Journal Name: Journal of Cardiovascular Disease and Medicine
Article Type: Review
Received date: 19 September, 2018
Accepted date: 08 October, 2018
Published date: 12 October, 2018
Citation: Das BK (2018) Assessment of Myocardial Perfusion and Viability-Basic Requirement for Optimal Cardiac Care. J Cardiovas Disea Medic Vol: 1, Issu: 1 (59-62).
Copyright: © 2018 Das BK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Prevalence of coronary artery disease like angina, heart attack and heart failure are rampant and appear to increase fast in our country. Coronary heart disease (CHD) is the major cause of heart failure. Severe left ventricular (LV) dysfunction was considered an irreversible condition, as regional akinesia was thought to represent infarcted or necrotic myocardial tissue. It is now understood that, among patients with ischemic cardiomyopathy, LV systolic dysfunction can result from myocardial necrosis and remodeling as also from myocardial hibernation, or repetitive myocardial stunning. While myocardial necrosis is irreversible, systolic dysfunction resulting from hibernation and stunning are potentially reversible states of ventricular dysfunction. Clinical studies have also shown that viable myocardium can be demonstrated in a substantial number of patients with CHD and LV dysfunction, even in the absence of angina. In patients with significant amounts of viable myocardium, LV function may improve markedly, and even normalize, following successful revascularization.
Keywords
Coronary heart disease, Severe left ventricular, cardiomyopathy, Cardiac Care, Myocardium.
Abstract
Prevalence of coronary artery disease like angina, heart attack and heart failure are rampant and appear to increase fast in our country. Coronary heart disease (CHD) is the major cause of heart failure. Severe left ventricular (LV) dysfunction was considered an irreversible condition, as regional akinesia was thought to represent infarcted or necrotic myocardial tissue. It is now understood that, among patients with ischemic cardiomyopathy, LV systolic dysfunction can result from myocardial necrosis and remodeling as also from myocardial hibernation, or repetitive myocardial stunning. While myocardial necrosis is irreversible, systolic dysfunction resulting from hibernation and stunning are potentially reversible states of ventricular dysfunction. Clinical studies have also shown that viable myocardium can be demonstrated in a substantial number of patients with CHD and LV dysfunction, even in the absence of angina. In patients with significant amounts of viable myocardium, LV function may improve markedly, and even normalize, following successful revascularization.
Keywords
Coronary heart disease, Severe left ventricular, cardiomyopathy, Cardiac Care, Myocardium.
Introduction
Prevalence of coronary artery disease like angina, heart attack and heart failure are rampant and appear to increase fast in our country. Coronary heart disease (CHD) is the major cause of heart failure. According to the WHO, the South Asian region has one of the highest cardiovascular mortality rates in the world [1]. An increasing high prevalence of CHD has been reported in India, varying from 1% -2% in 1960s to 8%-10% in late 1990s [2].
Living myocardium is characterized by preserved ventricular wall thickness, the presence of contractile reserve, cell membrane integrity, active myocyte metabolism and the existence of blood perfusion. Diagnostic techniques for studying myocardial viability are based on detecting one or more of these markers. In the past, severe left ventricular (LV) dysfunction was considered an irreversible condition. It is now understood that, among patients with ischemic cardiomyopathy, LV systolic dysfunction can result from myocardial necrosis and remodeling, myocardial hibernation, or repetitive myocardial stunning. While myocardial necrosis is irreversible, systolic dysfunction resulting from hibernation and stunning are potentially reversible states of ventricular dysfunction. The degree of contractile impairment remains strongly under the influence of the severity and duration of the ischemic event, with irreversible myocardial necrosis representing the end pathway of prolonged and significant coronary ischemia [3]. Hence, the primary priority in the management of acute coronary syndromes is to limit the extent of myocardial necrosis via reperfusion therapies. Most patients with chronic heart failure have an admixture of all three patho-physiologic entities namely myocardial necrosis and remodeling, myocardial hibernation or repetitive myocardial stunning.
Clinical studies have shown that viable myocardium can be demonstrated in a substantial number of patients with CHD and LV dysfunction, even in the absence of angina. Revascularization can improve prognosis in patients who have evidence of ischemic but viable myocardium. Observational studies have suggested that the presence of viable myocardium is directly associated with favorable progress of left ventricular function and good prognosis after revascularization. Patients who seem to benefit more from surgical revascularization are those with ischemic symptoms and severe left ventricular dysfunction [4].
An estimated 20 to 40 percent of patients with chronic ischemic LV dysfunction have the potential for significant improvement in LV function after revascularization Therefore, optimal assessment of myocardial viability is important [5]. Prospective delineation of viable from nonviable myocardium in patients with coronary artery disease is also an important factor in deciding whether a patient should be re-vascularized or treated medically.
Dobutamine stress echocardiography provides information on the contractile reserve and cardiac magnetic resonance imaging can delineate the trans-mural extent of scar [6]. In general, nuclear imaging techniques have a higher sensitivity for the detection of myocardial viability, whereas techniques evaluating contractile reserve display a lower sensitivity, may be with a higher specificity [7].
