Journal Name: Journal of Biomedical Research and Reviews
Article Type: Research
Received date: 10-March-2021
Accepted date: 10-March-2021
Published date: 10-March-2021
Citation: Carella S, Rossi C, Ribuffo D, Onesti MG (2021) The Use of Peripheral Blood-Mononuclear Cells in Scleroderma Patients: An Observational Preliminary Study. J Biomed Res Rev Vol: 4, Issu: 1. (22-31).
Copyright:© 2021 Carella S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Systemic sclerosis (SSc) is a systemic autoimmune disease characterized by vasculopathy and excessive production of collagen, which lead to skin and visceral fibrosis. The aim of our study is to assess the potential benefits of autologous peripheral blood mononuclear cells (PBMCs) implants in the treatment of clinical manifestations such as mouth impairment, hand disability, digital ulcers and Raynaud’s phenomenon in Scleroderma patients.
Methods: From February 2016 to May 2019, 10 female patients were enrolled from the outpatient clinic of the Plastic Surgery Unit of Sapienza University of Rome. Parameters evaluated were: patients’ disability, using the Health Assessment Questionnaire (HAQ) disability index (DI) and the scleroderma HAQ (sHAQ); mouth opening capacity, by measuring the maximum interincisal distance and the mouth perimeter; hand mobility, assessed with clinical exam and the Hand Mobility in Scleroderma (HAMIS) scale; Raynaud’s phenomenon, evaluated through nailfold capillaroscopy; digital ulcers, examined through their features and incidence of appearance. SPSS software was used for a simple descriptive statistical analysis performed by the Student’s paired t-test. P values less than 0.05 were considered statistically significant.
Results: The treatment showed a significant improvement of all the parameters evaluated at 1-year follow-up, it was well-tolerated by all the patients and the only complications noticed were small areas of ecchymosis.
Conclusion: With our preliminary study we tought to exploit PBMCs capability to induce angiogenesis widely described in literature in order to treat the vasculopathy-related manifestations of SSc, in patients with no chance for lipofilling. Our results suggest that PBMCs injection could represent a treatment option to take into account for SSc patients. The procedure we used is easy and fast to perform, minimally invasive and not-operator dependent. We hope our observational and preliminary study could be considered as a starting point for further research studies.
Keywords
Peripheral Blood Mononuclear Cells, Macrophages, Immunomodulation, Systemic sclerosis, Cell Therapy, Wound Healing.
List of Abbreviations:
SSc: Systemic sclerosis; PBMCs: peripheral blood mononuclear cells; HAQ-DI: Health Assessment Questionnaire Disability Index; sHAQ: scleroderma Health Assessment Questionnaire; HAMIS: Hand Mobility in Scleroderma; ACR: American College of Rheumatology; ACR/EULAR: American College of Rheumatology/ European League Against Rheumatism; SVF: Stromal Vascular Fraction; ASCs: Adipose-derived Stromal Cells; dcSSc: diffuse cutaneous Scleroderma; VAS: Visual Analogue Scale; FACS: Fluoresence-Activated Cell Sorting; TNCs: Total Nuclear Cells; MNCs: Mononuclear Cells; MSCs: Mesenchymal tromal Cells; GMP: Good Manufacturing Practice.
Abstract
Introduction: Systemic sclerosis (SSc) is a systemic autoimmune disease characterized by vasculopathy and excessive production of collagen, which lead to skin and visceral fibrosis. The aim of our study is to assess the potential benefits of autologous peripheral blood mononuclear cells (PBMCs) implants in the treatment of clinical manifestations such as mouth impairment, hand disability, digital ulcers and Raynaud’s phenomenon in Scleroderma patients.
Methods: From February 2016 to May 2019, 10 female patients were enrolled from the outpatient clinic of the Plastic Surgery Unit of Sapienza University of Rome. Parameters evaluated were: patients’ disability, using the Health Assessment Questionnaire (HAQ) disability index (DI) and the scleroderma HAQ (sHAQ); mouth opening capacity, by measuring the maximum interincisal distance and the mouth perimeter; hand mobility, assessed with clinical exam and the Hand Mobility in Scleroderma (HAMIS) scale; Raynaud’s phenomenon, evaluated through nailfold capillaroscopy; digital ulcers, examined through their features and incidence of appearance. SPSS software was used for a simple descriptive statistical analysis performed by the Student’s paired t-test. P values less than 0.05 were considered statistically significant.
Results: The treatment showed a significant improvement of all the parameters evaluated at 1-year follow-up, it was well-tolerated by all the patients and the only complications noticed were small areas of ecchymosis.
Conclusion: With our preliminary study we tought to exploit PBMCs capability to induce angiogenesis widely described in literature in order to treat the vasculopathy-related manifestations of SSc, in patients with no chance for lipofilling. Our results suggest that PBMCs injection could represent a treatment option to take into account for SSc patients. The procedure we used is easy and fast to perform, minimally invasive and not-operator dependent. We hope our observational and preliminary study could be considered as a starting point for further research studies.
Keywords
Peripheral Blood Mononuclear Cells, Macrophages, Immunomodulation, Systemic sclerosis, Cell Therapy, Wound Healing.
List of Abbreviations:
SSc: Systemic sclerosis; PBMCs: peripheral blood mononuclear cells; HAQ-DI: Health Assessment Questionnaire Disability Index; sHAQ: scleroderma Health Assessment Questionnaire; HAMIS: Hand Mobility in Scleroderma; ACR: American College of Rheumatology; ACR/EULAR: American College of Rheumatology/ European League Against Rheumatism; SVF: Stromal Vascular Fraction; ASCs: Adipose-derived Stromal Cells; dcSSc: diffuse cutaneous Scleroderma; VAS: Visual Analogue Scale; FACS: Fluoresence-Activated Cell Sorting; TNCs: Total Nuclear Cells; MNCs: Mononuclear Cells; MSCs: Mesenchymal tromal Cells; GMP: Good Manufacturing Practice.
Introduction
Systemic sclerosis (SSc) is a systemic autoimmune disease of unknown cause characterized by vasculopathy and excessive production of collagen [1]. Several pathophysiological processes are involved in its development such as cellular and humoral autoimmunity, vascular injury, and tissue fibrosis. They all lead to a connective tissue disease characterized by a pathological thickening and tethering of the skin and by the involvement of internal organs (gastrointestinal tract, heart, lungs and kidneys) [2].
Due to its low-frequency, insidious onset, variable presentation and the lack of uniformity in its diagnostic criteria, it is difficult to study its epidemiological features. However, its prevalence ranges from 7/million to 489/ million and its incidence from 0.6/million⋅year to 122/ million⋅year [2]. There are many geographical variations, with higher prevalence in the USA and Australia, followed by Japan and Europe. Age of onset is most commonly in the range of 30–50 years. The prevalence in first degree relatives is significantly higher than in the general population (1.6 vs 0.026%) and like other connective tissue disorders, SSc is also predominant in females with ratios of women to men between 5 and 14:1 [3].
