazepine, as well as for valproic acid and its amide derivatives [62,63].
Figure 4: Electrostatic potential maps of PHT, CBD, 7-COOH-CBD and 7-OH-CBDV in their minimum energy conformations.
Finally, the lack of stereoselectivity observed in mice for the anticonvulsant effect of natural (–)-(R,R)-CBD and its synthetic (+)-(S,S)-enantiomer [87–89] might be explained by a remarkably close overlap of the two enantiomeric molecules when superimposed (Figure 6). Reasonably, the cyclohexene ring assumes a half-chair conformation placing the bulky resorcinol moiety into the energetically favourable pseudo-equatorial position in both enantiomers, and this results in their similar overall shape. Oxidative metabolites of CBD enantiomers may also attain similar shapes, which are further tuned by electrostatic interactions with the target site involved in their anticonvulsant effects. While the effects of the trans-CBD enantiomers and their 7-OH and 7-COOH metabolites on various components of the endocannabinoid system and other targets have been studied [90–94], there are no pharmacological data on cis-CBD, that is the (R,S)- or (S,R)-CBD isomers [95–97]. The impact of chirality on sodium ion channel pore binding of PHT-related 5-(cyclo) alkyl-5-phenylhydantoins has been discussed [64].
Figure 5: Superposition 7-COOH-CBD (green carbon atoms) and Δ2(E)-VPA (gold carbon atoms).
Figure 6: Superpositions of (R,R)-CBD (green carbon atoms) and (S,S)-CBD (gold carbon atoms) based either on balanced steric + electrostatic properties (A), or by tethering the respective C-1’, C-3’ and C-5’ resorcinol atoms of the enantiomers (B). Hydrogen atoms are omitted.
Conclusion
Multiple lines of evidence indicate similarities between the anticonvulsant effects of PHT – and to some extent VPA – and CBD suggesting common mechanism of action. The molecular modeling studies presented here have revealed stereoelectronic similarities between PHT and the C–7- oxidized metabolites of CBD and CBDV. The lack of information on the precise molecular targets and on the structural requirements responsible for the anticonvulsant activity of CBD precludes a comparative SAR study of established AEDs and of CBD or its metabolites thus prevents the formulation of a valid pharmacophore model. Our hypothesis can be tested, for example, by applying the information obtained during the plethora of SAR studies of PHT and related AEDs to CBDtype substances. It would also be of interest to synthesize and test phenytoin–CBD hybrid molecules. Importantly, however, further pharmacological studies in vitro with 7-OH and 7-COOH metabolites of CBD and CBDV are required to establish the exact mechanism(s) responsible for their biological activity in general and their involvement in the anticonvulsant effect of cannabis-containing preparations in particular. In addition, the potential involvement of CBD metabolites in the anticonvulsant effect of the parent drug necessitates further studies on drug-drug interactions, in particular with medicines and xenobiotics which induce the formation of the primary oxidative metabolites discussed in this publication.
Funding and Author Disclosure Statement
No specific financial support was received from funding agencies. There is no competing financial interest.
The Global Burden of Disease Child and Adolescent Health Collaboration (2017) Child and adolescent health from 1990 to 2015. Findings from the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr171: 573-592. [ Ref ]
World Health Organization (2020) WHO Epilepsy Fact Sheet 2019. [ Ref ]
Merritt HH, Putnam TJ (1938) Sodium diphenyl hydantoinate in the treatment of convulsive disorders. JAMA 111: 1068-1073. [ Ref ]
Bialer M, White HS (2010) Key factors in the discovery and development of new antiepileptic drugs. Nature Rev Drug Discov 9: 68-82. [ Ref ]
Bialer M (2012) Chemical properties of antiepileptic drugs. Adv Drug Deliv Rev 64: 887-895. [ Ref ]
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, et al. (2017) Progress report on new antiepileptic drugs: A summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia 58: 181-221. [ Ref ]
Löscher W, Potschka H, Sisodiya SM, Vezzani A (2020) Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharm Rev 72: 606-638. [ Ref ]
Wolters K, St. Louis, MO (2017) Drug Facts and Comparisons 2017. Lippincott Williams and Wilkinspp, Philadelphia, United States. [ Ref ]
- Düsing RH (1992) Single-dose tolerance and pharmacokinetics of 2-n-propyl-2(E)-pentenoate (Δ2(E)-valproate) in healthy male volunteers. Pharm Weekbl Sci 14: 152-158.
