Journal Name: Journal of Biomedical Research and Reviews
Article Type: Clinical Trial
Received date: 24th-April-2018
Accepted date: 04th-May-2018
Published date: 11th-May-2018
Citation: Zavacka M, Pobehova J, Sabol F, Zavacky P (2018) The Impact of Angioplasty of the Renal Artery in Kidney Transplantation. J Biomed Res Rev Vol: 1, Issu: 1 (38-42).
Copyright: © 2018 Zavacka M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of this study was to analyse the benefit of the patients after renal transplantation with an assessment of the significance of different surgical techniques in the patients with renal transplantation (not only from the dead but also the living donors) with multiple arteries. 457 patients with end stage renal disease (ESRD) in the treatment using the extracorporeal elimination method (haemodialysis, or peritoneal dialysis), who in the period from 2005 to 2015 had a kidney transplant were included in our retrospective study. Our results confirm that the patients after kidney transplantation with cold ischemia time more than 12 hours have 2.5 times higher risk of delayed onset, possibly failure of graft function compared to those with cold ischemia time was less than 12 hours. This confirms our experience that the best option for the patient to achieve a stable graft function with long-term perspective is cold ischemia time of less than 12 hours and the realisation of renal artery angioplasty. In this case, the risk of delayed onset of transplanted kidney function, or graft failure, decreases 4.5 times compared to the respondents with cold ischemia time more than 12 hours without carrying out the arterial angioplasty.
Keywords
Kidney transplantation, Vascular anastomosis, Angioplasty, Graft function failure, Cold ischemic time.
Abstract
The aim of this study was to analyse the benefit of the patients after renal transplantation with an assessment of the significance of different surgical techniques in the patients with renal transplantation (not only from the dead but also the living donors) with multiple arteries. 457 patients with end stage renal disease (ESRD) in the treatment using the extracorporeal elimination method (haemodialysis, or peritoneal dialysis), who in the period from 2005 to 2015 had a kidney transplant were included in our retrospective study. Our results confirm that the patients after kidney transplantation with cold ischemia time more than 12 hours have 2.5 times higher risk of delayed onset, possibly failure of graft function compared to those with cold ischemia time was less than 12 hours. This confirms our experience that the best option for the patient to achieve a stable graft function with long-term perspective is cold ischemia time of less than 12 hours and the realisation of renal artery angioplasty. In this case, the risk of delayed onset of transplanted kidney function, or graft failure, decreases 4.5 times compared to the respondents with cold ischemia time more than 12 hours without carrying out the arterial angioplasty.
Keywords
Kidney transplantation, Vascular anastomosis, Angioplasty, Graft function failure, Cold ischemic time.
Introduction
Transplantation compared to the other methods of renal function replacement provides to patients with renal failure the highest possible quality of life while also being economically the cheapest way of treatment of renal failure with ESRD (end stage renal disease) [1-3].
Vascular graft in transplant surgery
The patency of the vascular graft is dependent on the quality of the vascular connections. Poor quality of the vessel wall, the intima damage, media snagging, dissection, contusion and subintimal haematoma are the indications for the vessel resection. Properly everted intima, the average knot traction, sufficiently large engagement of the wall, it all depends on the clinical experience of the operational erudition.
Mohamed et al. describes the renal vascular reconstruction in kidney transplants using polytetrafluoroethylene (PTFE) graft [4]. Up to now, however, no studies comparing long-term outcomes of biological vs. prosthetic vascular grafts in kidney transplantation have been known. The various techniques for the repair of short or damaged donor renal artery include an end-to-end anastomosis of artery graft, side-to-side anastomosis of branched artery, and the using of iliacal arterial graft. Short donor renal vein can be adjusted by transferring the recipient’s external iliac vein after ligation of internal iliac vein, extending the donor vein by dissection in the renal hilum and the use of venous grafts.
The types of adjustments of vascular pedicle:
Prolongation of the iliac artery graft
Revision of aortic patch with double kidney artery
Repairing of smaller damaged arteries: reimplantation into greater renal vessel or sewn by end to end to the donor vascular graft.
Combination of the types 1-3
Methods
Four hundred and fifty-seven patients with ESRD (end stage renal disease) in the treatment using the extracorporeal elimination method (haemodialysis, or peritoneal dialysis), who, between 2005 and 2015 underwent a kidney transplantation at the First Department of Surgery of UHLP and Medical School, University of P.J. Safarik in Kosice were included in our retrospective observational cohort study. Classifying criterion has been successfully implemented kidney transplant. Eliminating criteria were death, graftectomy and acute heart failure within 24 hours after kidney transplantation (KTx).