The ACR (American College of Rheumatology) criteria published in 1980 were the first used to classify these patients, but the 2013 EULAR (European League Against Rheumatism)/ACR classification criteria showed a higher sensitivity. The ACR criteria are limited by their lack of sensitivity for mild and early cases of SSc [4].
The term “Scleroderma” derives from the most characteristical feature of the disease – skin thickening. Raynaud’s phenomenon occurs as the first clinical feature of the pathology, simultaneously with skin thickening or shortly prior to it. It is tipically present in the digits of the hand but can also affect the feet, the earlobes, the tip of the nose and the tongue. In Scleroderma patients it often leads to the onset of trophic changes in the fingertips, in the form of necrosis, hard-to-heal erosions and ulcers, and residual scars. Finger contractures (sclerodactyly) develop with the progression of the disease. The range of finger motion is limited, while trophic disorders contribute to bone destruction and shortening of distal phalanges (acroosteolysis) [5].
Restricted hand movements include finger flexion and extension, abduction of the thumb, dorsal extension and volar flexion of the wrist, pronation and supination of the forearm, ability to make a thumb pincer grip and to make finger abduction [1-5].
Skin sclerosis may also affect the face, including the lips. The most typical facial features associated with SSc are teleangiectasias, shrunken nose, microcheily, reduced mouth opening (microstomy), and microglossy. In addition, cutaneous wrinkles around the mouth disappear and there may be a radial furrowing around the lips: the face of these patients appears inexpressive. Among other skin manifestations there are changes in skin pigmentation, hair loss, dryness due to the loss of sebaceous glands, and joint contracture [1-5].
Facial involvement and oral complications can lead to problems with oral hygiene and eating, aesthetic changes and impairment of the patient’s self-image. Moreover, sclerosis of the extremities is highly disabling and results in significant dysfunction. Due to these disabilities, several studies are concordant in remarking the importance of a multidisciplinary therapy and tailored approach. The usefulness of pharmacological, pathogenetic and symptomatic treatments (immunosuppressor, immunomodulators, antifibrotics, corticosteroids, plasmapheresis, phototherapy, anti-inflammatory, vasoactive, and analgesic drugs), as well as some precautionary measures and a proper life-style (such as avoiding cold or smoke), may be important to prevent and treat Scleroderma skin lesions [6].
In addition to these systemic approaches, local treatments should be considered in more severe and non-healing lesions [6]. Several regenerative cell-based techniques were described for the treatment of Scleroderma patients, mostly with autologous fat (lipofilling), stromal vascular fraction (SVF) and/or adipose-derived stomal cells (ASCs) from adipose tissue [7-12].
New point of care device based on peripheral blood selective filtration technology has been developed to produce fresh autologous peripheral blood-mononuclear cells (PBMCs) for use in human cell therapy applications. Cell concentrate produced by this innovative technology has been extensively studied in term of characterization and adequate potency in therapeutic angiogenesis in vitro and in vivo [13]. This tecnique is less invasive in comparison to adipose tissue transplantation, faster, not operator-dependent and userfriendly. Promising results on wound healing were obtained by this cellular concentrate in different clinical trials on critical limb ischemia patients, [14-16]. It has been observed that PBMCs are able to induce therapeutic angiogenesis to promote collateral vessel formation through paracrine effects [17,18]. PBMCs release growth factors, cytokines, messenger molecules as well as exosomes, demonstrated in a wound healing animal model [19-21]. Monocyte/macrophages and lymphocytes, in particular Treg populations, play a key role in tissue regeneration in non-healing trophic lesions through macrophage polarization from an inflammatory (M1) to regenerative (M2) phenotype [22]. Growing evidence also suggests a key role of limphocytes in angiogenesis and in tissue regeneration [23-26]. PBMCs could have an indication of use in auto-immune-based diseases where there is a vascular and/or microcirculation problem [27-29] not only for their angiogenic capacity but also for their ability to regenerate tissues and restore the correct M1/ M2 balance, always compromised in the non-healing lesions of patients suffering from these pathologies, as recently published [30].
For the first time at the best of our knowledge we report the experience on the use of PBMCs implants in order to treat open mouth impairment, hand disability, digital ulcers and Raynaud’s phenomenon.
Primary outcomes were: improvement of hand function and mouth opening. The secondary outcome was the gaining of a better quality of life.
Methods
From February 2016 to May 2019, 10 female patients, fulfilling ACR criteria and classified as having diffuse cutaneous Scleroderma (dcSSc), were enrolled from the outpatient clinic of the Plastic Surgery Unit of Sapienza University of Rome [31-33]. These patients had advanced SSc-related perioral thickening, mouth opening restriction (2 out of 10 patients) and hand mobility limitation, Raynaud’s phenomenon, and digital ulcers. The proposed treatement consisted of PBMCs injections in order to treat the above manifestations. The mean age was 50,2 years (range: 21-68) and the average disease length was 7 years (range: 3-11). They agreed by a written informed consent to participate in the study, which was conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki.
For each patient, during the first evaluation, demographic, anamnestic, and clinical data were collected and recorded, using a written form that was held securely, thus being accessible only to investigators involved in the study (Table 1).
Inclusion criteria
Age >18 years, stable phase of disease, SSc diagnosis with hands and/or perioral involvement; no possibility to perform lipofilling (lack of adipose tissue, BMI<20).
Exclusion criteria
Age >75 years, pregnancy, or lactation; immunomodulating or immunosuppressive therapy within the last 4 weeks and any topical therapy within the last 2 weeks except for the use of emollients, comorbidities contraindicating the treatment (active malignancy, bone marrow or hematologic disorders, active infections).
Disability evaluation
All patients were evaluated for the following parameters before the procedure (T0), and 1 week (T1), 1 month (T2), 3 months (T3), 6 months (T4) and 1 year (T5) after.
