Journal Name: Journal of Biomedical Research and Reviews
Article Type: Research
Received date: 05 May, 2019
Accepted date: 08 July, 2019
Published date: 2021-03-21
Citation: Olatunde OZ, Yang Yang Y, Yong J, Lu C (2019) The progress of Chemical Constituents Isolated from the Root of Actinidia chinensis Planch and their Biological Activities. J Biomed Res Rev Vol: 2, Issu: 2 (12-21).
Copyright: © 2019 Olatunde OZ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The Chinese traditional plant medicine Actinidia chinensis Planch roots has ever been widely used in clinical for its wide spectrum of biological activities, such as antitumor, antioxidant, antiviral, detoxifying, antidiuretic, anti-hepatitis and haemostatic activities, which owe to the different bioactive compounds (flavonoids, steroids, alkaloid, organic acids and polysaccharides) in the roots of Actinidia chinensis Planch. In this review, we mainly summarize the phytochemical progress as well as biological activities of A. chinensis root, to provide the reference for its further research and relevant knowledge towards drug development.
Keywords
Actinidia chinensis, chemical constituents, biological activities.
Abstract
The Chinese traditional plant medicine Actinidia chinensis Planch roots has ever been widely used in clinical for its wide spectrum of biological activities, such as antitumor, antioxidant, antiviral, detoxifying, antidiuretic, anti-hepatitis and haemostatic activities, which owe to the different bioactive compounds (flavonoids, steroids, alkaloid, organic acids and polysaccharides) in the roots of Actinidia chinensis Planch. In this review, we mainly summarize the phytochemical progress as well as biological activities of A. chinensis root, to provide the reference for its further research and relevant knowledge towards drug development.
Keywords
Actinidia chinensis, chemical constituents, biological activities.
Introduction
Actinidiaceae family has over fifty-four species, which are distributed mostly in tropical provinces of China [1,2]. It reported that Actinidiaceae family is rich in compounds of triterpenoids, anthraquinones, alkaloid, organic acids, flavonoids, steroids and polysaccharides [3-5]. Actinidia chinensis Planch roots commonly called Tengligen, used as Chinese folk medicine for treatment of tumors, such as liver cancer, gastric cancer and esophagus cancer [6-11] . In addition, it was also used as detoxifying, antidiuretic, anti-hepatitis and haemostatic agent [12], because A. chinensis roots is rich in these bioactive compounds [5,13-15], which possess a wide spectrum of biological activities listed above.
This review mainly focus on the phytochemicals of the A. chinensis roots as well as biological activities of extractions, pure compounds obtained from this folk plant medicine, to provide the reference for its further research and relevant knowledge towards drug development.
Chemical constituents