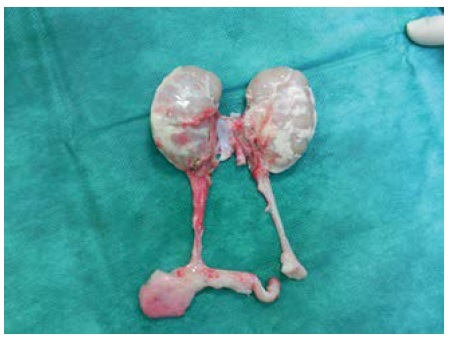
The kidney was transplanted from a dead or living donor. In each one we controlled: anatomical structures, the integrity of the intima of arteries and veins, the presence of accessory arteries, rinsing efficiency (Figure 1). If the accessory arteries were not damaged and were not far from the main arteries, we left them on the same target. If they were distant, damaged, or separated, we sewed them into the main artery “end to side” or have them rebuilt by vascular allografts (usually iliac vessels), or autografts taken from the recipient (arteria epigastrica inferior, or vena saphena magna).
Figure 1: Donors kidneys before transplantation (source: 1st Surgical Clinic, UHLP)
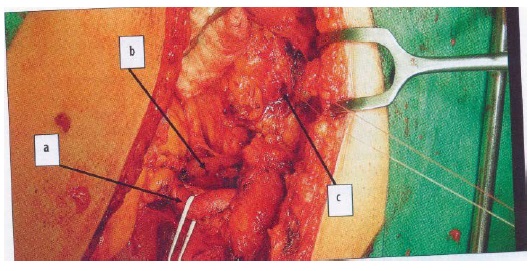
Kidney was inserted extra peritoneally into the iliac fossa, where the donor vessels were sewn end-to-side on the iliac arteries of the recipient following the non-absorbable suture material. We started with vein anastomosis, and after its checking followed the connection of arteries to a. iliaca interna, or a. iliaca externa (Figure 2). At sclerotic changes on the artery the anastomosis was sewn on at the place with the least disability. If this is not possible, we perform endarterectomy of the given part and then we sewed the anastomosis.
Figure 2: Dissected vasa iliaca externa: a-a. ilica, b-v. iliaca, c-bladder (Source: First Surgical Clinic, UHLP)
In the next step we reconstructed the urinary tract. After isolation of a part of the anterior and lateral wall of the bladder, laterally from vertex we implanted the ureter of the transplanted kidney into the bladder.
Results
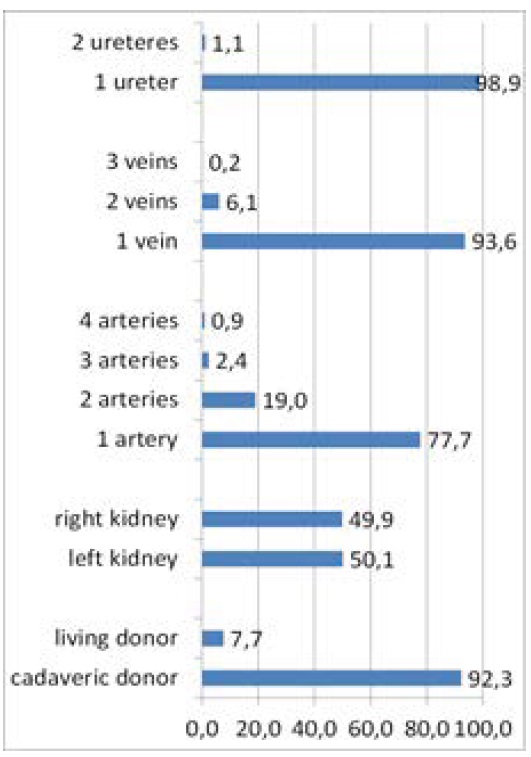
The research cohort consisted of 457 patients who underwent a kidney transplant (from a living or deceased donor) with a mean age of 47.92 (± 12.22) years (range 9–75). In the set the males were predominant with the percentage of 58.4 % (267), females were 41.6 % (190). Figure 3 shows the characteristics of set. Arterial angioplasty was performed in 22.3 % (102) of the patients, out of which the arteries individually sewn on were in 5.5 % (25), on the common target 9.4 % (43), and in 7.4 % (34) angioplasty was carried out as an implantation of pole renal arteries into the main strain.