Elmazar MMA, Hauck R-S, Nau H (1993) Anticonvulsant and neurotoxic activities of twelve analogues of valproic acid. J Pharm Sci 82: 1255-1258. [ Ref ]
Löscher W (2002) Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 16: 669- 694. [ Ref ]
Adams R, Pease DC, Clark JH (1940) Isolation of cannabinol, cannabidiol and quebrachitol from red oil of Minnesota wild hemp. J Am Chem Soc 62: 2194-2196. [ Ref ]
Jacob A, Todd AR (1940) Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J Chem Soc 649-653. [ Ref ]
Mechoulam R, Shvo Y, (1963) Hashish–I. The structure of cannabidiol. Tetrahedron 19: 2073-2078. [ Ref ]
Pertwee RG (2004) The pharmacology and therapeutic potential of cannabidiol. Cannabinoids. Landes Bioscience, Georgetown, Texas. [ Ref ]
Ibeas Bih C, Chen T, Nunn AVW, Bazelot M, Dallas M, et al. (2015) Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12: 699-730. [ Ref ]
Iffland K, Grotenhermen F (2017) An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2: 139-154. [ Ref ]
Morales P, Reggio PH, Jagerovic N (2017) An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front Pharmacol 8: 422. [ Ref ]
Arzimanoglou A, Brandl U, Cross JH, Gil-Nagel A, Members of The Cannabinoids International Experts Panel (2020) Epilepsy and cannabidiol: a guide to treatment. Epileptic Disord 2: 1-14. [ Ref ]
United States Federal Drug Administration (2020) Drug Approval Package: Epidiolex (Cannabidiol). [ Ref ]
European Medicines Agency. (2020) Epidyolex. Cannabidiol. [ Ref ]
GW Pharmaceuticals Announces Preliminary Results of Phase 2a Study for its Pipeline Compound GWP42006. [ Ref ]
White CM (2019) A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharmacol 59: 923-934. [ Ref ]
Cross JH, Cock H (2020) A perspective on cannabinoids for treating epilepsy: Do they really change the landscape? Neuropharmacology 170: 107861. [ Ref ]
Rocha L, Frías-Soria CL, Ortiz JG, Auzmendi J, Lazarowski A (2020) Is cannabidiol a drug acting on unconventional targets to control drugresistant epilepsy? Epilepsia Open 5: 36-49. [ Ref ]
Gray RA, Whalley BJ (2020) The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord 22 (Suppl 1): S10-S15. [ Ref ]
O’Shaughnessy WB (1843) On the preparations of the Indian hemp, or gunjah, (Cannabis Indica). Their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci 5: 363-369. [ Ref ]
- Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G (2016) Phytocannabinoids: a unified critical inventory. Nat Prod Rep 33: 1357-1392.
Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646-1647. [ Ref ]
Mechoulam R, Shani A, Edery H, Grunfeld Y (1970) Chemical basis of hashish activity. Science 169: 611-612. [ Ref ]
Williams CM, Jones NA, Whalley BJ (2014) Cannabis and epilepsy. Handbook of Cannabis. Oxford University Press: Oxford, pp. 547-563. [ Ref ]
Holtkamp M, Hamerle M (2017) Cannabis use in epilepsy—risks and benefits. Handbook of Cannabis and Related Pathologies. Academic Press: London, pp. 431-438. [ Ref ]
Stockings E, Zagic D, Campbell G, Weier M, Hall WD, et al. (2018) Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry 89: 741-753. [ Ref ]
Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, et al. (2020) Cannabisbased products for pediatric epilepsy: An updated systematic review. Seizure 75: 18-22. [ Ref ]
Karler R, Cely W, Turkanis SA (1974) Anticonvulsant properties of Δ9- tetrahydrocannabinol and other cannabinoids. Life Sci 15: 931-947. [ Ref ]
Katona I (2015) Cannabis and endocannabinoid signaling in epilepsy. Handb Exp Pharmacol 231: 285-316. [ Ref ]
Gray RA, Stott CG, Jones NA, Di Marzo V, Whalley BJ (2020) Anticonvulsive properties of cannabidiol in a model of generalized seizure are transient receptor potential vanilloid 1 dependent. Cannabis Cannabinoid Res 5: 145-149. [ Ref ]
Vieira de Assis Lima I, Quaglio Bellozi PM, Batista EM, Vilela LR, Brandão IL, et al. (2020) Cannabidiol anticonvulsant effect is partially mediated by the PI3Kγ pathway. Neuropharmacology 176: 108156. [ Ref ]
Ghovanloo M-R, Shuart NG, Mezeyova J, Dean RA, Ruben PC, et al. (2018) Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem 293: 16546-16558. [ Ref ]
Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, et al. (2014) Voltagegated sodium (Nav) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett 566: 269-274. [ Ref ]
Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N, Cummins TR (2016) Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain 139: 2164-2181. [ Ref ]
Mason ER, Cummins TR (2020) Differential inhibition of human NaV1.2 resurgent and persistent sodium currents by cannabidiol and GS967. Int J Mol Sci 21: 2454. [ Ref ]
Goodyer Sait L, Sula A, Hollingworth D, Whalley BJ, Rana RR, et al. (2020) Cannabidiol interactions with voltage-gated sodium channels. bioRxiv. [ Ref ]
Obach RS (2013) Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev 65: 578-640. [ Ref ]
Frédérich M, Pirotte B, Fillet M, de Tullio P (2016) Metabolomics as a challenging approach for medicinal chemistry and personalized medicine. J Med Chem 59: 8649-8666. [ Ref ]
Harvey DJ, Mechoulam R (1990) Metabolites of cannabidiol identified in human urine. Xenobiotica 20: 303-320. [ Ref ]
- Harvey DJ, Samara E, Mechoulam R (1991) Urinary metabolites of cannabidiol in dog, rat and man and their identification by gas chromatography–mass spectrometry. J Chromatogr 562: 299-322.
Ujváry I, Hanuš L (2016) Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res 1: 90-101. [ Ref ]
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G (2018) A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32: 1053-1067. Correction: idem (2019) CNS Drugs 33: 397. [ Ref ]
Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, et al. (2019) Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 60: 1586-1592. [ Ref ]
Wheless JW, Dlugos D, Miller I, Oh DA, on behalf of the INS011-14-029 Study Investigators (2019) Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs 33: 593-604. [ Ref ]
Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, et al. (2018) Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy Behav 88: 162-171. [ Ref ]
Morrison G, Crockett J, Blakey G, Sommerville K (2019) A phase I, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev 8: 1009-1031. [ Ref ]
Spindle TR, Cone EJ, Kuntz D, Mitchell JM, Bigelow GE, et al. (2019) Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD-dominant cannabis. J Anal Toxicol 44: 109-125. [ Ref ]
Tayo B, Taylor L, Shahebkar F, Morrison G (2020) A phase I, openlabel, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet 59: 747-755. [ Ref ]
Perucca E, Bialer M (2020) Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs 34: 795-800. [ Ref ]
Izgelov D, Davidson E, Barasch D, Regev A, Domb AJ, et al. (2020) Pharmacokinetic investigation of synthetic cannabidiol oral formulation in healthy volunteers. Eur J Pharm Biopharm 154: 108-115. [ Ref ]
Wang GS, Bourne DWA, Klawitter J, Sempio C, Chapman K, et al. (2020) Disposition of oral cannabidiol-rich cannabis extracts in children with epilepsy. Clin Pharmacokinet 59: 1005-1012. [ Ref ]
Stott C, Jones N, Whalley B, Stephens G, Williams C (2015) 7-OHCannabidiol (7-OH-CBD) and/or 7-OH-cannabidivarin (7-OH-CBDV) for use in the treatment of epilepsy. [ Ref ]
Whalley BJ, Stott C, Gray RA, Jones NA (2017) The human metabolite of cannabidiol, 7-hydroxy cannabidiol, but not 7-carboxy cannabidiol, is anticonvulsant in the maximal electroshock seizure threshold test (MEST) in mouse. 71st Annual Meeting of the American Epilepsy Society, Washington, DC. [ Ref ]
Tamir I, Mechoulam R, Meyer AY (1980) Cannabidiol and phenytoin: a structural comparison. J Med Chem 23: 220-223. [ Ref ]
Tasso SM, Bruno-Blanch LE, Estiú GL (2001) Pharmacophore model for antiepileptic drugs acting on sodium channels. J Mol Model 7: 231-239. [ Ref ]
Tasso SM, Moon SC, Bruno-Blanch LE, Estiú GL (2004) Characterization of the anticonvulsant profile of valproamide derivatives. Bioorg Med Chem 12: 3857-3869. [ Ref ]
Lipkind GM, Fozzard HA (2010) Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol Pharmacol 78: 631-638. [ Ref ]
- Lipinski CA (1986) Bioisosterism in drug design. Annu Rep Med Chem 21: 283-291.