Different kinds of constituents, including triterpenoids, anthraquinones, alkaloids, organic acids, flavonoids, steroids and polysaccharides in roots of A. chinensis have been isolated and reported, which are classified below.
Triterpenoids
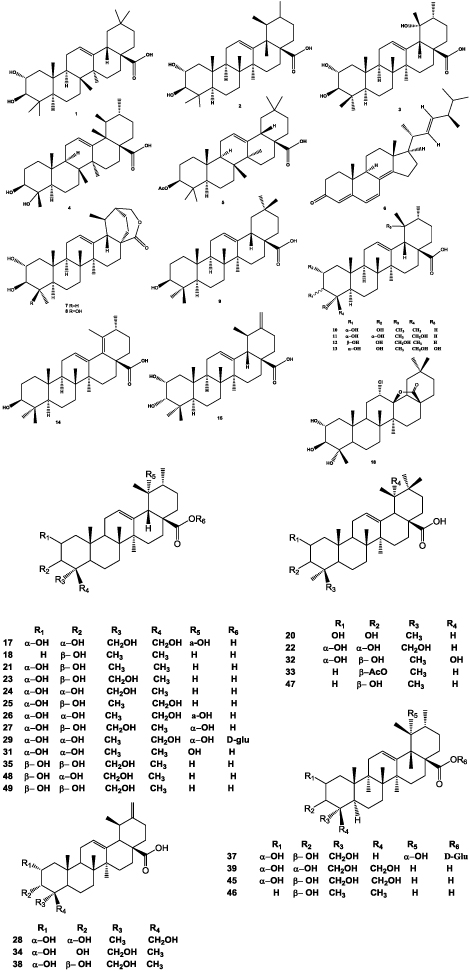
Triterpenoids are predominantly presence in the family of Actinidia species [16]. However, there are few triterpenoids reported to be presence in the A. chinensis root [13]. Six triterpenoids were also detected from the same part of this plant medicine, which were identified as 2α-hydroxyoleanolic acid (1), 2α-hydroxyursolic acid (2), euscaphic acid (3), 23-hydroxyursolic acid (4), 3β-O-acetylursolic acid (5) and ergosta-4,6,8,(14),22-tetraen-3-one (6) [17]. In addition, 2α,3β-dihydroxyurs-12-en-28,30-olide (7) and 2α,3β,24-trihydroxyurs-12-en-28,30-olide (8) with other eight known compounds, such as 12-en-28-oic acids of oleanane or ursane type triterpenoids including oleanolic acid (9), 3-epi-corosolic acid (10), 2α,3α,24-trihydroxyurs- 12-en-28-oic acid (11), 23-hydroxyursolic acid (4), asiatic acid (12), 2α,3α,19,24-tetrahydroxyurs-12-en-28-oic acid (13), 3β-hydroxyurs-12,18-dien-28-oic acid (14) and 2α,3α,23-trihydroxyursa-12,20,(30)-dien-28-oic acid (15) were also isolated and reported. Among them, compounds 2α,3β-dihydroxyurs-12-en-28,30-olide (7) and 2α,3β,24- trihydroxyurs-12-en-28,30-olide (8) were reported as the new compounds [16].
Xu et al. [18] isolated 12α-chloro-2α,3β,13β, 23-tetrahydroxyolean-28-oic acid-13-lactone (16) and 2α,3α,19α,23,24-pentahydroxyurs-12-en-28-oic acid (17) and reported as new triterpenoids with ursolic acid (18), pseudotaraxasterol (19), 2α,3β-dihydroxyolean- 12-en-28-oic acid (20), 2α,3β-dihydroxyurs-12-en-28- oic acid (21), 11, 2α,3α,24-trihydroxyolean-12-en-28-oic acid (22), 2α,3β,23-trihydroxyurs-12-en-28-oic acid (23), 2α,3α,23-trihydroxyurs-12-en-28-oic acid (24), 2α,3β,24- trihydroxyurs-12-en-28-oic acid (25), 2α,3α,19α,24- tetrahydroxyurs-12-en-28-oic acid (26), 2α,3β,19α,23- tetrahydroxyurs-12-en-28-oic acid (27), 2α,3α,24- trihydroxyurs-12,20(30)-dien-28-oic acid (28) and 2α,3α,19α,24-tetrahydroxyurs-12-en-28-oic acid 28-O-β- D-glucopyranoside (29) from the root of A. chinensis [18]. ɣ-quinide (30), 10, 18 were discovered from A. chinensis. ɣ-quinide (30) was isolated for the first time in this work [19].
Peng et al. [20] reported that the total contents of oleanolic acid (9) and ursolic acid (18) in roots of A. chinensis were 381.9 and 427.4 ng/g respectively. 2α,3α,19-trihydroxyurs-12- en-28-oic acid (31), 2α,3β-dihydroxyurs-12-en-28-oic acid (21), 2α, 3α,23-trihydroxyurs-12-en-28-oic acid (24), asiatic acid (12), ursolic acid (18), 2α,3α,19,24-tetrahydroxyurs- 12-en-28-oic acid (13), 2α,3β,19-trihydroxyolean-12-en- 28-oic acid (32), 2α, 3α, 24-trihydroxyolean-12-en-28-oic acid (22), oleanolic acid (9), 3β-O-acetyloleanolic acid (33), 2α,23-dihydroxylmicromeric acid (34) were isolated from roots of A. chinensis [21].