Nuclear Medicine Procedures
Two common techniques like single-photon emission computed tomography (SPECT) and positron emission computed tomography (PET) are used in nuclear medicine with various radiopharmaceuticals for the detection of myocardial viability.
Thallium-201 (201Tl) and technetium-99m (99mTc) labeled Sestamibi/Tetrophosmin are the common radiopharmaceuticals used in different protocols for myocardial perfusion SPECT studies whereas Fuoride-18 Deoxy glucose (18FFDG) and Rubidium-82 (82Rb) are most widely used in PET.
Use of Thallium for myocardial perfusion and viability studies have declined or have even been obsolete due to poor physical imaging qualities of the radio isotope (Thallium-201) and prolonged study periods.
Since PET-CT facilities are now available in many Nuclear Medicine Departments, it has been possible to conduct combination of myocardial SPECT studies using Te99m agents and PET-CT for better assessment of myocardial viability [8]. As mentioned above reliable information about viability is vital for appropriate and evidence-based management of many of the cardiac disorders.
What is Myocardial Viability
Contractile function is the hallmark of myocardium. Myocardial contractile dysfunction represents hibernating or stunned myocardium. Hibernating myocardium has depressed myocardial contractility at rest due to persistently impaired coronary blood flow [9]. The function can be partially or completely restored by improving coronary blood flow, by providing inotropic stimulation or by reducing oxygen demand. Several studies have shown that hibernating myocardium results from reduced myocardial blood flow. It is metabolically characterized by a switch from fat to glucose metabolism and accompanied by a reactivation of the fetal gene program. Myocardial territories that have normal blood flow at rest can also demonstrate depressed cardiac function if they undergo recurrent ischemic episodes with stress in a process known as repetitive myocardial stunning. These territories also improve with revascularization.
Viable myocardium thus can be defined as dysfunctional myocardium that resumes full contractile function if adequate blood supply is restored.
Methods of Assessment of Viability
Multiple imaging modalities exist for differentiating viable myocardium from scar in territories with contractile dysfunction. With the multiple modalities available, choosing the best modality for a specific patient can be at times difficult. The methods vary from echocardiography (Echo), cardiac magnetic resonance imaging(cMRI) nuclear imaging with single photon emission tomography (SPECT) and positron emission tomography (PET) imaging and cardiac computed tomography (CCT). Dobutamine echocardiography and MRI rely on identifying the property of contractile reserve in hibernating myocardium in response to low-dose inotropic agents. Cardiac magnetic resonance imaging (cMRI) and cardiac CT can assess the transmural extent of scar. This technology depends on an intact cellular membrane to prevent the extracellular contrast agent, Gadolinium/ iodinated contrast, from entering cells and thus allowing the Gadolinium/iodinated contrast to concentrate in areas of increased interstitial space. The ideal test for viability assessment would be easy availability, simple to perform, reasonably inexpensive, and safe with limited side effects. It would be reproducible and free from artifacts and also successfully differentiate patients who would benefit from revascularization from those who would not.
Nuclear medicine studies, including SPECT and PET rely on intact cellular membranes for active uptake of radiotracers like Thallium-201, intact sarcolemmal function to maintain electrochemical gradients across the cell membrane for radiotracer retention of Technetium-99m labeled compounds and intact glucose uptake [Fluorine-18- labeled deoxyglucose (FDG)]. Modalities that depend on cell membrane function, a process that occurs early in the underperfused state, show a low likelihood of recovery following revascularization if viability is not present (high sensitivity), while modalities that use contractile function, a change that occurs later in the under-perfused state, show a high likelihood of functional recovery if viability is present (high specificity). Commonly used technetium compounds include Tc 99m sestamibi, Tc 99m tetrofosmin, both are lipophilic compounds with considerable cardiac affinity, whose distribution in the myocardium is directly proportional to the regional coronary flow. Uptake of Tc 99m compounds depend on sarcolemmal integrity, mitochondrial function and an intact energy production pathway. Tc has a shorter half-life (6 h) than thallium (73 h) that allows for larger dose administration. To enhance the ability of technetium SPECT imaging to detect viability, several methods have been used, e.g. quantitation of uptake and using ectrocardiogram (ECG) gating and addition of agents, like nitrates and trimetazidine to enhance blood flow. The polar map of myocardial viability obtained by gated SPECT imaging accurately predicted functional recovery after coronary revascularization [10].
The biggest advantage of SPECT is that there is extensive clinical experience as well as a wealth of studies demonstrating reliability and reproducibility. SPECT imaging is widely available, easy to perform, and highly reproducible. Rest and stress perfusion scans can also be performed on the same day using the appropriate protocol. Apart from the magnitude of stress-induced ischemia, global LV systolic function, and LV volumes can also be assed. These factors influence post-revascularization recovery of function and determine patient management.