Figure 3: The characteristics of set.
The values of urea and creatinine in the relation to the onset type of graft function
Creatinine and urea values measured before surgery were not statistically significantly different (either as a continuous or as a categorical variable), according to the onset type of graft function. The opposite situation occurred in these values at the emission of the patients. The later onset of graft function, or its failure also reflected in the significantly different (worse) values of urea and creatinine at patient releasing from the hospital (p<0.001) (Table 1).
Table 1: A comparison of mean values of creatinine and urea before transplantation and after discharge in the group of patients with immediate, delayed onset, or without the onset of graft function (ANOVA).
| The onset of graft function | N | Mean (standard deviation) | ANOVA | |
|---|---|---|---|---|
| The onset of graft function | ||||
| Creatinine value before Tx | immediately | 266 | 597.7 (246.9) | F=0.676 |
| delayed | 171 | 622.0 (221.2) | p=0,509 | |
| without onset | 20 | 634.3 (211.3) | Ns | |
| Creatinine value after Tx | The onset of graft function | |||
| immediately | 266 | 136.4 (35.1) | F=306.857 | |
| delayed | 171 | 204.9 (115.1) | p=0.000 | |
| without onset | 18 | 665.8 (242.2) | p<0.001 | |
| Value of Urea before Tx | The onset of graft function | |||
| immediately | 266 | 14.7 (6.5) | F=0.781 | |
| delayed | 171 | 14.9 (6.2) | p=0.58 | |
| without onset | 20 | 16.6 (8.0) | Ns | |
| Value of Urea after Tx | The onset of graft function | |||
| immediately | 266 | 8.8 (3.6) | F=46.918 | |
| delayed | 171 | 11.8 (4.6) | p=0.000 | |
| without onset | 18 | 16.2 (7.3) | p<0.001 |
Similarly, the patient groups did not differ significantly according to the type of surgery and time of the cold ischemia in the values of creatinine after transplantation. However, comparison of the patients according to the type of surgery and the time of cold ischemia, we confirmed the statistically significant difference in the values of urea after surgery. The group without angioplasty with cold ischemia time more than 12 hours had significantly higher values of urea at their releasing from hospital compared to the group of patients without angioplasty, but transplantation was carried out to 12 hours (p<0.05).
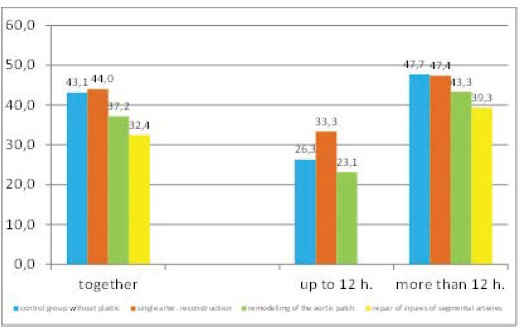
Causality of the type of surgical intervention, gender, or age with the risk of delayed onset or the onset, or failure of graft function has not been confirmed. On the other hand, the longer period of cold ischemia was significantly associated with the onset of graft function. Transplanted patients with cold ischemia time more than 12 hours had a 2 and a half times higher chance (OR = 2.49 / CI: 1.50 to 4.13) of delayed onset, or failure of graft function compared to those with cold ischemia time shorter than 12 hours (Figure 4).
Figure 4: The occurrence of delayed onset, or failure of the onset of graft function in the patient groups according to the type of surgical procedure, and the time of cold ischemia (in %).
The type of the surgery and the onset of graft function
The patients without angioplasty, but cold ischemia time was up to 12 hours had more than twice lower chance (OR/CI: 0.43/0.25 to 0.75) of delayed onset, or failure of graft function compared to the respondents with cold ischemia time more than 12 hours, but without necessity of performing angioplasty. Similarly, the patients with cold ischemia time of less than 12 hours, and they underwent angioplasty had four and a half times lower chance (OR/CI: 0.22/0.06-0.79) of delayed onset, or failure of graft function compared to those with cold ischemia time more than12 hours, but without necessity of carrying out angioplasty.