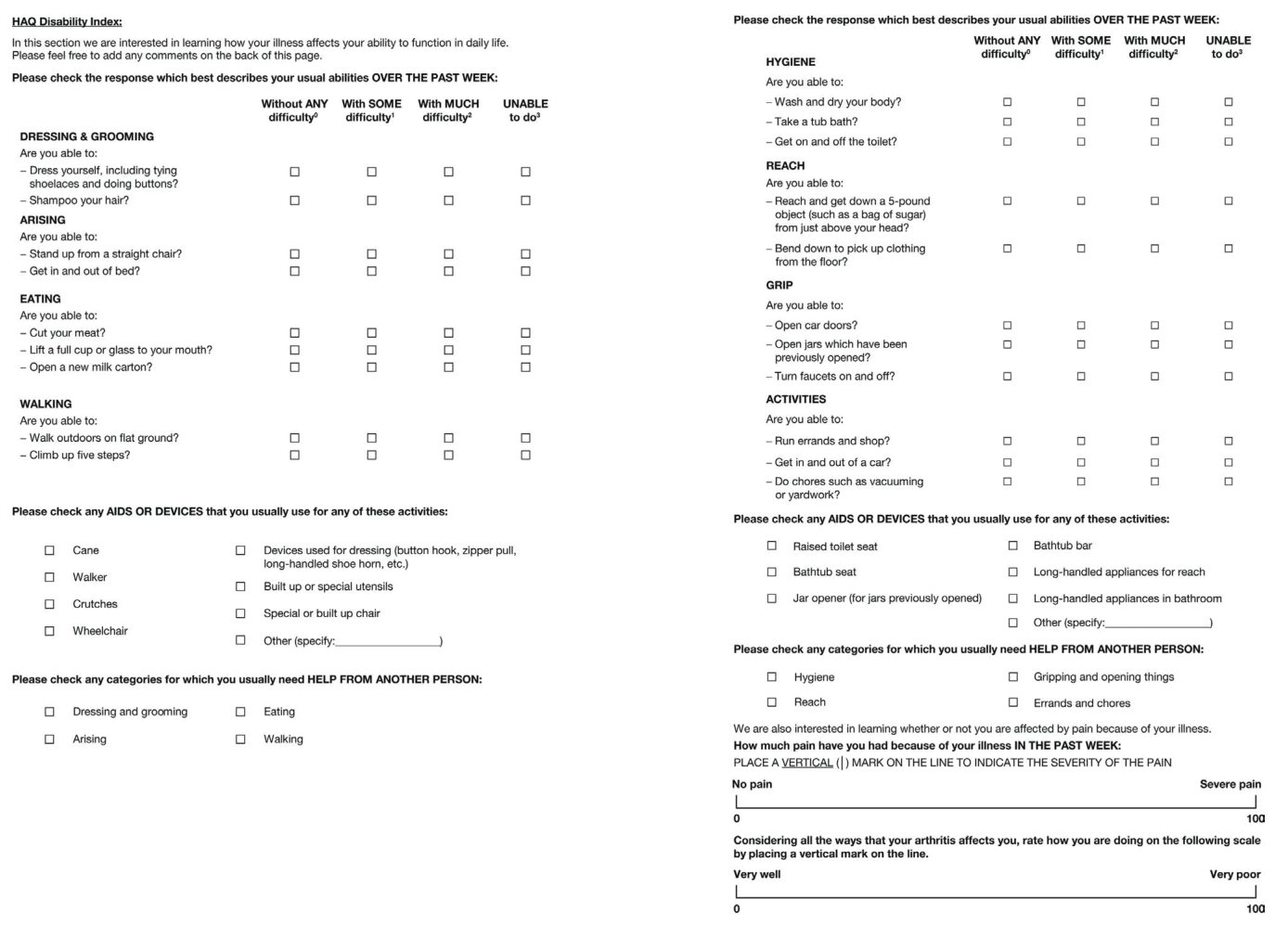
Health Assessment Questionnaire (HAQ) Disability Index (DI) and scleroderma HAQ (sHAQ):These instruments are increasingly utilized to assess Scleroderma patients in randomized trials. The HAQ-DI contains 8 domains of activity (dressing, arising, eating, walking, hygiene, reach, grip, and common daily activities) each of which has at least 2 questions, for a total of 20 items. For each item patients report the amount of difficulty experienced performing the activity with 4 possible responses, ranging from 0 (without any difficulty) to 3 (unable to do). The highest component score in each category determines the score for the category. The eight category scores are averaged into an overall HAQDI score on a scale from 0 (no impairment in function) to 3 (maximal impairment of function). The scale is not truly continuous. The HAQ-DI also contains a Visual Analogue Scale (VAS) that patients use to report the amount of pain experienced in the past week (Figure 1) [34]. The sHAQ is more specific for SSc, as it adds 5 visual analogue scales to HAQ, evaluating also Raynaud’s phenomenon, digital ulcers, gastrointestinal, lung symptoms, and overall disease severity [35].
Table 1: Patients' data.
| Patient | Age | Disease duration | Treated area |
|---|---|---|---|
| 1 | 68 | 6 years | Hands |
| 2 | 65 | 11 years | Hands |
| 3 | 49 | 8 years | Hands and mouth |
| 4 | 47 | 8.5 years | Hands |
| 5 | 39 | 4.5 years | Hands |
| 6 | 50 | 6 years | Hands |
| 7 | 44 | 7 years | Hands |
| 8 | 21 | 3 years | Hands |
| 9 | 56 | 9 years | Hands and mouth |
| 10 | 63 | 7 years | Hands |
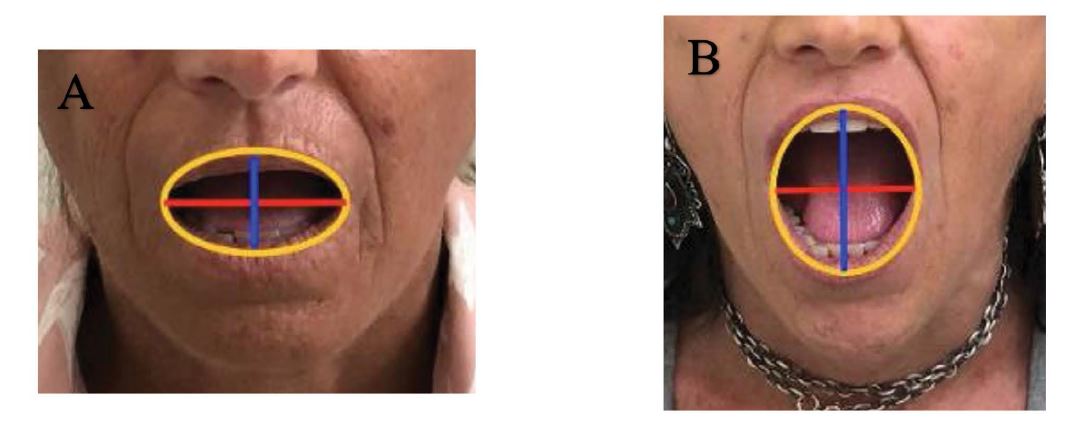
Mouth opening capacity [12]:it was evaluated by measuring, at baseline and at each follow-up, the maximum interincisal distance and the mouth perimeter. This latter one was derived by the ellipse geometrical formula, that is 2π√[(a2+b2)/2], where “a” stands for the semi-major axis (half of the distance between upper and lower lip, at maximally opened mouth), and “b” stands for the semiminor axis (half of the distance between the opposite lip commissures) (See Figures 4A-B). The software used was Microsoft Mathematics 4.0.
Hand Mobility in Scleroderma (HAMIS):this is a performance-based test, found to be a reliable and valid tool to assess hand function in SSc patients. It is composed of 9 items, assessing both hands (Table 2). The different performance areas of HAMIS test are composed of differentsized grips and different movements, all related to tools and activities that are part of daily occupations. Each exercise is graded on a 0–3 scale (from 0 = normal function to 3 = inability to perform the task), with a total possible score of 27 for each hand [36] (Table 2).
Cutaneous digital ulcers:its frequency and duration were assessed through patient reported data and its severity with nailfold capillaroscopy.