Cheng et al. [10] isolated 2β, 3β, 23-trihydroxyurs-12- ene-28-olic acid (35) from A. chinensis Radix. (2α, 3β)- 2,3,23-trihydroxyurs-13(18)-en-28-oic acid (36) (new discovered compound) and some known compounds such as 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oic acid 28-O-β-Dglucopyranoside (37), 2α,3β,23-trihydroxyursa-12,20(30)- dien-28-oic acid (38), 2α,3α,23,24-tetrahydroxyurs-12- en-28-oic acid (39) were also reported by others [25]. Jacoumaric acid (40) was isolated for the first time from the roots of A. chinensis [22]. Fupenzic acid (41), spathodic acid- 28-O-β-D-glucopyranoside (42), 12-diene-30-oic acid (43) and 3-β-(2-carboxybenzoyloxy) oleanolic acid (44) were isolated and reported [24].
Wei et al. [25] isolated sixteen triterpenoids from EtOH extract of A. chinensis roots, which included compounds 11, 15, 20, 23, 24, 26, 27, 28, 38, 39 reported above and 2α,3β,23,24-tetrahydroxyurs-12-en-28-oic acid (45), 3β-hydroxyurs-12-en-28-oic acid (46), 3β-hydroxyolean- 12-en-28-oic acid (47), 2β,3α,23-trihydroxyurs-12-en-28- oic acid (48), 2β,3β,23-trihydroxyurs-12-en-28-oic acid (49) (Figure 1).
Figure 1:
Structures of triterpenoids isolated from the roots of A. chinensis.
Flavonoids
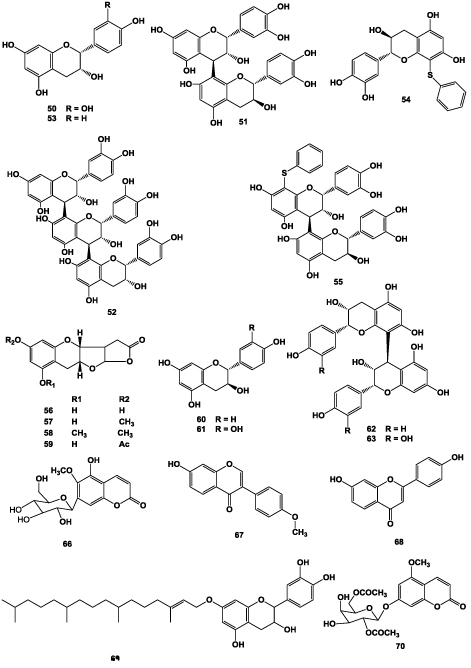
Michaud [26] reported the presence of (-)-epicatechin (50), its dimer (procyanidol B2 (51)) and trimer (procyanidol C1 (52)), (-)-epi-afzelechin (53), benzylthio- (-)-epi-catechin (54), 4′- benzylthioprocyanidol B2 (55) in the root of A. chinensis Planch. Twelve compounds were discovered from the EtOH extract of the root of A. chinensis, which were classified into planchols A-D (56- 59) and isomeric flavanoids ((-)-epi-afzelechin (53), (-)-epi-catechin (50), (+)-afzelechin (60), (+)-catechin (61), (-)-epi-afzelechin(4β→8)(-)-epi-afzelechin (62), (-)-epicatechin( 4β→8)(-)-catechin (63), (+)-afzelechin(4α→8) (+)-afzelechin (64), (+)-catechin(4α→8)(+)-catechin (65) [13]. (+)-catechin (61), (-)-epi-catechin (50) and 5-Hydroxy- 6-methoxy-7-O- β -D-glucosyl coumarin (66) were isolated with other compounds [4]. Chen et al. [19] also reported the presence of (-)-epi-catechin (50) in the root of A. chinensis. Formononetin (67) and 7,4’-dihydroxyflavone (68) were isolated from A. chinensis root, 7,4’-dihydroxyflavone (68) reported to be firstly obtained from this medicinal plant [27]. (+)-catechins-7-phytol (69), 5-methoxy-coumarin-7- β-D-glycosidase (70), (+)-catechins (61) were isolated with other four triterpenoids EtOAc extract of the root bark of A. chinensis [24] (Figure 2).
Figure 2: Structures of flavonoids isolated from the roots of A. chinensis.