Lipinski CA, Aldinger CE, Beyer TA, Bordner J, Burdi DF, et al. (1992) Hydantoin bioisosteres. In vivo active spiro hydroxy acetic acid aldose reductase inhibitors. J Med Chem 35: 2169-2177. [ Ref ]
Ujváry I, Hayward J (2012) Bioster: a database of bioisosteres and bioanalogues. Bioisosteres in Medicinal Chemistry. Wiley-VCH, Weinheim, pp. 55-74. [ Ref ]
Merritt HH, Putnam TJ (1945) Experimental determination of anticonvulsive activity of chemical compounds. Epilepsia B3: 51-75. [ Ref ]
Brown ML, Zha CC, Van Dyke CC, Brown GB, Brouillette WJ (1999) Comparative molecular field analysis of hydantoin binding to the neuronal voltage-dependent sodium channel. J Med Chem 42: 1537- 1545. [ Ref ]
Lenkowski PW, Batts TW, Smith MD, Ko SH, Jones PJ, et al. (2007) A pharmacophore derived phenytoin analogue with increased affinity for slow inactivated sodium channels exhibits a desired anticonvulsant profile. Neuropharmacology 52: 1044-1054. [ Ref ]
Oppenheimer JH; Tavernetti RR (1962) Displacement of thyroxine from human thyroxine-binding globulin by analogues of hydantoin. Steric aspects of the thyroxine-binding site. J Clin Invest 41: 2213-2220. [ Ref ]
Nitz R-E, Persch W, Schmidt A (1955) Zur Chemie und antikonvulsiven Wirkung neuer Hydantoinderivate. Arzneimittelforschung 5: 357-364. [ Ref ]
Piplani S, Verma PK, Kumar A (2016) Neuroinformatics analyses reveal GABAt and SSADH as major proteins involved in anticonvulsant activity of valproic acid. Biomed Pharmacother 81: 402-410. [ Ref ]
Agarwal SP, Blake MI (1968) Determination of the pKa’ value for 5,5-diphenylhydantoin. J Pharm Sci 57: 1434-1435. [ Ref ]
Lipinski CA, Fiese EF, Korst RJ (1991) pKa, Log P and MedChem CLOGP fragment values of acidic heterocyclic potential bioisosteres. Quant Struct-Act Relat 10: 109-117. [ Ref ]
Kleinpeter E (1997) The structure of hydantoins in solution and in the solid state. Struct Chem 8: 161-173. [ Ref ]
Abbott FS, Acheampong AA (1988) Quantitative structure– anticonvulsant activity relationships of valproic acid, related carboxylic acids and tetrazoles. Neuropharmacology 27: 287-294. [ Ref ]
Bhat SN, Rao R, Ranganayakulu K (1978) pKa values of protonated α,β- unsaturated cyclic carboxylic acids. Effect of ring size on acidities. Can J Chem 56: 2003-2007. [ Ref ]
Mastinu A, Ribaudo G, Ongaro A, Bonini SA, Memo M, et al. (2020) Critical review on the chemical aspects of cannabidiol (CBD) and harmonization of computational bioactivity data. Curr Med Chem (in print). [ Ref ]
Camerman A, Camerman N (1971) The stereochemical basis of anticonvulsant drug action. I. The crystal and molecular structure of diphenylhydantoin, a noncentrosymmetric structure solved by centric symbolic addition. Acta Cryst B27: 2205-2211. [ Ref ]
Wong MG, Defina JA, Andrews PR (1986) Conformational analysis of clinically active anticonvulsant drugs. J Med Chem 29: 562-572. [ Ref ]
Kane VV, Martin AR, Jaime C, Ōsawa E (1984) Dynamic nuclear magnetic resonance and empirical force field studies of cannabidiol. Tetrahedron 40: 2919-2927. [ Ref ]
Reggio PH, Bramblett RD, Yuknavich H, Seltzman HH, Fleming DN, et al. (1995) The design, synthesis and testing of desoxy-CBD: further evidence for a region of steric interference at the cannabinoid receptor. Life Sci 56: 2025-2032. [ Ref ]
- Consroe P, Martin A, Singh V (1981) Antiepileptic potential of cannabidiol analogs. J Clin Pharmacol 21: 428S-436S.