Raynaud’s phenomenon:they were examined through anamnestic and clinical findings. Their incidence of appearance was recorded.
Cell therapy: autologous PBMCs concentration
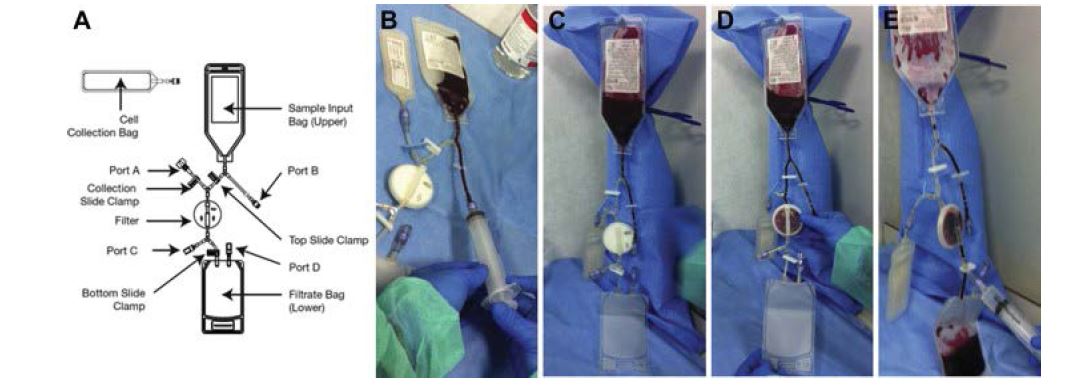
A disposable point-of-care selective filtration system certified for human cell therapy was used to concentrate PBMCs (Monocells – Pall Celeris – Athena Biomedical Innovations) (Figure 2). This system was fully characterized and the PBMCs obtained were tested positively for angiogenic potential in vitro and in vivo [13].
Fluoresence-activated cell sorting (FACS) analysis of total nuclear cells (TNCs) obtained by selective filtration confirmed the presence of CD45+CD3 T lymphocytes, CD45+CD14+ monocytes, CD45+ CD19+ B lymphocytes, CD45+CD66b+ neutrophil granulocytes, CD3-CD16+ CD56+ natural killer, CD45+CD34+ stem cell component, CD4+KDR+ and CD34+KDR+ endothelial progenitors. Pall Celeris filtration system cell concentrate output is enriched 2.97- fold in TNC, 4.2- fold MNCs, 3,94 fold monocytes, l 4,25 fold limphocytes, while CD34+ progenitor cells subpopulation are enriched 5,6 fold as reported [13].
Table 2: Hand Mobility In Scleroderma (HAMIS) test.
| Finger flection (All fingers must be tight to the object) 0 - Can bend fingers 2-5 around a pencil (5 mm diam) 1- Can bend fingers 2-5 around a piece of cutlery (15 mm diam) 2 - Can bend fingers 2-5 around handlebar (30 mm diam) 3 - Cannot manage the previous item |
| Finger extension 0 - Can feel the table completely with digits 2-5 1 - Can feel the pencil (5 mm diam) with digits 2-5 2 - Can feel the piece of cutlery (15 mm diam) with digits 2-5 3 - Cannot manage the previous item |
| Thumb abduction 0 - Can grip around a coffee package (90 mm diam) 1 - Can grip around a milk parcel (70 mm diam) 2 - Can grip around a bottle (60 mm diam) 3 - Cannot manage the previous item |
| Pincer grip 0 - Can form a round pincer grip 1 - Can form a D-shaped pincer grip 2 - Can form a long narrow pincer grip 3 - Cannot manage the previous item |
| Finger abduction 0 - Can spread the fingers and then fold the hands together to the bottom of the fingers 1 - Can spread the fingers and then fold the hands together to the first phalanx 2 - Can spread the fingers and then fold the hands together to the second phalanx 3 - Cannot manage the previous item |
| Volar flexion (The person stands with the arms alongside the body. The object is given from behind) 0 – Can grasp a spool of thread with a slight flexion of MCP and extended PIP and DIP joints 1 - Can grasp a spool of thread with a large flexion of MCP and ectended PIP and DIP joints 2 - Can grasp a spool of thread with a large flexion of MCP and flexion PIP 3 - Cannot manage the previous item |
| Dorsal extension 0 - Can hold the palms together and put the wrists against the stomach 1 - Can hold the palms together and put the thumbs against the throat 2 - Can hold the palms together and put the thumbs up to the mouth 3 - Cannot manage the previous item |
| Pronation 0 – Can put the palms of the hands on the table (MCP 2-5 must touch the surface) 1 - Can put the palms of the hands on the table (MCP 3-5 must touch the surface) 2 - Can put the palms of the hands on the table (MCP 4-5 must touch the surface) 3 - Cannot manage the previous item |
| Supination 0 - Can put the backs of the hands on the table (MCP 2-5 must touch the surface) 1 - Can put the backs of the hands on the table (MCP 3-5 must touch the surface) 2 - Can put the backs of the hands on the table (MCP 4-5 must touch the surface) 3 - Cannot manage the previous item |
| MCP: Metacarpophalangeal joints, PIP: Proximal interphalahgeal joints, DID: Distal interphalangeal joints |
In a sterile-operating environment PBMCs were obtained from a 100-120 mL venous blood sample following the original instruction for use. The procedure was performed as follows: 120 mL of ACD (Acid Citrate Dextrose) anticoagulated blood were transferred to the upper bag of the system which was hang up to let the blood flow by gravity through the filter below. The selective membrane retained TNCs and the residual blood flowed to the waste blood bag under the filter. After filtration, which took around 10-15 min, a 10 mL of saline solution backwash allowed to harvest the PBMCs from the filter, resulting in 8-10 mL of cell concentrate collected in a cell recovery bag and ready to be injected. Equal parts (0,25 mL) of the cell concentrate obtained were injected in each site, containing a mean average count of 20,8±0,1⋅106/mL TNC, 10,6±0,2⋅106 MNC and 137±0,3⋅103/mL CD34+ [13].
Cell therapy: autologous PBMCs implants
For hands, each injection was performed with a 21 G needle. Subcutaneous injections were performed at the bases of the fingers and proximally to the digital ulcers at perilesional skin level. The total amount of cell concentrate we injected varied from 3 to 5 mL, depending on each selective case. For mouth, injections were performed at subcutaneous level of selected perioral areas, using a blunt cannula of 2 mm in diameter. The cannula was inserted in 2 symmetric sites located just proximally to the labial commissures. Six areas were identfied: two in the upper lip and two in the lower lip (two lateral for each lip). The remaining 2 areas were represented from the nasolabial fold and a line extending from the labial commissure toward mandibular border. Perioral region was injected using as many radiating passages at subcutaneous level as needed for a total of 2 mL.
The study design foresaw the performance of the treatment once a month, for a total of 3 to 4 sessions, to be evaluated depending on the single case outcome.