Glucosides
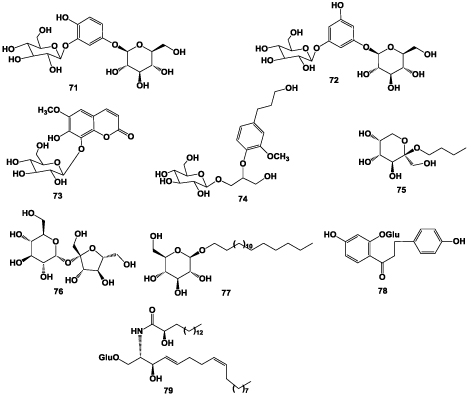
Tachioside (71), Isotachioside (72), Fraxin (73), 1-O-(β- D-glucosyl)-2-[2-methoxy-4-(ω-hydroxypropyl)-phenoxy]- propan-3-ol (74) and n-butyl-β-D-fructopyranoside (75) were reported to be presence in A. chinensis [4]. Chen et al. [19] isolated sucrose (76), n-butyl-β-D-fructopyranoside (75) and stearyl-β-D-glucopyranoside (77) from the same part of this plant. 4,4′-dihydroxyl-dihydrochalcone-2’- O-β-D-glucopyranoside (78) [18, 22] and sphingolipid (79) [25]. Liu et al. [28] isolated and identified acetylated monosaccharides from A. chinensis by gas chromatographymass spectrometry. Ding et al. [29] obtained polysaccharide liposomes in A. Chinensis root by preparative HPLC (Figure 3).
Figure 3: Structures of glucosides isolated from the roots of A. chinensis.
Anthraquinones
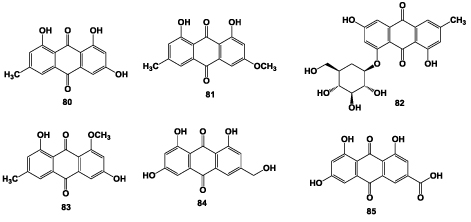
Ji and Liang [30] isolated the emodin (80), emodin-6- methyl ether (81), emodin 8-β-D- glucoside (82), emadin 8-methyl ether (83), ω-hydroxyemodin (84) and emodic acid (85) from the roots A. chinensis (Figure 4).
Figure 4: Structures of anthraquinones isolated from the roots of A. chinensis.
Steroids
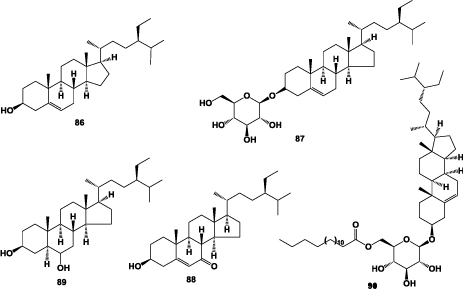
Few steroids were reported to be presence in A. chinensis roots, β-sitosterol (86) [4,17,19,30], daucosterol (87) [4,19,27], 3β-hydroxystigmast-5-en-7-one (88) [22], Stigmastane-3β,6α-diol (89) and sitoindoside I (90) [23] (Figure 5).
Figure 5: Structures of steroids isolated from the roots of A. chinensis.
Essential oils
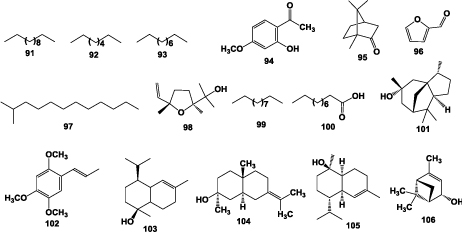
Yu et al. [31] reported forty one compounds from the roots of A. chinensis, in which dodecane (91) (29.39%), octane (92) (5.16%), decane (93) (2.94%), paeonal (94) (2.81%), camphor (95) (2.77%), furfural (96) (2.59%), methyldodecane (97) (2.45%), linalool oxide (98) (2.10%), undecane (99) (2.16%), n-decanoic acid (100) (2.64%), cedrol (101) (1.98%), asarone (102) (1.96%), tau-muurolol (103) (1.79%), juniper camphor (104) (1.52%), δ-cadinol (105) (1.33%), and verbenol (106) (1.06%) were the major constituents (Figure 6).
Figure 6: Structures of representative essential oils isolated from the roots of A. chinensis.