The kind of the surgery, duration of the cold ischemia, comorbidity and the onset of graft function
The patients with cold ischemia time more than 12 hours had 2.42 times higher probability, and the patients with comorbidity (CAD/DM) had more likely 1.65 times delayed start function, or graft failure compared to those with shorter cold ischemia and without the co-morbidity. Interaction of the influence of the type of surgery and comorbidities was not statistically significant. In other words, the type of surgery was not associated significantly with the onset of graft function, even when it was evaluated separately in the group with and without comorbidity.
Discussion
The aim of this study was to analyse the benefit of the renal transplant patients with an evaluation of the significance of the different surgical techniques. We followed up 457 patients who underwent the kidney transplantation in 2005–2015 at the First Surgical Clinic UHLP and the Medical Faculty of the University of P.J. Safarik in Kosice retrospectively.
Anatomically, there were most of the kidneys with 1 artery 1 (77.7 %), 19 % with two arteries, 2.4 % with 3 arteries, and 0.7 % with 4 arteries. The overwhelming number-93.6 % 1 vein was present, 6.1 % of donor kidney had 2 veins, and only 0.2 % of donated kidneys had 3 veins. Only in 1.1 % of the patients there were 2 ureters on the donor kidney. A lot of authors report the incidence of multiple renal arteries in the range of 18 % to 30 % [5- 7]. The incidence in the patients involved in the Aydin’s study from 2014 with renal transplantations was 12.8 %. The authors retrospectively evaluated 225 adult kidney transplants. 29 patients (12.8 %) had grafts with multiple renal arteries. For reconstruction of multiple renal arteries Aydin et al. describe various techniques [8]. So far however, none of the reconstruction technique has been shown to be the best, referring to complications and graft survival rate. Neither this study showed any significant differences in grafts and patients between the allograft with multiple and a single artery. When it was appropriate, Aydin et al. preferred connection of the artery extracorporeally as one renal artery to create a common disk. In the allografts with multiple renal arteries no significant differences were demonstrated in the graft survival in the patients with different reconstruction techniques. The mean systolic blood pressure, creatinine values in the first year and the last observation, and complication rate were in the acceptable range [8].
The values of urea and creatinine reflect the renal function [9]. Therefore, when we consider the fact that patients undergoing kidney transplantation, are in the final stages of CKD in need of renal replacement therapy, the analysis results did not confirm adequately the relationship between these values and the onset of graft function [3]. However, after the successful transplantation, at the time of the patient release, the parameters of kidney functioning are significantly associated with the onset of graft function, since there is a linear relationship between them [9]. This is related to the confirmation of a statistically significant inverse association between later onset of graft function, or its failure and higher levels of urea and creatinine at the time of release. The given statistically significant association between was have demonstrated also between the values of urea and creatinine with the type of surgery. After comparison of the groups of patients according to the type of plastic we confirmed a statistically significant difference in the values of urea after kidney transplantation. Namely the group of patients transplanted with the cold ischemia time more than 12 hours had significantly higher value of urea at their release, compared with the patients without, angioplasty, but the cold ischemia time of the kidney was less than 12 hours (p<0.05).
In this work we have also confirmed the significance of the performing the reconstruction of blood vessels in the kidneys of donors, which was not associated with gender and age, but with the level of urea. The group without angioplasty, with cold ischemia time more than 12 hours had significantly higher values of urea at their release compared to the group of patients without angioplasty, but the transplantation was performed within 12 hours. On the other hand, the association between the level of creatinine at release and the type of surgical procedure was confirmed only in the group of patients with severe comorbidity in terms of ischaemic heart disease and/or diabetes.
Moreover, we have shown the link between a longer period of cold ischaemia and the onset of graft function. The patients after kidney transplantation with cold ischemia time more than 12 hours had 2.5 times higher risk of delayed onset, or failure of graft function compared to those with cold ischemia time less than 12 hours. The patients without plastic, but the cold ischemic time was up to12 hours, had more than twice lower risk of delayed onset, or failure of graft function compared to respondents with cold ischemia time longer than 12 hours, but without a need of plastic.
This result explains the reasons for shortening of the cold ischemia time to the minimum.