Statistical analysis
SPSS software (IBM Corp., Armonk, NY) was used for a simple descriptive statistical analysis. Absolute scores and their changes from T0 to T5 were evaluated. The Shapiro– Wilk test was used to verify the normal distribution of continuous variables. Consequently, data were analyzed using Student’s paired t-test. P values less than 0.05 were considered statistically significant.
Results
The treatment showed a significant improvement of all the parameters evaluated (Table 3).
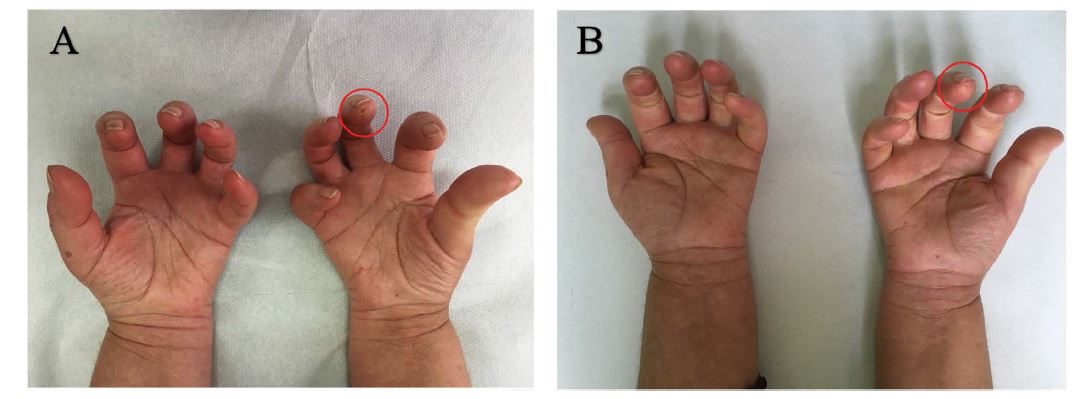
In all patients HAMIS scale decreased (p = 0,0026), reflecting a better hand function. The frequency of Raynaud’s phenomenon appeared to be reduced (p = 0,0023): in one patient expecially there was a reduction from an average of 15 episodes per day to a maximum of 5 episodes per day after the PBMCs therapy. Six patients were affected by multiple digital ulcers at baseline, after 12 months from the autologous implant only three of them had one digital ulcer left (p = 0,0044) (Figures 3A-B).
Table 3: Patients’ clinical outcomes.
| Patient | HAMIS Scale1(0-27) | Raynaud’s phenomenon (episodes per day) | Digital ulcers(annual incidence) | HAQ-DI score2 | ||||
|---|---|---|---|---|---|---|---|---|
| T0 (right/left) | T5 (right/left) | T0 | T5 | T0 | T5 | T0 | T5 | |
| 1 | 10/10 | 4/4 | 8 | 3 | 5 | 1 | 0.54 | 0.34 |
| 2 | 6/6 | 2/2 | 9 | 2 | 2 | 0 | 0.57 | 0.23 |
| 3 | 8/8 | 3/3 | 7 | 1 | 0 | 0 | 0.75 | 0.31 |
| 4 | 11/11 | 7/7 | 15 | 5 | 1 | 0 | 1.22 | 0.56 |
| 5 | 7/7 | 2/2 | 9 | 3 | 3 | 1 | 0.85 | 0.43 |
| 6 | 6/6 | 2/2 | 8 | 2 | 0 | 0 | 0.47 | 0.22 |
| 7 | 9/9 | 3/3 | 6 | 0 | 1 | 0 | 0.54 | 0.32 |
| 8 | 7/7 | 3/3 | 7 | 2 | 0 | 0 | 0.53 | 0.28 |
| 9 | 8/8 | 4/4 | 8 | 2 | 2 | 1 | 0.77 | 0.52 |
| 10 | 9/9 | 6/6 | 9 | 4 | 0 | 0 | 0.80 | 0.43 |
| 1 Hand Mobility In Scleroderma (HAMIS) Scale 2 Health Assessment Questionnaire (HAQ) Disability Index (DI) | ||||||||
Figure 1: Health Assessment Questionnaire (HAQ) Disability Index (DI).
The improvement of mouth opening was observed in two patients: an icreased mouth perimeter was recorded (patient A mouth perimeter: from T0 = 10,84 to T5 = 13,66; patient B mouth perimeter: from T0 = 9,72 to T5 = 12,87) (Figures 4A-B). Patients reffered improving in oral hygiene and bite function.
We also recorded a meaningful decrease of the HAQ-DI composite score (p = 0,0017).
The treatement was well-tolerated by all the patients, the only complications noticed were small areas of ecchymosis. Three patients claimed to be very satisfied with the procedure, five claimed to be satisfied, and two were moderately satisfied.
Figure 2: Pall Celeris system. A.) Filter’s components. B.) Step 1: 120 mL of ACD (Acid Citrate Dextrose) anticoagulated blood are transferred to the upper bag of the system. C.) Step 2: the system is hung up to let the blood flow by gravity through the filter below. D.) Step 3: the selective membrane retains TNCs and the residual blood flows to the waste blood bag under the filter. E.) Step 4: after filtration, which takes around 10-15 min, a 10 mL of saline solution backwash allows to harvest the PBMCs from the filter, resulting in 8-10 mL of cell concentrate collected in a cell recovery bag and ready to be injected.
Figure 3: Evolution of digital ulcers with treatment. A.) Pre-treatement appereance in patient with digital ulcer on the third right finger. B.) Healed ulcer after the treatement.
Figures 4A-B: Evolution of mouth impairement with treatment. A.) Pre-treatement appereance in patient with mouth opening impairement. Patient A – T0: half of the distance between the lip commissures (red line): 2 cm; half of the distance between upper and lower lip (blue line): 1,45 cm. Ellipse Perimeter = 10,84 cm. B.) Post-treatement appereance in patient with mouth opening impairement: improvement of mouth opening. Patient A – T5: half of the distance between the lip commissures (red line): 2 cm; half of the distance between upper and lower lip (blue line): 2,35 cm. Ellipse Perimeter = 13,66 cm.
Discussion
Over the years several alternatives to treat the cutaneous and vasculopathy-related manifestations of SSc were suggested. Autologous fat transplantation has become the first-choice technique for treating SSc cutaneous lesions. This approach, using the patient’s own body fat as a natural filler to achieve structural modifications, takes advantage of its abundance and accessibility and avoids complications associated with foreign materials. Elective liposuction for fat transplantation is nowadays considered a safe and welltolerated procedure, Unfortunatelly, adipose tissue is not available in every patient, as it happens for patient with low BMI (< 20) [12].
Nevertheless, some studies described the potential role of cell-based therapies, such as ASCs and SVF based therapy [10-12].They require less invasive harvesting techniques than the ones used when bone marrow is the primary source of mesenchymal stromal cells (MSCs) and are particularly useful in SSc patients with a degree of skin fibrosis that could not even permit the insertion of the smallest liposuction cannula.
Moreover, point-of-care devices have been placed on the market for the production of micro fragmented adipose tissue, also called nano graft, and adipose cells concentration system containing SVF [37].