Other components
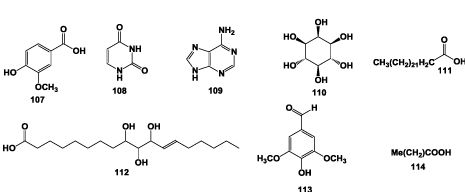
Vanillic acid (107), uracil (108), adenine (109) and myoinositol (110) were also isolated and identified from the root of A. chinensis [4]. Chen et al. [19] isolated lignoceric acid (111) from the root of A. chinensis. (Z)-9,10,11-trihydroxy-12-octadecenoic acid (112) and syringaldehyde (113) were isolated from the same part [27] and dotriacontanic acid (114) was also reported to be presence in roots of A. chinensis [22] (Figure 7).
Figure 7: Structures of others isolated from the roots of A. chinensis.
Biological Activities
Antitumor activity
Lin [32] reported that the polysaccharide isolated from A. chinensis exhibited anit-tumor effect. Chang and Case [13] studied the cytotoxicity of phenolic constituents of A. chinensis root, the results showed compounds (56-57) showed significant cytotoxic effect against leukaemia P-388 and adenocarcinomic human alveolar basal epithelial (A549) cell lines. It reported that 2α,3β-dihydroxyurs-12-en-28- oic acid (21) exhibited weaker cytotoxicity against LOVO (IC50 =2.9 μg/mL) and HepG2 (IC50=9.2 μg/mL) cell lines, and 2α,3β-dihydroxyolean-12-en-28-oic acid (20) showed weaker cytotoxicity against LOVO (IC50 = 6.0 μg/mL) cell line [20]. The round extraction of A. chinensis root showed potential inhibition to proliferation of carcinoma such as colon cancer and gastric cancer, leukemia and hepatic cancer [7, 37].
Zhang and Han [34] reported that the polysaccharide of A. chinensis root can reduce the H22 tumor cell proliferation, with the inhibition rate (68.5%) at the highest dose and reduce the Proliferating Cell Nuclear Antigen (PCNA) of 51.20 %. It is reported that the roots of A. chinensis usually used as crude drug in China for the treatment of all kinds of cancer [35], while polysaccharide derived from A. chinensis root inhibited the growth of human gastric cancer SGC-7901 adenocarcinoma cells and induced apoptosis [36].
Cheng et al. [10] evaluated 2β, 3β, 23-trihydroxy-urs-12- ene-28-olic acid (35) against the lung cancer cell lines and it can trigger the apoptosis through blocking the activation of Nuclear factor- κB expression level and enhancing the activation of IκBα in NCI-H460 cells. The results showed that this compound can be regarded as the candidate for the treatment of lung cancer.
Xu et al. [22] studied the rude extraction and six main isolated compounds from A. chinensis root against P450 enzyme. The results showed that 12α-chloro 2α,3β,13β,23-tetrahydroxyolean-28-oic acid-13-lactone (16), 2α,3β-dihydroxyurs-12-en-28-oic acid (21), 2α,3α,24-trihydroxyurs-12-en-28-oic acid (11), 2α,3α,24- trihydroxyolean-12-en-28-oic acid (22), and 2α,3β,23- trihydroxyurs-12-en-28-oic acid (23) exhibited distinct inhibition to cytochrome P450 enzyme. While 12α-chloro- 2α, 3β, 13β, 23-tetrahydroxyolean-28-oic acid-13-lactone (16) showed a significant inhibitory effect on the CYP3A4. The rude EtOH extraction of the root of A. chinensis showed strong inhibition against SW480 cells and could trigger apoptosis of tumor cell in vitro [37].
Wang et al. [38] obtained the total triterpenoids from the A. chinensis root and evaluated its inhibition to the proliferation rates of human colon cancer SW480 cells of colorectal carcinoma and LOVO cells, human hepatoma SMMC -7721 cells and human breast cancer MCF -7 cells. The results showed that the total triterpenoids inhibited cancer cells proliferation at Gap (G) and Synthesis (S) phase. While the rude extraction from the root of A. chinensis possessed anti-tumor efficacy and could significantly inhibited the cholesterol metabolism in hepatocellular carcinoma cell lines by enhancing Proprotein convertase subtilisin/ Kexin type 9 (PCSK9) expression and decreasing Low- Density Lipoprotein Receptor (LDLR) expression at posttranscriptional level [39].