Measurement of myocardial perfusion can be assessed using Rubidium-82 or N-13 ammonia at rest and with pharmacologic stress to assess for stress-induced ischemia. Similarly, myocardial metabolism can be assessed by, C-11 acetate (oxidative metabolism) or C-11 palmitate (fatty acid metabolism). An on-site Cyclotron is necessary for these agents [11]. Most commonly, however, myocardial metabolism is assessed by FDG, a glucose analog that is taken up by the glucose transporters on the myocytes and metabolized by hexokinase to F-18 FDG 6-phosphate, which is no longer metabolized and becomes trapped within the myocytes. FDG imaging is usually performed following a glucose load and intravenous insulin administration to improve image quality. In viable but jeopardized cells, FDG uptake increases due to a shift to anaerobic metabolism and a preference for glucose rather fatty acid metabolism.
Combination of MPS with MIBI and FDG- PET
PET-CT facilities are now available in many Nuclear Medicine departments. It has been possible to conduct combination of myocardial SPECT studies using Te99m agents and PET-CT for better assessment of myocardial viability. The comparison between segmental myocardial perfusion and metabolism provides information regarding the amount of normal, hibernating, and necrotic myocardium [10]. We have been using this combination technique for patients in whom SPECT studies alone could not provide sufficiently acceptable results. Another advantage of PET study is to identify infiltrative/inflammatory diseases of the myocardium like Sarcoidosis. The underlying pathophysiology of cardiac sarcoidosis involves formation of non-caseating granulomas, which may involve any part of the heart, although the left ventricle is the most commonly affected chamber (Figures 1 and 2).
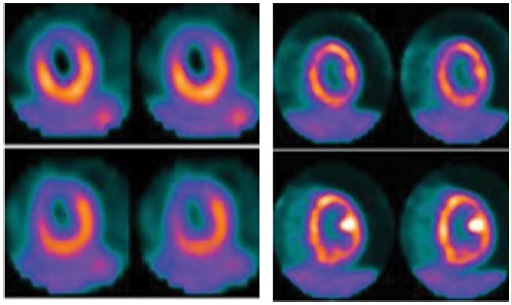
Figure 1: It shows a typical study of myocardial viability using combination of MPS with MIBI and FDG PET-CT. MPS finding shows a transmural infarct with irreversible ischemia. The PET-CT study reveals viable hibernating myocardium in that area.
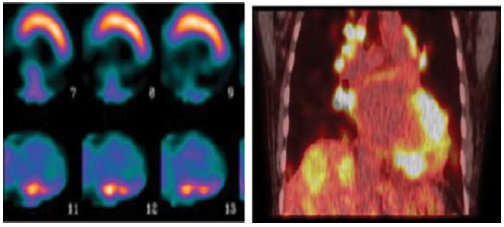
Figure 2: It shows one case of myocardial infiltration of Sarcoidosis along with findings of MPS with MIBI. After a course of steroid therapy patient’s myocardial function improved significantly thus confirming the diagnosis of Sarcoidosis. The focal patchy absence of perfusion in the infero-lateral, inferior and basal segments is likely to be caused by infiltration which is clearly demonstrated by findings of PET study.
The primary clinical manifestations of cardiac sarcoidosis, in order of frequency, include conduction abnormalities and arrhythmias, congestive heart failure, and sudden death [12].
In these patient’s myocardial perfusion study alone may lead to the diagnosis of non-viable myocardium which in reality may be due to infiltrative or inflammatory processes. For example, three patients with strong indication of nonviable myocardium in 4 segments in MPS-SPECT subjected to FDG Study showed Sarcoidosis infiltration to myocardium revealing the cause of MPS findings.
Summary
Prevalence of coronary artery disease like angina, heart attack and heart failure are rampant and appear to increase fast in our country. Coronary heart disease (CHD) is the major cause of heart failure. Severe left ventricular (LV) dysfunction was considered an irreversible condition, as regional akinesia was thought to represent infracted or necrotic myocardial tissue. It is now understood that, among patients with ischemic cardiomyopathy, LV systolic dysfunction can result from myocardial necrosis and remodeling as also from myocardial hibernation, or repetitive myocardial stunning. While myocardial necrosis is irreversible, systolic dysfunction resulting from hibernation and stunning are potentially reversible states of ventricular dysfunction. Clinical studies have also shown that viable myocardium can be demonstrated in a substantial number of patients with CHD and LV dysfunction, even in the absence of angina. In patients with significant amounts of viable myocardium, LV function may improve markedly, and even normalize, following successful revascularization.
Therefore, optimal assessment of myocardial viability is important. Prospective delineation of viable from nonviable myocardium in patients with coronary artery disease is also an important factor in deciding whether a patient should be re-vascularized or treated medically. Among the multiple imaging modalities existing for differentiating viable myocardium from scar in territories with contractile dysfunction, a combination of myocardial perfusion study (MPS) and myocardial metabolism with PET appears to be the best choice. Since PET-CT facilities are now available in many Nuclear Medicine Departments, it is now possible to conduct such combination of studies using Te99m agents for MPS and PET-CT with FDG for better assessment of myocardial viability and evidence-based management of the cardiac disorders.
There is no references