The main disadvantages of ASCs-based therapy are represented by high costs, due to cell preparation in certified Good Manufacturing Practice (GMP) laboratories, and its need to be performed in two separate sessions, thus increasing the patient discomfort.
Notably, several papers showed that a main mechanism of action of MSCs from adipose tissue, is to promote tissue regeneration through M2 polarization, but hypoxia reduces their capability to polarize macrophages in the M2 phenotype while for PBMCs hypoxia is a physiological trigger for angiogenesis [38-46].
Moreover, Navarro et al recently showed that the angiogenic capacity of adipose tissue is related to the monocytes CD14+ population contained in SVF, which is more efficient in inducing angiogenesis than the ASCs [47].
PBMCs rappresent a new promising autologous therapy used in critical limb ischemia, diabetic foot and chronic ulcers [14-16,48-53]. The angiogenic and arteriogenic capacity of monocytes is well described [17,18,20,21,54]. More recently several studies also indicate an angiogenic function of specific limphocytes populations [25,55-58].
It is interesting to note that PBMCs efficacy was also reported in a large meta-analisys in no-option critical limb ischemia patients showing that PBMCs may outperform bone marrow–mononuclear cells and mesenchymal stem cells in reducing significately limb amputation [48].
With our preliminary study we sought to exploit PBMCs capability to induce angiogenesis widely described in literature in order to treat the typical vasculopathyrelated manifestations of SSc, in patients with no chance for lipofilling [21,59-61].
The selective filtration based technology we used produces an autologous cell concentrate wich contains PBMCs plus around 1% of CD34+ [13]. Despite that the efficiency in CD34+ hematopoietic stem cell enrichment with the use of Bone Marrow Point of Care system is comparable to the enrichment obtained by Pall Celeris system, several studies showed that limb salvage did not correlate to CD34+ concentration [16,48,62-64].
In addition to their angiogenic ability, PBMCs mechanism of action is based also on tissue regeneration due to immunemodulation and paracrine release of growth factors, cytokines and chemokines [19,20].
Monocytes give rise to mature macrophages which are also heterogeneous themselves, although the physiological relevance of this is not completely understood [65]. Macrophages display a wide range of phenotypes and physiological properties depending on the cytokines inducing their maturation [66]. They can adopt a variety of different phenotypes in response to different stimuli. Two of the best-characterized in vitro phenotypes are a proinflammatory “M1” phenotype, produced by exposure to IFN-γ and TNF-α and hypoxia, and an anti-inflammatory “M2” phenotype, produced by IL-4 or IL-13 [30]. M1 is the predominant population present during the first few days after injury, corresponding to the inflammatory and early proliferative phases, whereas M2 population is the primary effector of the later stages of repair or the later proliferative and remodeling phases. In fact, M2 are frequently termed “wound healing” macrophages, as they express factors that are important for tissue repair [30,67,68].
Macrophages interact with other cell types through paracrine factors to control reepithelialisation, angiogenesis and extracellular matrix remodelling, and several studies have analysed the difficulties in skin wound healing upon macrophage depletion [69-72]. Macrophages play key roles in tissue homeostasis and immune surveillance in response to microbial assault and are fundamental in promoting wound healing to repair damaged tissue [22,73]. Failure to resolve macrophage activation can lead to chronic inflammation and fibrosis, and ultimately to disease generation. PBMCs implant induces tissue regeneration through immune-modulation (M1 to M2 polarization) [22,51,74,75]. In particular PBMCs produced by selective filtration implanted perilesionally in non healing diabetic foot ulcers were able to polarize M1 to M2 phenotype inducing complete healing [51]. Activated M1 macrophages have been implicated in the pathogenesis of SSc [54,55]. Thererefore targeting therapeutic interventions directed against SSc inflammatory M1 macrophages may ameliorate inflammation and fibrosis [76].
PBMCs ability to induce the shift from M1 to M2 causing tissue regeneration could explain the results we obtained regarding the healing of the digital ulcers. We recorded a lower frequency of Raynaud’s phenomenon, together with a significant improvement in hand function. We also observed a mouth opening improvement in the two patients who received the treatment in this area. Furthermore, we observed a significant improvement of all the parameters evaluated, including a meaningful decrease of the HAQ-DI composite score.
Two aspects of this work have to be resolved in future studies: the low number of treated patients, and the fact that this is a preliminary and observational study lacking a control group. Despite this, the preliminary results on these critical patients, non-responders to standard therapies and with no chance for lipofilling, are encouraging. Our results are in agreement with the observations found in two case reports showing an improvement in vascularization and digital ulceration in SSc patients [77,78]. Results are also in line with suggestive result of PBMCs on others auto-immune disease like Thromboangitis Obliterans [16,77].
Conclusion
The great vasculogenic/angiogenic capacity of monocytes/macrophages together with their key role in immune-modulation have been demonstrated in numerous different clinical settings [79-85]. On the whole, evidence highlights M1 and M2 macrophages as important targets for immunotherapy [30]. In our preliminary study, PBMCs injection effectively increased all the primary outcomes of the study despite a low level of evidence due to the small sample size. These results suggest that PBMCs injection could represent a treatment option for SSc patients. The procedure we used, is easy and fast to perform, minimally invasive, not-operator dependent, safe and effective in treating mouth opening, Raynaud’s phenomenon, digital ulcers and hand movement impairment. Further studies are necessary to confirm these preliminary positive outcomes.
Declarations
Ethics approval and consent to participate
Patients agreed to participate in the study by signing a written informed consent.
The study was conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki. This study is part of a project approved by Sapienza University of Rome.
Consent for publication
Consent for publication was obtained from all the patients.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author, [S.C.]. The data are not publicly available because the containing information could compromise the privacy of the research participants.
Competing interests
The authors declare that they have no competing interests regarding the publication of this article.
Funding Statement
The authors received no specific funding for this work.
Authors’ contribution
All Authors read and approved the manuscript. S.C. enrolled the patients and was a contributor in treating the patients. She drafted and revised the manuscript. C.R. analyzed and interpreted the patient data regarding the Scleroderma, the treatement and the follow-up visits. She was a major contributor in writing the manuscript. D.R. read and approved the manuscript. M.G.O. performed the treatement and revised and approved the manuscript.
Acknowledgement
Not applicable.