Wei et al. [25] investigated the cytotoxicity of pure compounds isolated from the root of A. chinensis to HepG2, A549, MCF-7, SK-OV-3 and Hela cell lines. The results showed that 2α, 3α, 23,24-tetrahydroxyursa-12,20(30)- dien-28-oic acid (15) is the most active compound to these five cancerous cell lines with IC50s: 19.62 ± 0.81, 18.86 ± 1.56, 45.94 ± 3.62, 62.41 ± 2.29 and 28.74 ± 1.07 μM, respectively.
Hou et al. [40] tested the rude extraction from the roots of A. chinensis against Hepatocellular Carcinoma (HCC). The results revealed that the extraction inhibited HepG2 proliferation by affecting the S-phase cell cycle. Fang et al. [41] studied the extraction of A. chinensis root anticancer effect and the results showed that the extraction could significantly inhibit the proliferation, migration, clonality, invasion and epithelial mesenchymal transition of hepatocellular carcinoma. It also promoted the apoptosis of Hepatocellular Carcinoma (HCC) cells by cellular decrease DLX2 expression. Lv et al. [42] validated that the extraction of A. chinensis root could significantly inhibit lung cancer cell proliferation and metastasis via phosphatidylinositol 3-kinase/oligoadenylate synthetase L (PI3K/OASL) signal pathway.
Hepatoprotective activity
Some bioactive compounds in A. chinensis root possess hepatoprotective activity [9]. Asiatic acid (12) and ursolic acid (18) have been studied and the results showed that which possessed good hepatoprotective efficacy [43-44], these compounds are of much content in A. chinensis root. Zhou et al. [18] reported that 2α, 3β-dihydroxyurs-12- en-28,30-olide (7) and 2α,3β,24-trihydroxyurs-12-en- 28,30-olide (8) isolated from A. chinensis showed strong hepatoprotective activity. In China, oleanolic acid (9) has been used as clinical drug for treatment of chronic liver and human acute diseases [45], while the oleanolic acid is also of much content in A. chinensis root, which indicate that A. chinensis root can also be used for treatment of chronic liver and human acute diseases.
Antiviral activitiy
Zhang et al. [24] tested some compounds isolated from A. chinensis root for their antiviral activity and the results showed that spathodic acid-28-O-β-D-glucopyranoside (42) and 5-methoxy-coumarin-7-β-D-glycosidase (70) exhibited the higher anti-phytoviral activity with inactivation effect range from 56.76% to 42.56% than other isolated compounds, including ningnanmycin (used as a control).
Antioxidant activity
Yang and He [46] revealed that the extract from the root of A. chinensis could remove the DPPH, superoxide anion radicals effectively and strongly inhibit the hydroxyl radicals production.
Antiangiogenic activities
Zhu et al. [21] evaluated the bioactive compounds isolated from the root of A. chinensis to the human umbilical vein endothelial cells (HUVEC) proliferation and tube formation. The results revealed that 2α,3β-dihydroxyurs-12-en-28-oic acid (21), 2α,3α,23-trihydroxyurs-12-en-28-oic acid (24), asiatic acid (12) and 2α,3α,24-trihydroxyolean-12-en-28-oic acid (22) exhibited strong antiangiogenic effect.
Conclusion
Extractions and some pure compounds from A. chinensis root have showed effective biological activities such as antitumor, antioxidant, antiviral, detoxifying agent, antidiuretic, anti-hepatitis and haemostatic agent in agreement with folkloric uses of the root of this medicinal plant. Studies have revealed that triterpenoids are abound in the root of A. chinensis, which are probably responsible for its anticancer capacity. To ascertain this hypothesis, further analysis and research should be carried out. For example, more and more pure compounds should be isolated from the root of A. chinensis and their biological evaluation should be studied, in vitro and in vivo antitumor efficacy and the mechanisms to provide the evidence for development of new drug or drug candidates.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21875252) and the Project of The Plan of Xiamen Science and Technology (3502ZCQ20171000).
There are no references