Ooms F, Wouters J, Oscari O, Happaerts , Bouchard G, et al. (2002) Exploration of the pharmacophore of 3-alkyl-5-arylimidazolidinediones as new CB1 cannabinoid receptor ligands and potential antagonists: synthesis, lipophilicity, affinity, and molecular modeling. J Med Chem 45: 1748-1756. [ Ref ]
Muccioli GG, Martin D, Scriba GKE, Poppitz W, Poupaert JH, et al. (2005) Substituted 5,5’-diphenyl-2-thioxoimidazolidin-4-one as CB1 cannabinoid receptor ligands: synthesis and pharmacological evaluation. J Med Chem 48: 2509-2517. [ Ref ]
Leite JR, Carlini EA, Lander N, Mechoulam R (1982) Anticonvulsant effects of the (–) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology 24: 141-146. [ Ref ]
Consroe P, Martin A, Mechoulam R (1985) Anticonvulsant effects of cannabidiol stereoisomers and analogs in rats. Proceedings of the Oxford Symposium on Cannabis. Oxford. [ Ref ]
Martin AR, Consroe P, Kane VV, Shah V, Singh V, et al. (1987) Structureanticonvulsant activity relationships of cannabidiol analogs. NIDA Res Monogr 79: 48-58. [ Ref ]
Bisogno T, Hanuš L, de Petrocellis L, Tchilibon S, Ponde DE, et al. (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134: 845-852. [ Ref ]
Fride E, Feigin C, Ponde DE, Breuer A, Hanuš L, et al. (2004) (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur J Pharmacol 506: 179-188. [ Ref ]
Fride E, Ponde D, Breuer A, Hanuš L (2005) Peripheral, but not central effects of cannabidiol derivatives: Mediation by CB1 and unidentified receptors. Neuropharmacology 48: 1117-1129. [ Ref ]
Hanuš LO, Tchilibon S, Ponde DE, Breuer A, Fride E, et al. (2005) Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem 3: 1116-1123. [ Ref ]
Kozela E, Haj C, Hanuš L, Chourasia M, Shurki A, et al. (2016) HU-446 and HU-465, derivatives of the non-psychoactive cannabinoid cannabidiol, decrease the activation of encephalitogenic T cells. Chem Biol Drug Des 87: 143-153. [ Ref ]
Korte F, Dlugosch E, Claussen U (1966) Zur chemischen Klassifizierung von Pflanzen, XXIX (Haschisch, VIII). Synthese von dl-Cannabidiol und seinem Methylhomologen. Justus Liebigs Ann Chem 693: 165-170. [ Ref ]
Handrick GR, Razdan RK, Uliss DB, Dalzell HC, Boger E (1977) Hashish. Synthesis of (±)-Δ1- and Δ6-3,4-cis-cannabidiols and their isomerization by acid catalysis. J Org Chem 42: 2563-2568. [ Ref ]
Inoue S, Kosugi C, Lu ZG, Sato K (1992) New synthesis of Δ9- and Δ8- tetrahydrocannabinol using [2,3]sigmatropic rearrangement and the intramolecular Diels-Alder cyclization reaction. Nippon Kagaku Kaishi. 1992: 45-52. [ Ref ]