Coral-Alvarado P, Pardo AL, Castaño-Rodriguez N, Rojas-Villarraga A, Anaya JM (2009) Systemic sclerosis: A world wide global analysis. Clin Rheumatol 28: 757-765. [ Ref ]
Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J (2008) Incidence and Prevalence of Systemic Sclerosis: A Systematic Literature Review. Semin Arthritis Rheum 37: 223-235. [ Ref ]
Gaubitz M (2006) Epidemiology of connective tissue disorders. Rheumatology (Oxford) 45(S3): 3-4. [ Ref ]
Jordan S, Maurer B, Toniolo M, Michel B, Distler O (2015) Performance of the new ACR/EULAR classification criteria for systemic sclerosis in clinical practice. Rheumatology (Oxford) 54: 1454-1458. [ Ref ]
Sobolewski P, Maślińska M, Wieczorek M, Łagun Z, Malewska A, et al. (2019) Systemic sclerosis – multidisciplinary disease: clinical features and treatment. Reumatologia/Rheumatology 57: 221-233. [ Ref ]
Giuggioli D, Manfredi A, Lumetti F, Colaci M, Ferri C (2018) Scleroderma skin ulcers definition, classification and treatment strategies our experience and review of the literature. Autoimmun Rev 17: 155-164. [ Ref ]
Song JI, Volz S, Liodaki ME, Mailander P, Kalousis K (2017) Stem cells therapy: the future in the management of systemic sclerosis? A case report. Hell J Nucl Med 20 Suppl: 164. [ Ref ]
Guillaume-Jugnot P, Daumas A, Magalon J, Sautereau N, Veran J, et al. (2016) State of the art. Autologous fat graft and adipose tissue-derived stromal vascular fraction injection for hand therapy in systemic sclerosis patients. Curr Res Transl Med 64: 35-42. [ Ref ]
Magalon G, Daumas A, Sautereau N, Magalon J, Sabatier F, et al. (2015) Regenerative Approach to Scleroderma with Fat Grafting. Clin Plast Surg 42: 353-364. [ Ref ]
Magalon J, Velier M, Simoncini S, François P, Bertrand B, et al. (2019) Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann Rheum Dis 78: 391- 398. [ Ref ]
Scuderi N, Ceccarelli S, Onesti MG, Fioramonti P, Guidi C, et al. (2013) Human Adipose-Derived Stromal Cells for Cell-Based Therapies in the Treatment of Systemic Sclerosis. Cell Transplant 22: 779-795. [ Ref ]
Onesti MG, Fioramonti P, Carella S, Fino P, Marchese C, et al. (2016) Improvement of Mouth Functional Disability in Systemic Sclerosis Patients over One Year in a Trial of Fat Transplantation versus Adipose- Derived Stromal Cells. Stem Cells Int 2016: 2416192. [ Ref ]
Spaltro G, Straino S, Gambini E, Bassetti B, Persico L, et al. (2015) Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. J Cytotherapy 17: 1302-1313. [ Ref ]
De Angelis B, Gentile P, Orlandi F, Bocchini I, Di Pasquali C, et al. (2015) Limb Rescue: A New Autologous-Peripheral Blood Mononuclear Cells Technology in Critical Limb Ischemia and Chronic Ulcers. Tissue Eng Part C Methods 21:423-435. [ Ref ]
Persiani F, Paolini A, Camilli D, Mascellari L, Platone A, et al. (2018) Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single-Center Experience. Ann Vasc Surg 53: 190-196. [ Ref ]
Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y, et al. (2009) Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv 2: 245-254. [ Ref ]
Patel AS, Smith A, Nucera S, Biziato D, Saha P, et al. (2013) TIE2- expressing monocytes/macrophages regulate revascularization of the ischemic limb. EMBO Mol Med 5: 858-869. [ Ref ]
Krishnasamy K, Limbourg A, Kapanadze T, Gamrekelashvili J, Beger C, et al. (2017) Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun 8: 952. [ Ref ]
Beer L, Mildner M, Gyöngyösi M, Ankersmit HJ (2016) Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 21: 1336-1353. [ Ref ]
Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M (2013) Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Experimental Cell Research 319: 1626-1634. [ Ref ]
Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, et al. (2018) Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 37: e97786. [ Ref ]
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9: 419. [ Ref ]
Li J, Tan J, Martino MM, Lui KO (2018) Regulatory T-cells: Potential regulator of tissue repair and regeneration. Front Immunol 9: 585. [ Ref ]
Stabile E, Kinnaird T, La Sala A, Hanson SK, Watkins C, et al. (2006) CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 113: 118-124. [ Ref ]
Hellingman AA, Zwaginga JJ, Van Beem RT, Hamming JF, Fibbe WE, et al. (2011) T-cell-pre-stimulated monocytes promote neovascularisation in a murine hind limb ischaemia model. Eur J Vasc Endovasc Surg 41: 418-428. [ Ref ]
Sharma A, Rudra D (2018) Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front Immunol 9: 883. [ Ref ]
Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y (2004) Defective vasculogenesis in systemic sclerosis. Lancet 364: 603-610. [ Ref ]
Fleming JN, Nash RA, McLeod DO, Florentino DF, Shulman HM, et al. (2008) Capillary regeneration in scleroderma: Stem cell therapy reverses phenotype? PLoS One 3: e1452. [ Ref ]
Avouac J, Wipff J, Goldman O, Ruiz B, Couraud PO, et al. (2008) Angiogenesis in systemic sclerosis: Impaired expression of vascular endothelial growth factor receptor 1 in endothelial progenitor-derived cells under hypoxic conditions. Arthritis Rheum 58: 3550-3561. [ Ref ]
Funes SC, Rios M, Escobar-Vera J, Kalergis AM (2018) Implications of macrophage polarization in autoimmunity. Immunology 154: 186-195. [ Ref ]
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, et al. (2013) 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 72: 1747-1755. [ Ref ]
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, et al. (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15: 202-205. [ Ref ]
LeRoy EC, Medsger TA (2001) Criteria for the classification of early systemic sclerosis. J Rheumatol 28: 1573-1576. [ Ref ]
Johnson SR, Hawker GA, Davis AM (2005) The health assessment questionnaire disability index and scleroderma health assessment questionnaire in scleroderma trials: An evaluation of their measurement properties. Arthritis Rheum 53: 256-262. [ Ref ]
Steen VD, Medsger Jr. TA (1997) The value of the health assessment questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 40: 1984-1991. [ Ref ]
Del Rosso A, Maddali-Bongi S, Sigismondi F, Miniati I, Bandinelli F, et al. (2010) The Italian version of the Hand Mobility in Scleroderma (HAMIS) test: evidence for its validity and reliability. Clin Exp Rheumatol 28(5 Suppl 62): S42-S47. [ Ref ]
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, et al. (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22: 2063-2077. [ Ref ]
Pilny E, Smolarczyk R, Jarosz-Biej M, Hadyk A, Skorupa A, et al. (2019) Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue. Stem Cell Res Ther 10: 93. [ Ref ]
Heo JS, Choi Y, Kim HO (2019) Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int 2019: 7921760. [ Ref ]
Xiaoning He, Zhiwei Dong, Yina Cao, Han Wang, Shiyu Liu, et al. (2019) MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int 2019: 7132708. [ Ref ]