However, isolated vessel plastic was not independently associated with the graft function at the evaluation without cold ischemia time and comorbidities. These results offer an ideal option that is: the cold ischemia time under 12 hours occurring concurrently and performing the renal artery angioplasty. In this case, the risk of delayed onset of the transplanted kidney function, or graft failure decreases 4.5 times compared to the respondents with the cold ischemia time above 12 hours without angioplasty. Based upon the analyses the important information follows which is profiting from the reconstruction of donor kidney arteries, while the benefit rises more in the patients with co-morbidities than without them [10,11]. On the other hand, not only the very longer period of cold ischemia and non-implementation of angioplasty of the donor kidney if there are options for it, increase the risk of delayed onset, or loss of graft function as well as co-morbidity. Patients with co-morbidity in terms of ischemic heart disease and diabetes of first and/or second type had almost a 2 times higher probability of delayed onset of graft function, or its failure compared to those with shorter cold ischemia time and without comorbidity. An association among the renal function (measured according to the serum creatine values) after the first year from kidney transplantation, and carrying out the plastic, and/or shorter ischemia time has not been confirmed. To these results testifies the fact that after stabilisation period of three months the aspect of surgical intervention does not come into focus. For the given period, the adequate immunosuppression, the absence of infection, rejection, and other complications are the important factors for preservation of the kidney function [12]. The results in connection with a statistically significant association between male sex and impaired graft were confirmed by several authors [13,14]. In our set, we demonstrated that men in comparison with women have approximately 2 times higher chance of deterioration of the graft function after 3 months and after 1 year from kidney transplantation.
Statistically significant differences were confirmed only in the control groups without angioplasty with different cold ischemia time in month 3 after transplantation. The control group without angioplasty with the cold ischemia time up to 12 hours had significantly lower mean values of creatinine after 3 months from the transplantation in comparison with the control group with the cold ischemia time more than 12 hours.
Even though no other significant association was demonstrated between the graft function and performing angioplasty, or cold ischemia time after the first year from the kidney transplantation, based upon the results the trend of the development of creatinine level was recorded, while the lowest mean values after 3 months had the patients with the cold ischemia time up to 12 hours and had only one artery, or without angioplasty.
Conclusion
In the study we confirmed the significance of performing the reconstruction of blood vessels in the donor kidneys that was not associated with sex and age, but with the serum urea level. The strong point of the work is comprehensive compilation of transplanted patients in the period of ten years within one transplant centre in Slovakia, with the majority carrying out kidney transplantation. Longitudinally of the data processing strengthens the meaningful results.
Port FK, Wolfe RA, Mauger EA (1993) Comparison of survival probabilities for dialysis patient’s vs cadaveric renal transplant recipients. JAMA 270:1339-1343. [ Ref ]
Wolfe RA, Ashby VB, Milford EL (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725-1730. [ Ref ]
Seghal AR (2002) What is the best treatment for end-stage renal disease? Am J Med 112:735-736. [ Ref ]
Mohamed HK, Anil AT, Ponusamy MH, David PH (2007) Renal vessel reconstruction in kidney transplantation using a polytetrafluor ethylene (PTFE) vascular graft. Journal of surgical case reports 22:1030-1032. [ Ref ]
Suthanthiran M, Strom TB (1994) Renal transplantation. N Engl J Med 331:365-376. [ Ref ]
Navrátil P (2005) Praktická urológia u nemocných v dialyzační léčbe, před a po transplantaci ledviny Hradec Králové. vydavatelství Oľga Čermáková. [ Ref ]
Viklický O, Janoušek L, Balaáž P (2008) Transplantace ledviny v klinické praxi. Grada Publicing, Praha. [ Ref ]
Aydin NM, Berber I, Altaca G, Yigit B, Titiz I (2004) The outcome of kidney transplants with multiple renal arteries. BMC surgery 4:4-8. [ Ref ]
Jafri SSA, Younas M, Chughtai MN (2009) Surgical aspect and outcomes of Kidney transplantation with multiple renal arteries. Annals 15: 122- 128. [ Ref ]
Levey AS, Stevens LA (2010) Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am J Kidney Dis 55:622-627. [ Ref ]
Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, et al. (2010) Banff ‘09 meeting report: Antibody mediated graft deterioration and implementation of banff working groups. Am J Transplant 10: 464-471. [ Ref ]
Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, et al. (2008) Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) Conference. Clin J Am Soc Nephrol 3: 471- 480. [ Ref ]
Franke GH, Reimer J, Philipp T, Heemann U (2003) Aspects of quality of life through end-stage renal disease. Qual Life Res 12:103-115. [ Ref ]
Benjamins MR, Hummer RA, Eberstein IW, Nam CB (2004) Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Soc Sci Med 59: 1297-1306. [ Ref ]