Faulknor RA, Olekson MA, Ekwueme EC, Krzyszczyk P, Freeman JW, et al. (2017) Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2 transition. Technol (Singap World Sci) 5: 81-86. [ Ref ]
Moreno-Moral A, Bagnati M, Koturan S, Ko J-H, Fonseca C, et al. (2018) Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann Rheum Dis 77: 596-601. [ Ref ]
Kubo M, Li TS, Suzuki R, Shirasawa B, Morikage N, et al. (2008) Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Circ Physiol. 294: H590-H595. [ Ref ]
Kudo T, Kubo M, Katsura S, Nishimoto A, Ueno K, et al. (2014) Hypoxically preconditioned human peripheral blood mononuclear cells improve blood flow in hindlimb ischemia xenograft model. Am J Transl Res 6: 570-579. [ Ref ]
Liu YX, Guo XM, Li JF, Meng Y, Zhang HT, et al. (2014) Restoration of tissue damage, and never activity after hypoxia-ischemia by implantation of peripheral blood mononuclear cells. Brain Res 1546: 34-45. [ Ref ]
Vaibhav K, Braun M, Khan MB, Fatima S, Saad N, et al. (2018) Remote ischemic post-conditioning promotes hematoma resolution via AMPKdependent immune regulation. J Exp Med 215: 2636-2654. [ Ref ]
Navarro A, Marín S, Riol N, Carbonell-Uberos F, Miñana MD (2014) Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties. Stem Cell Res Ther 5: 50. [ Ref ]
Rigato M, Monami M, Fadini GP (2017) Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res 120: 1326-1340. [ Ref ]
Dubsky MD, Jirkovská A, Bem R, Nemcová A, Fejfarová V, et al. (2017) Cell therapy of critical limb ischemia in diabetic patients-State of art. Diabetes Res Clin Pract 126: 263-271. [ Ref ]
Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, et al. (2013) Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev 29: 369-376. [ Ref ]
Di Pardo A, Cappello E, Pepe G, Marracino F, Carrieri V, et al. (2017) Infusion of autologous-peripheral blood mononuclear cells : a new approach for limb salvage in patients with diabetes. 7th International Diabetic Foot Congress abu Dhabi. IFD Congress Abu Dhabi 4-8 December 2017, Abu Dhabi. [ Ref ]
Colonna MR, Flavia L, Gabriele D, et al. (2016) Regenerative Approaches in Wound Healing : New Alternatives for Older Tools. In: IntechOpen, editor. Wound Healing- New Insight into Ancient Challenges. Intech 155-163. [ Ref ]
Palermo C, Sanfiorenzo A, Trigona C, Bernardini G, Veroux P (2018) LEA 15. Role of Monocytes in the Treatment of Chronic Limb Ischemia and “Hard to Heal” Ulcers. J Vasc Surg 68: E119-E120. [ Ref ]
Fung E, Helisch A (2012) Macrophages in collateral arteriogenesis. Front Physiol 3: 353. [ Ref ]
Lee C, Schlereth SL, Park EY, Emami-Naeini P, Chauhan SK, et al. (2014) A Novel Pro-Angiogenic Function for Interferon-Y-Secreting Natural Killer Cells. Investig Opthalmology Vis Sci 55: 2885-2892. [ Ref ]
Liang C, Yang KY, Chan VW, Li X, Fung THW, et al. (2020) CD8+ T-cell plasticity regulates vascular regeneration in type-2 diabetes. Theranostics 10: 4217-4232. [ Ref ]
Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih T-Y, White D, et al. (2019) Treating ischemia via recruitment of antigen-specific T cells. Sci Adv 5: eaav6313. [ Ref ]
Van Weel V, Toes REM, Seghers L, Deckers MML, De Vries MR, et al. (2007) Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol 27: 2310-2318. [ Ref ]
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, et al. (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116: 829-840. [ Ref ]
Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, et al. (2013) Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation 127: 710-719. [ Ref ]
Jalees R, Jingling L (2003) Peripheral Blood “Endothelial Progenitor Cells” Are Derived From Monocyte/Macrophages and Secrete Angiogenic Growth Factors. Circulation 107: 1164-1169. [ Ref ]
Prochazka V, Gumulec J, Jaluvka F, Salounova D, Jonszta T, et al. (2010) Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant 19: 1413-1424. [ Ref ]
Liotta F (2018) Therapeutic Efficacy of Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors in Patients With Critical Limb Ischemia ―THE SCELTA TRIAL. Circ J 82: 1688-1698. [ Ref ]
Dong Z, Fang Y, Pan T, Liu H, Wei Z, et al. (2019) Autotransplantation of Purified CD34+Cells for Critical Limb Ischemia Caused by Buerger Disease. Cytotherapy 21(5S): S55. [ Ref ]
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953-964. [ Ref ]
Rodero MP, Khosrotehrani K (2010) Skin wound healing modulation by macrophages. Int J Clin Exp Pathol 3: 643-653. [ Ref ]
Novak ML, Koh TJ (2013) Macrophage phenotypes during tissue repair. J Leukoc Biol 93: 875-881. [ Ref ]
Wynn TA, Vannella KM (2016) Review Macrophages in Tissue Repair, Regeneration, and Fibrosis. 2016. Immunity 44: 450-462. [ Ref ]
Rodero MP, Licata F, Poupel L, Hamon P, Khosrotehrani K, et al. (2014) In vivo imaging reveals a pioneer wave of monocyte recruitment into mouse skin wounds. PLoS One 9: e108212. [ Ref ]
Rodero MP, Legrand JMD, Bou-Gharios G, Khosrotehrani K (2013) Wound-associated macrophages control collagen 1α2 transcription during the early stages of skin wound healing. Experimental Dermatology 22: 143-145. [ Ref ]
De La Durantaye M, Piette AB, Van Rooijen N, Frenette J (2014) Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res 32: 279-285. [ Ref ]
Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, et al. (2010) Differential Roles of Macrophages in Diverse Phases of Skin Repair. J Immunol 184: 3964-3977. [ Ref ]
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496: 445-455. [ Ref ]
Arnold L, Henry A, Poron F, Baba-Amer Y, Van Rooijen N, et al. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057-1069. [ Ref ]
Italiani P, Boraschi D (2014) From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front Immunol 5: 514. [ Ref ]
Toledo DM, Pioli PA (2019) Macrophages in Systemic Sclerosis: Novel Insights and Therapeutic Implications. Curr Rheumatol Rep 21: 31. [ Ref ]
Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, et al. (2007) Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford) 46:882-884. [ Ref ]
Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, et al. (2009) Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford) 48: 61-64. [ Ref ]
Julier Z, Park AJ, Briquez PS, Martino MM (2017) Promoting tissue regeneration by modulating the immune system. Acta Biomater 53: 13-28. [ Ref ]
Oishi Y, Manabe I (2018) Macrophages in inflammation, repair and regeneration. Int Immunol 30: 511-528. [ Ref ]
Chazaud B (2014) Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 219: 172-178. [ Ref ]
Ogle ME, Segar CE, Sridhar S, Botchwey EA (2016) Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood) 241: 1084-1097. [ Ref ]
Spiller KL, Koh TJ (2017) Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev 122: 74-83. [ Ref ]
Godwin JW, Pinto AR, Rosenthal NA (2017) Chasing the recipe for a proregenerative immune system. Semin Cell Dev Biol 61: 71-79. [ Ref ]
Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, et al. (2020) An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577: 405-409.. [ Ref ]